長期探尋的Au23(S-Adm)16 結構及未曾預期的摻雜效應
摘要:一鍋同時獲得單個金屬原子摻雜的納米團簇與母體團簇富有挑戰性。這樣的合成可排除微量雜質的影響,使得摻雜和未摻雜納米團簇的性質對比更加合理可靠。在此,我們首次實現了這種合成,得到了長期追尋的納米團簇Au23(SAdm)16和其單鎘摻雜的Au22Cd1(S-Adm)16納米團簇,并通過單晶X射線晶體學解析了其結構。令人驚訝的是,與以前的報道結果相反,Au23(S-Adm)16比Au22Cd1(S-Adm)16更穩定。另一方面,由于摻入鎘原子后,內核Au―Au鍵長度增加,光激發電子轉移阻力增加,導致Au22Cd1(S-Adm)16吸收和發射強度明顯下降。因而,不僅團簇的穩定性,而且團簇的吸收和發射強度也與內核Au―Au鍵的長度關聯。這項工作表明了兩種團簇結構上的微小差異就可導致光學、熱穩定性等方面的顯著區別,也為研究金屬納米團簇的構效關系提供了良好的借鑒。
關鍵詞:金屬納米團簇;合成;Au―Au鍵長;性質;構效關系
中圖分類號:O641
Long-Pursued Structure of Au23(S-Adm)16 and the Unexpected Doping Effects
Abstract: Metal nanoclusters are rising stars in material science, and one advantageis their atomically precise tunability. It is well known that metal doping can efficientlymodify the properties of metal nanoclusters. In particular, without altering the parentnanocluster framework, doping a single heterometal atom can tailor the properties ofmetal nanoclusters and aid investigations of the structure–property relationship of metalnanoclusters. To our knowledge, the simultaneous synthesis of a single heterometaldopednanocluster and its parent nanocluster is challenging and has not been previouslyreported; however, this is highly desirable because it can prevent the influence of traceimpurities and allow comparison between doped and undoped nanoclusters. The singleCd-doped gold nanocluster Au22Cd1(S-Adm)16 (S-Adm = 1-adamantanethiolate) hasbeen previously synthesized and structurally elucidated. However, the structure of theparent nanocluster, Au23(S-Adm)16, remains unknown, inspiring this investigation. In this study, we synthesized Au23(SAdm)16 and its single-doped Au22Cd1(S-Adm)16 nanocluster in one pot for the first time, and we resolved their structuresusing single-crystal X-ray crystallography. The structure of Au22Cd1(S-Adm)16 is similar to that of Au23(S-Adm)16 except thata kernel Au atom in Au23(S-Adm)16 is replaced with a Cd atom. This Cd replacement causes the kernel Au―Au bond lengthto increase owing to the loosening of the original closely packed structure. In contrast to prior reports, Au23(S-Adm)16 issurprisingly more stable than Au22Cd1(S-Adm)16, as determined via ultraviolet visible-near infrared (UV-Vis-NIR)spectroscopy at 80 °C. This stability was attributed to the decrease in the kernel Au―Au bond length. Although themaximum absorption of Au22Cd1(S-Adm)16 slightly red-shifted from 605 to 608 nm after Cd doping, the molar extinctioncoefficient of Au23(S-Adm)16 at 605 nm was approximately twice that of Au22Cd1(S-Adm)16 at 608 nm. Thus, the increasein kernel Au―Au bond length may decrease the photoexcitation electron transfer efficiency owing to lengthening of thephotoexcitation electron transfer pathway. As further support for this opinion, although the two nanoclusters showed similaremission profiles and maxima (750 nm for Au23(S-Adm)16 and 760 nm for Au22Cd1(S-Adm)16), they exhibited obviousemission intensity differences. Specifically, the quantum yield of Au23(S-Adm)16 (approximately 3.160 × 10?5) was found tobe 1.13 times that of Au22Cd1(S-Adm)16 (approximately 2.793 × 10?5). Thus, the stability and absorption and emissionintensities correlate with the kernel Au―Au bond length.
This study shows that two metal nanoclusters with slight structural differences can exhibit different properties in terms of optical and thermal stability, providing a good reference for studying their structure–property relationships.
