Using a novel hemostatic peptide solution to prevent bleeding after endoscopic submucosal dissection of a gastric tumor
Kuniyo Gomi,Yorimasa Yamamoto,Erika Yoshida,Misako Tohata,Masatsugu Nagahama
Abstract BACKGROUND Endoscopic mucosal dissection has become the standard treatment for early gastric cancer.However,post-endoscopic submucosal dissection (ESD) ulcer occurs in 4.4% of patients.This study hypothesized whether applying PuraStat,a novel hemostatic peptide solution,prevents post-ESD bleeding.AIM To investigate the preventive potential of PuraStat,a hemostatic formulation,against bleeding in post-ESD gastric ulcers.METHODS Between May 2022 and March 2023,101 patients (Group P) underwent ESD for gastric diseases at our hospital and received PuraStat (2 mL) for post-ESD ulcers.We retrospectively compared this group with a control group (Group C) comprising 297 patients who underwent ESD for gastric diseases at our hospital between April 2017 and March 2021.P values <0.05 on two-sided tests indicated significance.RESULTS Post-ESD bleeding occurred in 6 (5.9%) (95%CI: 2.8–12.4) and 20 (6.7%) (95%CI:4.4–10.2) patients in Groups P and C,respectively,with no significant betweengroup difference.The relative risk was 1.01 (95%CI: 0.95–1.07).The lesser curvature or anterior wall was the bleeding site in all 6 patients who experienced postoperative bleeding in Group P.In multivariate analysis,the odds ratios for resection diameter ≥ 50 mm and oral anticoagulant use were 6.63 (95%CI:2.52–14.47;P=0.0001) and 4.04 (1.26–0.69;P=0.0164),respectively.The adjusted odds ratio of post-ESD bleeding and PuraStat was 1.28 (95%CI: 0.28–2.15).CONCLUSION PuraStat application is not associated with post-ESD bleeding.However,the study suggests that gravitational forces may affect the effectiveness of applied PuraStat.
Key Words: Endoscopic submucosal dissection;PuraStat;Bleeding;Gastric cancer;hemostatic forceps;Proton pump inhibitor;hemostatic peptide solution
INTRODUCTION
Endoscopic mucosal dissection (ESD) has become the standard treatment for early gastric cancer.However,bleeding from the post-ESD ulcer occurs in 4.4% of patients[1].To prevent this complication,recommended measures include coagulating blood from the remaining vessels on the ulcer surface using hemostatic forceps or a similar device and admin-istering proton pump inhibitors[2].However,it is essential to note that excessive vascular coagulation increases the risk of delayed perforation,necessitating caution.With the aging population,the number of patients taking oral antithrombotic drugs will likely increase,leading to more cases of larger post-ESD ulcers due to the expansion of ESD-adapted lesions.As a result,controlling post-ESD bleeding poses a considerable challenge.
PuraStat (3D-Matrix Europe Ltd.,France) is a novel hemostatic peptide solution designed to reduce the need for cauterization using hemostatic forceps in managing exudative bleeding during gastrointestinal endoscopy.The material comprises peptide molecules comprising three amino acids (arginine,alanine,and aspartic acid) that rapidly form fibers and transform into peptide hydrogels on contact with body fluids such as blood.By covering the bleeding point with this hemostat,the collapsed parenchymatous organ and superficial portions of the blood vessels are physically occluded,and blood coagulation occurs to stop bleeding.
We aim to investigate whether applying PuraStat to post-ESD gastric ulcers can prevent post-ESD bleeding.
MATERIALS AND METHODS
Patients and methods
From May 2022 to March 2023,101 patients (Group P) who underwent ESD for gastric diseases at our hospital received PuraStat 3 mL formulation.PuraStat (1 mL) was used to stop bleeding during ESD,and after ESD,bleeding from the remaining blood vessels in the post-ESD ulcer was stopped by initiating coagulation using hemostatic forceps.Subsequently,the remaining 2 mL of PuraStat was applied to the post-ESD ulcer (Figure 1).Two experienced endoscopists(over 300 ESD cases) applied the medication to the post-ESD ulcers.Each patient received a proton pump inhibitor (PPI)for 8 wk starting from the day of the ESD.An endoscopic examination was performed on the day following the ESD day to address any potential bleeding.Hemostatic treatment with argon plasma coagulation or clips was performed in cases where bleeding was identified.

