Risk of hepatitis B reactivation in patients with myeloproliferative neoplasms treated with ruxolitinib
Adeniyi Abraham Adesola,Matei-Alexandru Cozma,Yong-Feng Chen,Bahadar Singh Srichawla,Mihnea-Alexandru G?man
Abstract Classical Philadelphia-negative myeloproliferative neoplasms (MPNs),i.e.,polycythemia vera,essential thrombocythemia,and primary/secondary myelofibrosis,are clonal disorders of the hematopoietic stem cell in which an uncontrolled proliferation of terminally differentiated myeloid cells occurs.MPNs are characterized by mutations in driver genes,the JAK2V617F point mutation being the most commonly detected genetic alteration in these hematological malignancies.Thus,JAK inhibition has emerged as a potential therapeutic strategy in MPNs,with ruxolitinib being the first JAK inhibitor developed,approved,and prescribed in the management of these blood cancers.However,the use of ruxolitinib has been associated with a potential risk of infection,including opportunistic infections and reactivation of hepatitis B.Here,we briefly describe the association between ruxolitinib treatment in MPNs and hepatitis B reactivation.
Key Words: Ruxolitinib;Myeloproliferative neoplasms;Hepatitis B;Polycythemia vera;Myelofibrosis;JAK inhibitor
INTRODUCTION
Introduction to hepatitis B virus reactivation
Hepatitis B virus (HBV) infection is the most common chronic viral infection in the world.It affects more than 350 million people worldwide as chronic carriers,and more than 2 billion (30% of the world’s population) people show evidence of past exposure.Additionally,HBV infection has accounted for roughly half of total liver cancer mortality in 2010[1,2].Once contacted,the virus cannot be eliminated,even with proper and rapid antiviral treatment,but the infection is selflimiting in more than 95% of immunocompetent adults.These patients are now known as carriers 'anti-HBc positive'.They do not require specific management or monitoring unless immunosuppression is suspected[3].
If HBV persists for more than 6 mo in the body,the affected individual is considered to have chronic hepatitis B.Its incidence depends on the time of exposure: 95% of newborns,20%-30% of children aged 1 to 5 years,and less than 5% of adults[3].The reason for this dormant state of HBV is the presence of covalently closed circular viral DNA (cccDNA) that penetrates and persists indefinitely in hepatocyte DNA[2-4].This cccDNA acts as a template for future viral components in the case of HBV reactivation (HBVr).Viral transmission has been greatly slowed recently by the advent of a safe and effective vaccine,available since 1981 and introduced in 2011 in routine vaccination schedules in more than 180 countries[1,5].
DEFINITION,EPIDEMIOLOGY AND MANIFESTATIONS OF HBVR
The number of cases of HBVr after treatment with immunosuppressive agents is increasing worldwide,mostly attributed to an increase in the prevalence of positive HBV serology and,at the same time,an increase in the number of clinical indications for potent immunosuppression,including solid malignancies,inflammatory bowel disease,autoimmune disorders,blood cancers,e.g.myeloproliferative neoplasms (MPNs),and rheumatic diseases[3].
There are,although very similar,several definitions of HBVr,proposed by several medical associations from around the globe.All of them take into account both virological and serological criteria and describe HBVr as either an exacerbation of chronic hepatitis B or a reactivation of past hepatitis B infection.The most used definition is the one proposed by the American Association for the Study of Liver Diseases,last updated in 2020,which defines HVBr according to the virological status of the patient[4,6-8].
For HBsAg-positive patients with or without detectable HBV DNA: (1) At least 2 Log (or 100-fold) increase in HBV DNA compared to the baseline level;(2) HBV DNA at least 3 Log (or 1000) IU/mL in patients with previously undetectable HBV DNA;or (3) HBV DNA at least 4 Log (or 10000) IU/mL if the baseline level is unavailable[4,6-8].
For patients with HBsAg negative and HBV DNA negative: (1) HBV DNA becomes detectable;or (2) reverse HBsAg seroconversion (reappearance of HBsAg)[4,6-8].
The natural history of HBVr depends,among others,on the underlying disease requiring immunosuppressives,host immunity and the immunosuppressive agents used.Evolution can be classified into multiple stages[4,6-8].
After the initiation of immunosuppressive therapy,viral replication resumes,leading to a gradual increase in serum HBV DNA levels.The patient is still asymptomatic and,in general,HBVr-related hepatitis,described as an increase in alanine transaminase (ALT) or aspartate transaminase (AST) to 3 times upper limit of normal (ULN),does not develop[4,6-8].