Key Words: Metal nanoclusters; Synthesis; Au―Au bond length; Property; Property–structure correlation
1 Introduction
Metal nanoclusters have recently attracted extensive attention,partly due to their tunability with atomic precision 1–16. Metaldoping is well-realized for efficiently modulating theproperties 17–25, especially, single-heterometal atom doping 26–28without changing the framework of parent nanoclusters providesan excellent opportunity not only for subtly tailoring theproperties, but also for insightfully investigating the structure(composition)-property correlation. However, the classic mixedsalts reduction method leads to the doped nanoclusters, whoseparent nanoclusters are difficult to be obtained in some cases 29–31.For instance, single cadmium-doped gold nanoclusterAu22Cd1(S-Adm)16 (S-Adm = 1-adamantanethiolate) waspreviously synthesized and structurally resolved, while its parentnanocluster Au23(S-Adm)16 remains mysterious (ubi infra) 32. Onthe other hand, the various synthesis systems may bring aboutsome uncertainties for the property comparison due to thepotential enclosure of trace solvents or some other impurities,thus the co-current synthesis of the single-atom dopednanoclusters and their precursor (parent) nanoclusters is highlydesired, whilst challenging. To the best our knowledge, such asynthesis was not achieved until now, which inspires our studyenthusiasm. Especially, we are interested in the co-currentsynthesis of Au23(S-Adm)16 and Au22Cd1(S-Adm)16 mentionedabove, since the structure of Au23(S-Adm)16 is notexperimentally resolved. Early in 2014, Dass et al. 33 detectedthe species of Au23(S-Adm)16 by mass spectrometry, and theypredicted the instability of Au23(S-Adm)16 basing on localchemical bonding and surface coverage effects. In 2018, Huanget al. 34 claimed the successful synthesis of Au23(S-Adm)16mainly basing on the UV-Vis-NIR spectrometry (Au23(CHT)16as a reference, CHT = cyclohexanethiolate) over 7 day’sreaction, however, they didn’t provide single crystal X-raystructurefor the assignment. Subsequently, in 2020, Jin et al. 32facilely synthesized Au22Cd1(S-Adm)16 and resolved itsstructure, but they did not obtain Au23(S-Adm)16 in their work,which was attributed to the instability of Au23(S-Adm)16 on thebasis of ionization potential and electron affinity analysis. Thus,it is still a question whether the atomically mono-disperseAu23(S-Adm)16 can be obtained. The recently introduced antigalvanicreduction (AGR) 35–38 was validated to be a veryversatile method to tune the compositions and structures of metalnanoclusters, especially, this method can be utilized tosynthesize mono-metal nanoclusters 35 as well as doped metalnanoclusters 26, which provides the prerequisite for the cocurrentsynthesis of single-heterometal atom-doped nanoclustersand their parent nanoclusters. The known Au23(SR)16 39–42structure has some relation with the star nanocluster Au25(SR)18,and the transformation from Au25(SeR)18 to Au23(SeR)16 wasalso reported 43, thus the well-known Au25(SR)18 nanoclusterwas selected as the starting nanocluster. Fortunately, wesuccessfully co-synthesized Au23(S-Adm)16 and Au22Cd1(SAdm)16 by two-phase anti-galvanic reduction method 38 and thuscan compare them rationally.
2 Experimental and computational section
2.1 Chemicals
Tetrachloroauric(III) acid (HAuCl4?4H2O, gt; 99.9% metalsbasis), tetrahydrofuran (THF, 99.0%), acetonitrile (CH3CN,99.0%), methanol (CH3OH, 99.5%), dichloromethane (CH2Cl2,99.0%), petroleum ether (99.0%) and toluene (Tol, 99.0%) werepurchased from Sinopharm chemical reagent Co. Ltd.Tetraoctylammonium bromide (TOAB, 98.0%) was purchasedfrom Aladdin Co. Ltd. Sodium borohydride (NaBH4) waspurchased from Shanghai Chemical Reagent Co. Ltd. 1-Adamantanethiol (1-AdmSH, 95%) and 2-phenylethanethiol(PET) were purchased from Sigma-Aldrich (USA).Cd(NO3)2?4H2O (99.5%) was purchased from Adamas(Switzerland).