Figure 1 Applying PuraStat to post-endoscopic submucosal dissection gastric ulcers. A: Post-endoscopic submucosal dissection gastric ulcer;B:Applying PuraStat with a special catheter;C: Ulcer after application.
A control group (Group C) com-prising 297 patients who underwent ESD for gastric diseases at our hospital from April 2017 to March 2021 was retrospectively compared with group P.
Consultations with physicians were conducted to consider the discontinuation of antithrombotic medications.In cases where discontinuation was not feasible,ESD was performed while maintaining the continuation of aspirin.Antiplatelet medications eligible for resumption were restarted 2 d after the ESD procedure.Warfarin use was not discontinued during ESD.In the case of direct-acting oral anticoagulants,they were not administered on the day of ESD but resumed on the following day.
This study was approved by the Showa University Institutional Review Board (2023-052-A) and complied with the 1989 revised version of the Declaration of Helsinki.
Outcome parameters
Post-ESD bleeding was the primary study endpoint of the study.In contrast,the secondary endpoints included the duration from ESD to the onset of post-ESD bleeding and adverse events associated with PuraStat administration.Post-ESD bleeding was defined as bleeding from a post-ESD ulcer that required emergency endoscopic hemostasis or a ≥ 2 g/dL reduction in hemoglobin at week 8 after ESD.
Statistical analyses
The primary endpoint,post-ESD bleeding,was analyzed using theχ2test.Mann–WhitneyU-test was employed for the duration from ESD to the onset of post-ESD bleeding.Logistic regression analysis was performed for multivariate analysis.Pvalues <0.05 on two-sided tests were considered statistically significant.JMP Pro 16 (SAS Institute Inc.,North Carolina,United States) for Windows was used for the statistical analyses.
RESULTS
Patients' background characteristics in Groups P and C were comparable (Table 1).ESD lesions were comparable between the groups.Notably,non-experts conducted 71 (70.3%) and 143 (48.1%) of the ESD procedures in Groups P and C,respectively,with a higher proportion in Group P (P=0.0001).The median resection times for Groups P and C were 64(10–320) and 68 (7–445) min,respectively,exhibiting comparability.However,lesions with resection diameters ≥ 50 mm were lower in Group PvsC [3 (3.0%)vs32 (10.8%);P=0.0167].Theen blocand the completeen blocresection rates for Groups P and C were comparable (Table 2).