HBVr-related hepatitis
ALT or AST increases to ≥ 3 times ULN (in some cases between 5-10 times ULN).Although most patients may remain asymptomatic,a small number might experience constitutional symptoms,such as pain in the right upper quadrant and jaundice.In rare cases,hepatic injury could further progress and cause liver failure,fulminant hepatitis or even death[4,6-8].
Spontaneous or antiviral-induced resolution
Normalization of serum ALT and AST levels,due to completion of immunosuppressive therapy,due to antiviral therapy,or due to host immunological mechanisms[4,6-8].
Acute liver failure/persistent liver injury
Found in a small number of individuals who continue to have a progressive decline in liver function,it is characterized by increased levels of bilirubin,prolonged prothrombin time,and,in very rare cases,even signs and symptoms of acute liver failure and hepatic decompensation (ascites and encephalopathy)[4,6-8].
MECHANISMS OF HBVR
As previously mentioned,after entering the hepatocytes,the viral genome is converted into plasmid-like cccDNA which can persist in liver cells in a latent state,serving as a reservoir for HBVr,in spite of active anti-HBV immune response.Compared to the hepatitis C virus (HCV) infection,complete eradication of both HBV cccDNA and integrated DNA is impossible with current antiviral treatment with nucleos(t)ide analogs.Thus,these cells constitute a reservoir of persistent HBV.Although HBVr can occur in a variety of settings,immunosuppressive therapies are the most commonly reported.A detailed description of the HBVr induction mechanisms of immunosuppressive therapies is provided in Table 1[3,4,6-12].
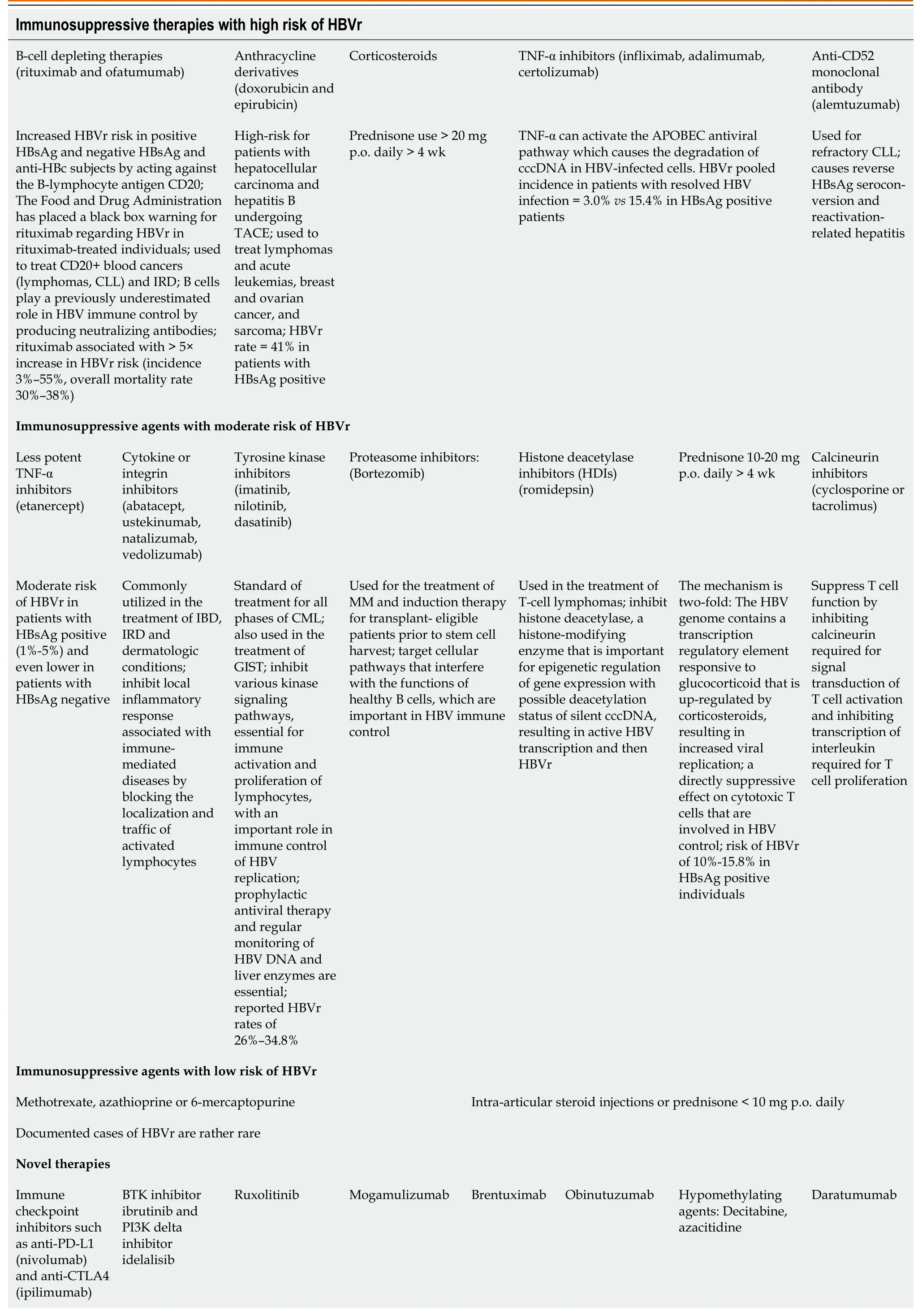
Table 1 Immunosuppressive agents associated with HBVr
RISK FACTORS FOR HBVR
Host-related risk factors for HBVr include male sex,younger age and older age (the elderly are more likely to have HBsAg seroclearance but persistent levels of total HBV DNA and cccDNA in the liver) and have been associated with increased risk of HBVr.Preexisting conditions,for example,cirrhosis or MPNs,also play a role in HBVr.HBVr has been reported in patients with MPNs,lymphomas,myeloma,and acute myeloid leukemia.However,it is not yet clear whether this association is attributed to the underlying disease or to the potent immunosuppressants used in the management of these blood cancers[7-9].