2.2 Synthesis of Au23(S-Adm)16 and Au22Cd1(S-Adm)16nanoclusters
As mentioned above, the two-phase anti-galvanic reductionmethod 38 was adopted for the co-current synthesis (seesupporting information for the experimental details). Briefly, theorganic phase was prepared by dissolving Au25(SCH2CH2Ph)18?TOA+ (short for Au25(SCH2CH2Ph)18?) in toluene, in which 1-adamantanethiol was added. The water phase was prepared bydissolving Cd(NO3)2?4H2O in water. The organic phase wasadded to the water phase and floated on the surface of the waterphase. The reaction temperature was held at 70 °C, and thegrowth of the gold nanoclusters continued for 12 h. After thereaction was complete, the organic phase was washed bymethanol. The Au23(S-Adm)16 and Au22Cd1(S-Adm)16nanoclusters were isolated by preparative thin-layerchromatography 44 (PTLC, CH2Cl2 : petroleum = 1 : 4, ether wasused as the developing solvent). Single crystals of Au22Cd1(SAdm)16 nanoclusters were formed by slowly evaporating amethylbenzene/methanol solution for two days. Single crystalsof Au23(S-Adm)16 nanoclusters were formed by slowlyevaporating a methylbenzene/acetonitrile solution for one week.The yield of Au22Cd1(S-Adm)16 is a little higher than that ofAu23(S-Adm)16 (8% vs. 6%, on the basis ofAu25(SCH2CH2Ph)18?). Note that, the single-phase anti-galvanicreduction method under otherwise similar conditions can onlylead to a white emulsion, and the two-phase ligand exchangeunder otherwise similar conditions except for the absence ofCd(NO3)2?4H2O provides a dominant product with featurelessabsorption (see Fig. S1, Supporting Information), indicating thesuperiority of the two-phase AGR method in the cosynthesis ofAu23(S-Adm)16 and Au22Cd1(S-Adm)16 compared with the othertwo in this work.
2.3 Characterization
UV-Vis/NIR measurements were conducted on a AnalytikJena 210 plus spectrophotometer (Germany) (CH2Cl2 as solvent)in the range of 190–1100 nm at room temperature. Thephotoluminescence (PL) properties of the nanoclusters intoluene were recorded on confotec TMMR520 (Republic ofBelarus) with EX = 532 nm. The PL lifetime of Au23(S-Adm)16and Au22Cd1(S-Adm)16 in toluene were measured on Horibafluoromax plus (USA) at room temperature. Characterizations ofenergy dispersive spectrometer (EDS) were carried out onHitachi SU-8020 (Japan) under the accelerating voltage of 5 kV.The single crystal diffraction data of Au23(S-Adm)16 andAu22Cd1(S-Adm)16 were recorded on a Stoe Stadivari X-rayDiffractometer (Stoe, Germany). Au23(S-Adm)16 was measuredby graphite monochromatic Mo-Kα (λ = 0.71073 ?) radiation.Au22Cd1(S-Adm)16 was measured by graphite monochromaticCu-Kα (λ = 1.54178 ?) radiation.
3 Results and discussion
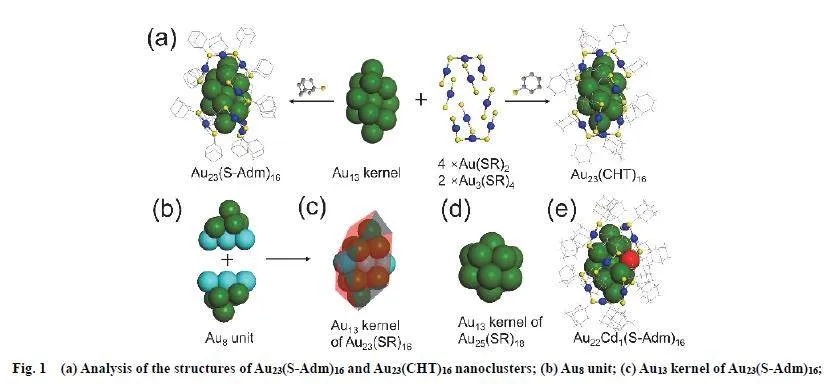