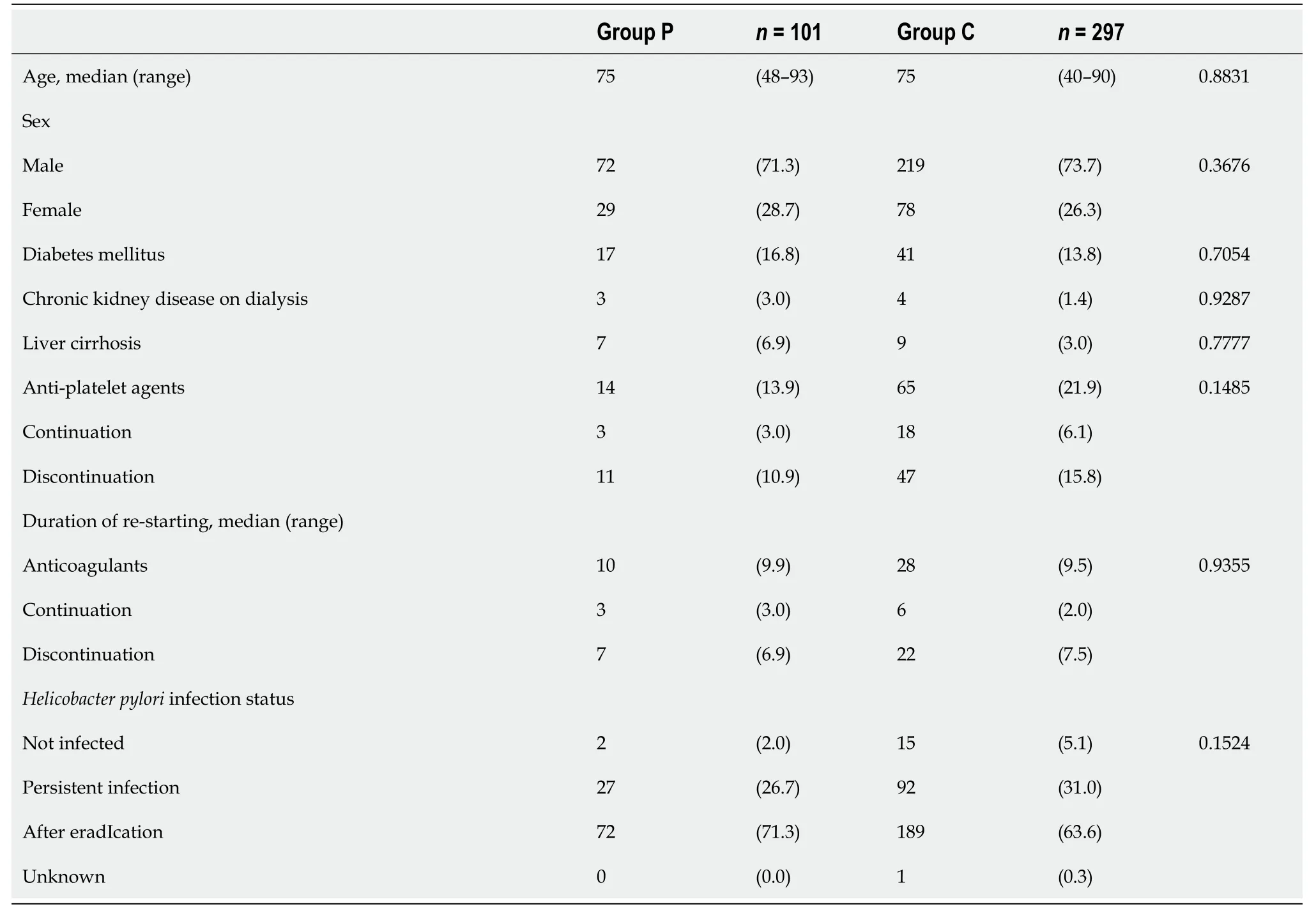
Table 1 Clinical characteristics of the patients,n (%)

Table 2 Characteristics of the lesions and treatment outcomes,n (%)
Post-ESD bleeding occurred in 6 (5.9%) (95%CI: 2.8–12.4) and 20 (6.7%) (95%CI: 4.4–10.2) patients in Groups P and C,respectively,with no significant between group difference (P=0.7804) (Table 2).The relative risk as 1.01 (95%CI:0.95–1.07).No adverse events were observed with PuraStat application.In addition,the median number of days between when ESD was performed and when post-ESD bleeding started was 2 (1–12) and 7.5 (1–14) days in Groups P and C,respectively,with no significant difference between the groups (Figure 2).Other complications were not significantly different between the groups (Table 2).

Figure 2 Date of post-endoscopic submucosal dissection bleeding.
Multivariate analysis was performed for the factors associated with postoperative bleeding due to PuraStat application,with ESD practitioner,resection diameter ≥ 50 mm,oral antiplatelet drugs,and oral anticoagulant drugs as explanatory variables.The odds ratio for resection diameter ≥ 50 mm and oral anticoagulant use were 6.63 (95%CI: 2.52–14.47;P=0.0001) and 4.04 (1.26–0.69;P=0.0164),respectively.Adjusted OR of post-ESD bleeding and PuraStat was 1.28 (95%CI:0.28–2.15;P=0.6363) (Table 3).

Table 3 Factors involved in post-operative bleeding
DISCUSSION
PuraStat is an absorbable local hemostatic agent for reducing the requirement of cauterization using hemostatic forceps to stop exudative bleeding in gastrointestinal endoscopic treatment.PuraStat is highly useful for combating intraoperative bleeding.When the peptide molecules in the hemostatic material contact body fluids,such as blood,they rapidly form fibers and become peptide hydrogels,covering bleeding points and physically occluding collapsed parenchymatous organs and superficial blood vessels,enabling blood coagulation.