Virological factors include HBsAg and HBeAg positivity (adding a 5-to 8-fold risk for HBVr),non-A HBV genotypes,elevated HBV DNA levels before starting immunosuppressive therapy,and co-infection of HBV with other viruses such as HIV and HCV[4,7,8].
Type of immunosuppression: the greatest risk of HBVr is represented by the use of B-cell depleting therapies,used in the therapeutic armamentarium of blood and solid cancers and in the setting of bone marrow or solid organ transplantation[3,4,6-12].More details are presented in Table 1.
PREVENTION OF HBVR
Identifying infected individuals is the first and most important step for HBVr prophylaxis.According to the latest specialty guidelines,HBV infection screening must be performed in all patients who are receiving immunosuppressive treatment.Furthermore,all patients who are HBcAg positive,regardless of the status of HBsAg or the HBV DNA values,must receive prophylactic antiviral treatment.In numerous studies,prophylactic antiviral treatment has been shown to reduce the rate of HBVr,liver failure,and death in these categories of patients.Even if lamivudine was the first and for many years the most used oral antiviral agent for HBVr prophylaxis,YMDDgene mutations cause a high incidence of viral resistance if used for > 6 mo.This is why entecavir or tenofovir are recommended as therapies for HBVr prevention if intended for longer periods of time[4,6-8].
Duration of antiviral prophylaxis
In general,the duration of antiviral therapy varies depending on the type of immunosuppressives used.General recommendations include the use of antiviral therapy for at least 6 mo after the last dose of immunosuppressive agents is administered.However,in the case of B cell-depleting therapies (such as rituximab or obinutuzumab),it is recommended that antiviral prophylaxis be continued up to 12 mo after the last dose.Another important step is routine testing for HBV DNA and serum ALT and AST 3-6 mo after discontinuation of immunosuppressives[3,7].
Moreover,particular attention should be given to preventive measures,such as instructing patients to withdraw from alcohol consumption,as well as close monitorization of liver function tests in subjects who are prescribed pharmacological agents with a potentially hepatotoxic effect[13,14].According to the findings of the Dionysos Study,individuals diagnosed with HBV who consume alcohol experience elevated rates of hepatic fibrosis and death[13].
HBVR RISK IN MPNS TREATED WITH RUXOLITINIB
Ruxolitinib is a commonly used medication to treat MPNs,a group of blood disorders characterized by excessive blood cell production in the bone marrow.One of the common manifestations of MPNs is splenomegaly.Ruxolitinib acts by inhibiting Janus kinases (JAK1 and JAK2),which are enzymes involved in signaling pathways associated with cytokine receptors.By inhibiting these enzymes,ruxolitinib effectively helps control MPNs,particularly intermediate and high-risk myelofibrosis (MF) and high-risk polycythemia vera (PV).Importantly,its effect is not specific to any particular mutation.Ruxolitinib shows good oral bioavailability and reaches its maximum plasma concentration within 1-2 h after administration.Plasma half-life of this drug is approximately 3 h when administered at a maximum tolerated dose of 100 mg once a day.It is mainly metabolized through the CYP3A4 pathway,an important liver enzyme system involved in drug metabolism.Consequently,ruxolitinib has the potential for interactions with medications that induce or inhibit the CYP3A4 pathway.Ruxolitinib is primarily eliminated from the body through metabolism in urine and feces.Therefore,dosage adjustments are necessary for patients with renal or liver impairments,as these conditions can affect the clearance of the drug from the body[15].