Single crystal X-ray crystallography (SCXC) 45–49, assistedwith EDS (Fig. S2), reveals the total structures of Au23(SAdm)16 and Au22Cd1(S-Adm)16 (Fig. S3). Specifically, Au23(SAdm)16 nanoclusters crystallize in a centrosymmetric spacegroup P21/n with four nanoclusters in a cubic unit cell (Fig. S4).The atomic structure of Au23(S-Adm)16 is similar to that ofAu23(CHT)16 50, consisting of an Au13 kernel protected by fourAu(S-Adm)2 staples and two Au3(S-Adm)4 staples (Fig. 1a). The ligand has obvious influence on the optical properties. As shownin Fig. S5, the peak at 450 nm in the UV-Vis-NIR spectrum ofAu23(S-Adm)16 is enhanced and the peak at 605 nm is blueshiftedto 575 nm when all the S-Adm ligands on Au23(S-Adm)16are replaced with CHT ligands; the emission peak of Au23(SAdm)16 at ~751 nm is slightly red-shifted to ~762 nm, but theemission intensity of Au23(S-Adm)16 dramatically decreases by76.7% after the ligand replacement with CHT. Note that, thecuboctahedral Au13 kernel of Au23(S-Adm)16, which is closepackedand composed of two same Au8 unit shared with threeatoms (Fig. 1b,1c), is different from the icosahedron Au13 kernelin Au25(SR)18 nanoclusters 51,52 (Fig. 1d), and the Au8 unit lookslike an equilateral triangle viewed from the front, protected withone Au(S-Adm)2 and one Au3(S-Adm)4 staple motifs as well astwo shared Au(S-Adm)2 staple motifs (Fig. S6). Compared withAu23(S-Adm)16, Au22Cd1(S-Adm)16 has similar kernel andstaples with only one atom difference in the kernel (Cd vs Au)(Figs. 1e and S7). Careful inspection reveals that the averageAu―Au bond length in the Au13 kernel of Au23(S-Adm)16 (2.881?) is shorter than that in the Au12Cd1 kernel of Au22Cd1(SAdm)16 (2.890 ?), indicating that the doped Cd pries the closepackingof kernel atoms due to the mismatch between Au and Cd(Fig. S8). Such an influence might decrease the stability ofAu22Cd1(S-Adm)16, which was confirmed by the thermalstability tests.
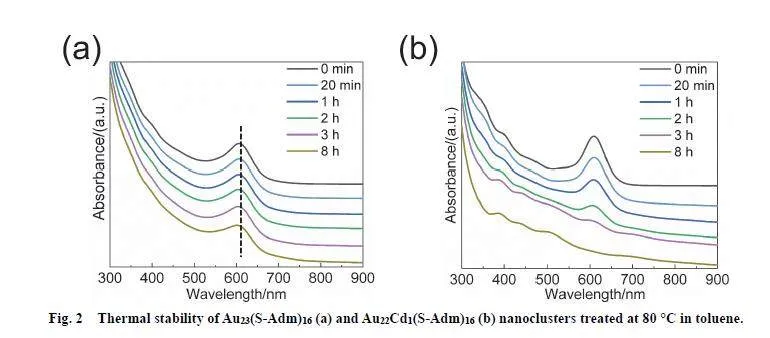
The UV-Vis-NIR spectrum of Au23(S-Adm)16 doesn’t show obvious change even after 8 h heating under 80 °C, whilst thatof Au22Cd1(S-Adm)16 displays notable alterations in 2 h undersimilar conditions, demonstrating that Au23(S-Adm)16 isobviously more stable than Au22Cd1(S-Adm)16 (Fig. 2). Theligand also influences the nanocluster stability. As shown in Fig.S5c), Au23(S-Adm)16 exhibits obviously higher thermostabilitythan Au23(CHT)16. This finding is surprising, since it is inverseto the previous reports 32. The two nanoclusters are obtained in apot, and their crystals are employed for comparison, thus thecomparison in this work should be reliable. The stabilitydeparture in various syntheses indicates that the nanoclusterstability is reaction conditions-sensitive, i.e., the nanoclusters arestable in some reaction conditions, while they are not in someothers.