Several randomized controlled trials have compared vascular coagulation procedures during ESD.In one randomized controlled trial,the PuraStat group exhibited a significantly shorter duration of coagulation treatment device usage than the control group (49.3%vs99.6%,P<0.001)[3].In another trial[4],the mean number of coagulation procedures using a hemostat was significantly lower in the PuraStat group than in the control group (1.0 ± 1.4vs4.9 ± 5.2,P<0.001),provingthe efficacy of PuraStat in managing intraoperative bleeding.
PuraStat is expected to accelerate the healing of post-ESD ulcers and reduce the post-ESD bleeding rate[5,6].Additionally,PuraStat has been confirmed to prevent post-ESD bleeding in the United States and Europe.However,only one report[6] of its usefulness in preventing post-ESD bleeding and its impact on post-ESD bleeding rate exists.PuraStat was applied to 65 Lesions in the esophagus (n=8),stomach (n=22),duodenum (n=10),ampulla of Vater (n=3),colon (n=7),and rectum (n=15).Therefore,our study aimed to investigate the effect of applying PuraStat to post-ESD gastric ulcers,assessing its potential in preventing exudative bleeding following ESD.
PuraStat was administered to post-ESD gastric ulcers immediately after ESD in 101 patients who underwent ESD for gastric disease at our hospital from May 2022 to March 2023.However,the postoperative bleeding rate was not reduced with PuraStat use compared with previous ESD cases in our hospital.Significantly,PuraStat application was not associated with post-ESD bleeding.
An essential observation was that PuraStat did not demonstrate a sustained effect.Therefore,we suspect it had a minimal hemostatic impact on postoperative bleeding over time.We expected reduced postoperative bleeding within the first few days after ESD;however,no significant difference was observed in the occurrence of postoperative bleedingbetween the PuraStat and non-PuraStat groups.This suggests that PuraStat's hemostatic effect might be minimal and not enduring,warranting further investigation into its efficacy and potential limitations in post-ESD bleeding.
The bleeding site for all 6 patients who experienced postoperative bleeding in the PuraStat group was consistently identified as the lesser curvature or anterior wall (Table 4).Comparatively,in previous cases at our hospital,post-ESD bleeding originated from the lesser curvature in 35.0%,anterior abdominal wall in 15.0%,greater curvature in 20.0%,and posterior abdominal wall in 30.0% of cases.This discrepancy suggests that gravitational forces may affect the efficacy of applied PuraStat.Specifically,PuraStat appeared less effective for lesions on the anterior wall and lesser curvature than lesions on the greater curvature and posterior wall.Adjusting the patient’s position during application could potentially enhance PuraStat’s effectiveness by preventing the hemostatic material from flowing away from the targeted area.

Table 4 Location of post-operative bleeding lesions,n (%)
The limitations of this study include its single-center basis and retrospective design.Therefore,conducting large-scale,multicenter prospective studies on this subject is highly desirable to provide more comprehensive and generalizable insights.
CONCLUSION
Our findings indicate that PuraStat application is not associated with post-ESD bleeding.However,we infer that gravitational forces may affect the effectiveness of applied PuraStat.As a result,we aim to explore and develop strategies to prevent PuraStat from flowing away from the target area of interest in further investigation.Addressing this aspect may contribute to optimizing the hemostatic efficacy of PuraStat in the context of post-ESD procedures.
ARTICLE HIGHLIGHTS
Research background
Endoscopic mucosal dissection (ESD) has become the standard of care for early gastric cancer,but bleeding from ulcers after ESD occurs in 4.4% of patients.We aim to minimize post-ESD bleeding to the greatest extent possible.PuraStat (3DMatrix Europe Ltd.,France) is a novel hemostatic peptide solution aiming to reduce the need for cautery with hemostatic forceps in treating exudative bleeding during gastrointestinal endoscopy.We hypothesized that applying PuraStat to gastric ulcers after ESD could prevent post-ESD bleeding.