It is important to note that this pharmacological agent exhibits immunomodulatory effects,meaning that it can modify the functioning of the immune system.As a result,ruxolitinib treatment may increase susceptibility to opportunistic infections in patients prescribed this drug.Thus,regular monitoring for signs of infection is important when subjects diagnosed with MPNs start taking this medicine[16].In particular,this pharmacological agent exhibits immunomodulatory and anti-inflammatory actions and can interfere with or impair the innate/adaptive immune response due to its interplay with dendritic cells,regulatory/T-helper lymphocytes or natural killer cells[17,18].
In a case report by Sjoblomet al[19],a patient with a history of PV received initial treatment with hydroxyurea.However,due to progressive splenomegaly and fatigue,his treatment was changed to pegylated interferon.Furthermore,to more effectively manage his symptoms,ruxolitinib was introduced.The patient experienced HBVr while on ruxolitinib,which was confirmed by abnormal liver function test results,positive viremia,and newly positive surface antigen for hepatitis B (HbsAg).With the initiation of tenofovir disoproxil,the patient's liver function gradually normalized,indicating successful management of HBVr[19].In another report by Shenet al[20],a patient with MF and a history of HBV infection experienced HBVr during ruxolitinib treatment.The initial elevation in transaminase levels was mistakenly attributed to drug toxicity.Subsequent detection of high plasma levels of HBV DNA confirmed the reactivation.Ruxolitinib was discontinued and antiviral therapy was started,resulting in a gradual decrease in transaminase levels[19,20].Additionally,in another report by Passucciet al[21],a patient with PMF and previous HBV infection achieved resolution of splenomegaly with ruxolitinib therapy.However,HBVr occurred after the patient discontinued prophylactic lamivudine.De-escalation of ruxolitinib and the initiation of anti-HBV therapy led to a gradual decline in HBV DNA levels without signs of active hepatitis[21].Kiritoet al[22] highlight the importance of considering prophylactic antiviral therapy in patients with chronic HBV infection before starting treatment with ruxolitinib,as such a proactive measure can help prevent HBVr,as observed in their patient[22].
Ruxolitinib has an immunosuppressive effect,leading to an increased risk of serious infections.The immunosuppressive effect of ruxolitinib is due to its interaction with multiple pathways of the immune system,affecting both adaptive and innate immune responses.This can result in the reactivation of silent infections such as tuberculosis,HBV,and varicella-zoster virus.Therefore,proactive infection surveillance,baseline screening for latent infections,and considering prophylactic or preventive interventions for specific infections such as varicella-zoster virus and HBV virus are crucial[23].A pilot study conducted by Crodelet al[24] investigated the frequency of infections in patients with MPNs.The study included multiple centers and relied on patient-reported data.The findings revealed that over 50% of MPN patients experienced one or more episodes of infection within a 12-mo period.The most frequently reported infections were upper respiratory tract infections,herpes virus infections,and gastrointestinal infections.Among the different subtypes of MPNs,subjects with MF had the highest percentage of infectious events,followed by PV and essential thrombocythemia[24].Furthermore,Lussanaet al[25] conducted a systematic review and meta-analysis examining the safety and efficacy of ruxolitinib in the treatment of MF and PV.The study specifically focused on the incidence of infections in patients receiving ruxolitinib.It was found that ruxolitinib,with its immunosuppressive effects,can affect immune functions and increase the risk of infections.Herpes zoster,pneumonia,bronchitis,and urinary tract infections were among the most frequently reported infectious complications.The aforementioned quantitative assessment emphasized the importance of carefully evaluating infection risk before initiating ruxolitinib therapy and highlighted the need to monitor and address infections in patients receiving ruxolitinib for MF and PV[25].