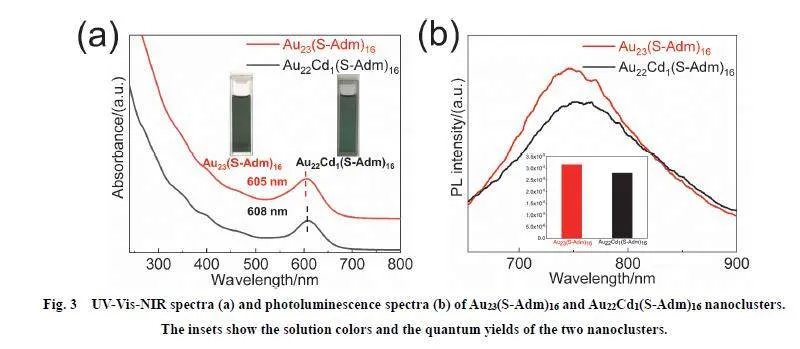
It is known that the rod-like metal nanoclusters exhibit kernelbasedmiddle-to-both ends sp ← sp photoexcitation electrontransfer 53, thus the kernel doping should influence thephotoexcitation electron transfer. Especially, the kernel Au―Aubond length increase might decrease the photoexcitation electrontransfer rate (efficiency) due to the pathway lengthening, whichas a result weakens the absorption or emission intensity. Totestify this, we measured the molar extinction coefficients of thetwo nanoclusters at the maximum absorption wavelengths in thenear-infrared region (605 nm for Au23(S-Adm)16, and 608 nm forAu22Cd1(S-Adm)16) (Figs. 3a and S9). Indeed, it is found that themolar extinction coefficient of Au23(S-Adm)16 at 605 nm is almost twice that of Au22Cd1(S-Adm)16 at 608 nm, preliminarilyconfirming our conjecture (ε(Au23(S-Adm)16) = 10526L?(mol·cm2)?1 vs. ε(Au22Cd1(S-Adm)16) = 5410 L?(mol·cm2)?1.Note that, the slight red-shift of the maximum absorption ofAu23(S-Adm)16 from 605 to 608 nm after Cd-doping is causedby the more electron delocalization from Cd 5s2 compared withAu 6s1, and it is expected that the molar extinction coefficientincreases with the maximum absorption redshift, for that morelow-energy photons can be absorbed after the photoexcitationenergy gap is decreased. However, the photoexcitation electrontransfer might be inhibited by the kernel Au―Au bond lengthincrease for Au22Cd1(S-Adm)16 compared with Au23(S-Adm)16,which interprets that Au23(S-Adm)16 exhibits larger molarextinction coefficient than Au22Cd1(S-Adm)16 regardless of theslight blueshift of the maximum absorption.
Interestingly, although the two nanoclusters have similaremission profiles and maximums (750 nm for Au23(S-Adm)16,760 nm for Au22Cd1(S-Adm)16) (Fig. 3b), they have obviousemission intensity differences: the quantum yield of Au23(SAdm)16 (~3.160 × 10?5) is 1.13 times that of Au22Cd1(S-Adm)16(~2.793 × 10?5), further confirming that the kernel bond lengthincrease retards the photoexcitation electron transfer rate(efficiency) here. In addition, the photoluminescence lifetime 54of Au23(S-Adm)16 (799.586 ns (43.18%), 2351.55 ns (51.73%)and 146.863 ns (5.09%)) is longer than that of Au22Cd1(SAdm)16 (654.45 ns (54.84%), 106.192 ns (2.36%) and 2006.31ns (42.8%)) (see Fig. S10).
It is also worth noting that the structural resolution of Au23(SAdm)16 means a unique series of single-same metal atomaugment for metal nanoclusters in size (Au22(S-Adm)16, Au23(SAdm)16, and Au24(S-Adm)16) is experimentally fulfilled 5,36 (seeScheme S1, Supporting Information) and an interesting findingis that merely one metal atom can stimulate the structuraltransformation from bioctahedral Au10-kernelled Au22(S-Adm)16to cuboctahedral Au13-kernelled Au23(S-Adm)16, demonstratingthe diversity and tunability of metal nanocluster structures.。
4 Conclusions
In conclusion, we developed a two-phase AGR method andfor the first time co-synthesized the doped metal nanocluster(Au22Cd1(S-Adm)16) and the parent nanocluster (Au23(SAdm)16) in a pot for rational comparisons. The long pursuednanocluster Au23(S-Adm)16 was structurally resolved by SCXC,which reveals that Au23(S-Adm)16 exhibits a similar structurewith the doped nanocluster Au22Cd1(S-Adm)16 except that a Cdatom in Au22Cd1(S-Adm)16 is replaced by a Au atom. The kernelAu―Au bond length increase in Au22Cd1(S-Adm)16 revealed bySCXC indicates that the Cd doping agitates the close-packing ofkernel atoms and decreases the nanocluster stability, which wasconfirmed by the thermal stability comparison betweenAu22Cd1(S-Adm)16 and Au23(S-Adm)16. Note that, our stabilitycomparison result is inverse to the previous results, whichindicates that the nanocluster stability is reaction conditionssensitive The kernel Au―Au bond length increase might alsoretard the photoexcitation electron transfer, which wasconfirmed by the intensity decreases of both absorption andemission. Thus, the kernel bond length change was successfullycorrelated to the alternations of nanocluster stability, andabsorption and emission intensity, which provides insightfulviews for understanding and predicting the stability, andabsorption (emission) intensity change of metal nanoclusters onthe basis of the structure. Overall, our work has importantimplications for the nanocluster synthesis, and structurepropertycorrelation, and is expected to trigger more work in therelated fields in the future.