Research motivation
Reducing post-ESD bleeding is a crucial goal.If PuraStat can be applied to post-ESD gastric ulcers to prevent post-ESD bleeding,it may have broader applications in gastrointestinal bleeding.
Research objectives
The purpose of this study is to determine whether the application of PuraStat to gastric ulcers after ESD can prevent post-ESD bleeding.
Research methods
From May 2022 to March 2023,101 patients (Group P) who underwent ESD for gastric diseases at our hospital received PuraStat (2 mL) applied to their post-ESD ulcer.We retrospectively compared this group with a control group (Group C)com-prising 297 patients who underwent ESD for gastric diseases at our hospital between April 2017 and March 2021.Post-ESD bleeding was the primary endpoint,while the secondary endpoints included the number of days from ESD to post-ESD bleeding and adverse events associated with PuraStat administration.
Research results
Post-ESD bleeding occurred in 6 (5.9%) (95%CI: 2.8–12.4) and 20 (6.7%) (95%CI: 4.4–10.2) patients in Groups P and C,respectively,with no significant between-group difference.The relative risk was 1.01 (95%CI: 0.95–1.07).Therefore,PuraStat application was not associated with post-ESD bleeding.The lesser curvature or anterior wall was the bleeding site in all 5 patients who experienced postoperative bleeding in the PuraStat group.This suggests that gravitational forces may affect the efficacy of applied PuraStat.Specifically,PuraStat seemed less effective for lesions on the anterior wall and lesser curvature than those on the greater curvature and posterior wall.Adjusting the patient’s position during its application could potentially enhance PuraStat’s effectiveness by preventing the hemostatic material from flowing away from the targeted area.
Research conclusions
PuraStat application is not associated with post-ESD bleeding.
Research perspectives
We infer that gravitational forces may affect the efficacy of applied PuraStat.Hence,we aim to explore and develop strategies to prevent PuraStat from flowing away from the targeted areas of interest in further investigation.
ACKNOWLEDGEMENTS
The authors thank Eisuke Inoue,PhD,Professor of Showa University,for advice on statistical analysis.
FOOTNOTES
Author contributions:Gomi K and Yamamoto Y designed the research study;Gomi K,Yamamoto Y,Yoshida E and Tohata M performed the research;Gomi K and Nagahama M analyzed the data and wrote the manuscript;All authors have read and approve the final manuscript.
Institutional review board statement:This study was reviewed and approved by the Showa University Institutional Review Board (2023-052-A).
Clinical trial registration statement:The clinical trial is registered with UMIN Clinical Trials Registry,using identifier UMIN000053481.Details can be found at https://center6.umin.ac.jp/cgi-bin/ctr/ctr_view_reg.cgi?recptno=R000061029.
Informed consent statement:All study participants or their legal guardians,provided informed written consent prior to study enrollment.
Conflict-of-interest statement:All authors declare no potential conflicting interests related to this paper.
Data sharing statement:No additional data are available.
CONSORT 2010 statement:The authors have read the CONSORT 2010 statement,and the manuscript was prepared and revised according to the CONSORT 2010 statement.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:Japan
ORCID number:Kuniyo Gomi 0000-0002-8914-1170.
Corresponding Author's Membership in Professional Societies:Japan Gastroenterological Endoscopy Society,37251731.
S-Editor:Liu JH
L-Editor:A
P-Editor:Zhang YL
 World Journal of Gastrointestinal Endoscopy2024年3期
World Journal of Gastrointestinal Endoscopy2024年3期
- World Journal of Gastrointestinal Endoscopy的其它文章
- Computed tomography for prediction of esophageal variceal bleeding
- Methods to increase the diagnostic efficiency of endoscopic ultrasound-guided fine-needle aspiration for solid pancreatic lesions: An updated review
- Future directions of noninvasive prediction of esophageal variceal bleeding: No worry about the present computed tomography inefficiency
- Precision in detecting colon lesions: A key to effective screening policy but will it improve overall outcomes?
- Computed tomography for the prediction of oesophageal variceal bleeding: A surrogate or complementary to the gold standard?
- Could near focus endoscopy,narrow-band imaging,and acetic acid improve the visualization of microscopic features of stomach mucosa?