In a paper by Perriconeet al[26],two case reports of HBVr in MF patients treated with ruxolitinib are discussed.The immunosuppressive effects of ruxolitinib,particularly in dendritic cells and T cells,may contribute to an increased risk of infections,including HBVr.The article emphasizes the need for vigilance among physicians when considering infectious causes when using immunosuppressive agents such as ruxolitinib[26].In a prospective study by Gillet al[27],40 patients with MPNs were included.Among the 37 subjects who were negative for HBsAg,15 tested positive for anti-HBc antibodies,indicating occult HBV infection.Prophylactic treatment for HBV was administered to the three HBsAg positive patients.During a median follow-up of 19.2 mo,four patients (26.7%) experienced HBVr,occurring at a median of 10.5 mo after starting ruxolitinib therapy.The estimated cumulative incidence rates of HBVr at 6 and 12 mo were 7.7%and 30.8%,respectively.This investigation emphasizes the need to monitor HBVr in patients with occult HBV infection who receive ruxolitinib therapy[27].Garcia-Hortonet al[28] conducted a retrospective cohort study involving 1171 individuals with MPNs to evaluate the risk of HBVr in subjects treated with ruxolitinib.Among the 58 patients with prior HBV infection,20 received ruxolitinib.Only one patient experienced HBVr during ruxolitinib therapy,and their HBV DNA levels peaked,but subsequently returned to undetectable levels without interrupting or reducing the ruxolitinib dose[28].Duanet al[29] conducted a retrospective analysis to evaluate the incidence of HBVr in MPN patients treated with ruxolitinib.The study included 62 patients with a history of HBV infection,56 with resolved infection and 6 with chronic HBV infection.Among patients with chronic HBV infection,two experienced HBVr and hepatitis flare-up after ruxolitinib therapy.None of the patients with resolved HBV infection experienced reactivation.In particular,the two patients with chronic HBV infection did not receive antiviral prophylaxis[29].Caocciet al[30] presented a case report of a patient with MF who experienced HBVr during treatment with ruxolitinib.The patient had a history of HBV infection and initially received ruxolitinib for symptoms related to MF.Although there was improvement in MF symptoms,HBVr was observed through increased levels of HBV-DNA.Adjusting the dose of ruxolitinib resulted in an improvement in symptoms,but HBV-DNA levels remained fluctuating.This case report raises concerns about the management of MF patients with HBV infection receiving ruxolitinib and emphasizes the importance of careful monitoring and potential prophylactic treatment[30].
A schematic representation between the benefits and risks of ruxolitinib use in terms of opportunistic infections in MPNs is depicted in Figure 1.

Figure 1 Benefits and risks of ruxolitinib use in terms of opportunistic infections in myeloproliferative neoplasms.MPNs: Myeloproliferative neoplasms;RUX: Ruxolitinib;HBVr: Hepatitis B virus reactivation;NK cells: Natural killer cells.
CONCLUSION
In conclusion,ruxolitinib is an effective medication to manage MPNs such as MF and PV,particularly in intermediate and high-risk cases.By inhibiting JAK1 and JAK2,ruxolitinib helps control excessive blood cell production and reduce splenomegaly.However,its use carries certain risks and considerations.The interaction of ruxolitinib with the immune system can increase the susceptibility to opportunistic infections,highlighting the need for vigilant monitoring and timely intervention.Furthermore,there is a potential for HBVr,especially in patients with a history of HBV infection.Close monitoring of liver function and proactive measures,such as prophylactic antiviral therapy,are crucial to managing these risks.In general,ruxolitinib offers therapeutic benefits for MPNs,but careful evaluation of infection risk,regular monitoring,and appropriate interventions are essential to ensure patient safety.
FOOTNOTES
Author contributions:Adesola AA,Cozma MA,Chen YF,Srichawla BS,Gaman MA reviewed the literature and drafted the manuscript;Bahadar SS,Cozma MA and Gaman MA provided overall intellectual input,reviewed the literature,and edited the final version of the manuscript;all authors approved the final version to be published.
Supported byCompetitiveness Operational Programme (COP) A1.1.4.ID: P_37_798 MYELOAL-EDIAPROT,No.149/26.10.2016,(MySMIS2014+: 106774).
Conflict-of-interest statement:All the authors declare no conflict of interest.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Romania
ORCID number:Matei-Alexandru Cozma 0000-0002-4998-0105;Bahadar Singh Srichawla 0000-0002-5301-4102;Mihnea-Alexandru G?man 0000-0001-7133-8875.
S-Editor:Liu JH
L-Editor:A
P-Editor:Liu JH
 World Journal of Hepatology2023年11期
World Journal of Hepatology2023年11期
- World Journal of Hepatology的其它文章
- Metabolomics in chronic hepatitis C: Decoding fibrosis grading and underlying pathways
- Evaluation of a protocol for rifaximin discontinuation in critically ill patients with liver disease receiving broad-spectrum antibiotic therapy
- Global burden of cirrhosis and other chronic liver diseases due to nonalcoholic fatty liver disease,1990-2019
- Function of macrophage-derived exosomes in chronic liver disease:From pathogenesis to treatment
- Budd-Chiari syndrome in children: Challenges and outcome
- Letter to editor ‘Non-invasive model for predicting high-risk esophageal varices based on liver and spleen stiffness’