Author Contributions: Single crystal data analysis, ZeminZhu; Characterization, Lei Feng and Ying Yang; Synthesis, LeiFeng, Jiafeng Zou and Zongbin He; Writing – Original DraftPreparation, Yan Zhao and Man-Bo Li; Writing – Review amp;Editing, Zhikun Wu.
Supporting Information: available free of charge via theinternet at http://www.whxb.pku.edu.cn.
References
(1) Jadzinsky, P. D.; Calero, G.; Ackerson, C. J.; Bushnell, D. A.;Kornberg, R. D. Science 2007, 318 (5849), 430.doi: 10.1126/science.1148624
(2) Lu, Y.; Chen, W. Chem. Soc. Rev. 2012, 41 (9), 3594.doi: 10.1039/C2CS15325D
(3) Zhu, M.; Li, M.; Yao, C.; Xia, N.; Zhao, Y.; Yan, N.; Liao, L.; Wu,Z. Acta Phys. -Chim. Sin. 2018, 34 (7), 792. [祝敏, 李漫波, 姚傳好,夏楠, 趙燕, 閆楠, 廖玲文, 伍志鯤. 物理化學學報, 2018, 34 (7),792.] doi: 10.3866/PKU.WHXB201710091
(4) Zeng, C.; Zhou, M.; Chen, Y. X.; Jin, R. Chem. Rev. 2016, 116 (18),10346. doi: 10.1021/acs.chemrev.5b00703
(5) Qian, H.; Wu, Z.; Zhu, Y.; Zhu, M.; Mohanty. A.; Garg, N.; Jin, R.J. Phys. Chem. Lett. 2010, 1 (19), 2903. doi: 10.1021/jz100944k
(6) Wu, Y.-G.; Huang, J.-H.; Zhang, C.; Guo, X.-K.; Wu, W.-N.; Dong,X.-Y.; Zang, S.-Q. Chem. Commun. 2022, 58 (52), 7321.doi: 10.1039/D2CC00794K
(7) Li, J.-J.; Liu, Z.; Guan, Z.-J.; Han, X.-S.; Shi, W.-Q.; Wang, Q.-M.,J. Am. Chem. Soc. 2022, 144 (2), 690. doi: 10.1021/jacs.1c11643
(8) Tian, S.; Cao, Y.; Chen, T.; Zang, S.; Xie, J. Chem. Commun. 2020,56 (8), 1163. doi: 10.1039/C9CC08215H
(9) Yang, J.-S.; Zhang, M.-M.; Han, Z.; Li, H.-Y.; Li, L.-K.; Dong, X.-Y.; Zang, S.-Q.; Mak, T. C. W. Chem. Commun. 2020, 56 (16), 2451.doi: 10.1039/C9CC09439C
(10) Salorinne, K.; Man, R. W. Y.; Lummis, P. A.; Hazer, M. S. A.;Malola, S. J.; Yim, C.-H.; Veinot, A. J.; Zhou, W.; H?kkinen, H.;Nambo, M.; et al. Chem. Commun. 2020, 56 (45), 6102.doi: 10.1039/D0CC01482F
(11) Si, W.-D.; Li, Y.-Z.; Zhang, S.-S.; Wang, S.; Feng, L.; Gao, Z.-Y.;Tung, C.-H.; Sun, D. ACS Nano 2021, 15 (10), 16019.doi: 10.1021/acsnano.1c04421
(12) Jin, R. Acta Phys. -Chim. Sin. 2019, 35 (3), 245. [金榮超. 物理化學學報, 2019, 35 (3), 245.] doi: 10.3866/PKU.WHXB201803213
(13) Zhang, Q.-F.; Williard, P. G.; Wang, L.-S. Small 2016, 12 (18), 2518.doi: 10.1002/smll.201600407
(14) Chakrahari, K. K.; Silalahi, R. P. B.; Chiu, T.-H.; Wang, X. P.;Azrou, N.; Kahlal, S.; Liu, Y.-C.; Chiang, M.-H.; Saillard, J.-Y.; Liu,C. W. Angew. Chem. Int. Ed. 2019, 58 (15), 4943.doi: 10.1002/anie.201814264
(15) Li, Y., Kim, H. K., McGillicuddy, R. D.; Zheng, S.-L.; Anderton, K.J.; Stec, G. J.; Lee, J.; Cui, D.; Mason, J. A. J. Am. Chem. Soc. 2023,145 (16), 9304. doi: 10.1021/jacs.3c02458
(16) Yu, Y.; Rao, P.; Feng, S.; Chen, M.; Deng, P.; Li, J.; Miao, Z.; Kang,Z.; Shen, Y.; Tian, X. Acta Phys. -Chim. Sin. 2023, 39 (8), 2210039.[于彥會, 饒鵬, 封蘇陽, 陳民, 鄧培林, 李靜, 苗政培, 康振燁,沈義俊, 田新龍. 物理化學學報, 2023, 39 (8), 2210039.]doi: 10.3866/PKU.WHXB202210039
(17) Hossain, S.; Niihori, Y.; Nair, L. V.; Kumar, B.; Negishi, Y. Acc.Chem. Res. 2018, 51 (12), 3114. doi: 10.1021/acs.accounts.8b00453
(18) Liu, Z.; Meng, X.; Gu, W.; Zha, J.; Yan, N.; You, Q.; Xia, N.; Wang,H.; Wu, Z. Acta Phys.-Chim.Sin. 2023, 39 (12), 2212064. [劉真, 孟祥福, 古萬苗, 查珺, 閆楠, 尤青, 夏楠 王輝, 伍志鯤. 物理化學學報, 2023, 39 (12), 2212064.] doi: 10.3866/PKU.WHXB202212064
(19) Li, T.; Li, Q.; Yang, S.; Xu, L.; Chai, J.; Li, P.; Zhu, M. Chem.Commun. 2021, 57 (38), 4682. doi: 10.1039/D1CC00577D
(20) Hossain, S.; Suzuki, D.; Iwasa, T.; Kaneko, R.; Negishi, Y. J. Phys.Chem. C 2020, 124 (40), 22304. doi: 10.1021/acs.jpcc.0c06858
(21) Ito, E.; Ito, S.; Takano, S.; Nakamura, T.; Tsukuda, T. J. Am. Chem.Soc. Au 2022, 2 (11), 2627. doi: 10.1021/jacsau.2c00519
(22) Yao, C.; Chen, J.; Li, M.-B.; Liu, L.; Yang, J.; Wu, Z. Nano Lett.2015, 15 (2), 1281. doi: 10.1021/nl504477t
(23) Kzan, R.; Müller, U.; Bürgi, T. Nanoscale 2019, 11 (6), 2938.doi: 10.1039/C8NR09214A
(24) Ghosh, A.; Mohammed, O. F.; Bakr, O. M. Acc. Chem. Res. 2018, 51(12), 3094. doi: 10.1021/acs.accounts.8b00412
(25) Sun, Y.; Cheng, X.; Zhang, Y.; Tang, A.; Cai, X.; Liu, X.; Zhu, Y.Nanoscale 2020, 12 (35), 18004. doi: 10.1039/D0NR04871B
(26) Yao, C.; Lin, Y. J.; Yuan, J.; Liao, L.; Zhu, M.; Weng, L.-H.; Wu, Z.J. Am. Chem. Soc. 2015, 137 (49), 15350. doi: 10.1021/jacs.5b09627
(27) Zheng, Y.; Jiang, H.; Wang, X. Acta Phys. -Chim. Sin. 2018, 34 (7),740. [鄭有坤, 姜暉, 王雪梅. 物理化學學報, 2018, 34 (7), 740.]doi: 10.3866/PKU.WHXB201712111
(28) Zou, J.; Fei, W.; Qiao, Y.; Yang, Y.; He, Z.; Feng, L.; Li, M.-B.; Wu,Z. Chin. Chem. Lett. 2023, 34 (4), 107660.doi: 10.1016/j.cclet.2022.07.003
(29) Tang, L.; Ma, A.; Zhang, C.; Liu, X.; Wang, S.; Jin, R. Angew. Chem.Int. Ed. 2021, 133 (33), 18113. doi: 10.1002/ange.202106804
(30) Ma, X.; Xiong, L.; Qin, L.; Tang, Y.; Ma, G.; Pei, Y.; Tang, Z. Chem.Sci. 2021, 12 (38), 12819. doi: 10.1039/D1SC03679C
(31) Du, Y.; Guan, Z.-J.; Wen, Z.-R.; Lin, Y.-M.; Wang, Q.-M. Chem.Eur. J. 2018, 24 (60), 16029. doi: 10.1002/chem.201886065
(32) Li, Y.; Cowan, M. J.; Zhou, M.; Luo, T.; Jin, R. J. Am. Chem. Soc.2020, 142 (48), 20426. doi: 10.1021/jacs.0c09110
(33) Crasto, D.; Barcaro, G.; Stener, M.; Sementa, L.; Fortunelli, A.; Dass,A. J. Am. Chem. Soc. 2014, 136 (42), 14933. doi: 10.1021/ja507738e
(34) Ren, X.; Lin, X.; Fu, X.; Liu, C.; Yan, J.; Huang, J. Acta Phys. -Chim.Sin. 2018, 34 (7), 825. [任秀清, 林欣章, 付雪梅, 劉超, 閆景輝,黃家輝. 物理化學學報, 2018, 34 (7), 825.]doi: 10.3866/PKU.WHXB201712013
(35) Wu, Z. Angew. Chem. Int. Ed. 2012, 51 (12), 2934.doi: 10.1002/anie.201107822
(36) Wang, M.; Chu, Z.; Yang, J.; Yao, C.; Wu, Z. Chem. -Asian. J. 2014,9 (4), 1006. doi: 10.1002/asia.201301562
(37) Gan, Z.; Xia, N.; Wu, Z. Acc. Chem. Res. 2018, 51 (11), 2774.doi: 10.1021/acs.accounts.8b00374
(38) Zhuang, S.; Chen, D.; Liao, L.; Zhao, Y.; Xia, N.; Zhang, W.; Wang,C.; Yang, J.; Wu, Z. Angew. Chem. Int. Ed. 2019, 132 (8), 3097.doi: 10.1002/ange.201912845
(39) Muhammed, M. A. H.; Verma, P. K.; Pal, S. K.; Kumar, R. C. A.;Paul, S.; Omkumar, R. V.; Pradeep, T. Chem. -Eur. J. 2009, 15 (39),doi: 10.1002/chem.200901425
(40) Wan, X.-K.; Yuan, S.-F.; Tang, Q.; Jiang, D.-E.; Wang, Q.-M.Angew. Chem. Int. Ed. 2015, 54 (20), 5977.doi: 10.1002/anie.201500590
(41) Kang, X.; Xiang, J.; Lv, Y.; Du, W.; Yu, H.; Wang, S.; Zhu, M.Chem. Mater. 2017, 29 (16), 6856.doi: 10.1021/acs.chemmater.7b02015
(42) Liu, C.; Ren, X.; Lin, F.; Fu, X.; Lin, X.; Li, T.; Sun, K.; Huang, J.Angew. Chem. Int. Ed. 2019, 58 (33), 11335.doi: 10.1002/anie.201904612
(43) Song, Y.; Chai, J.; Abroshan, H.; Kang, X.; Kim, H.; Zhu, M.; Jin, R.Chem. Mater. 2017, 29 (7), 3055.doi: 10.1021/acs.chemmater.7b00058
(44) Zhao, Y.; Zhuang, S.; Liao, L.; Wang, C.; Xia, N.; Gan, Z.; Gu, W.;Li, J.; Deng, H.; Wu, Z. J. Am. Chem. Soc. 2020, 142 (2), 973.doi: 10.1021/jacs.9b11017
(45) Wan, X.-K.; Yuan, S.-F.; Tang, Q.; Jiang, D.-E.; Wang, Q.-M.Angew. Chem. Int. Ed. 2015, 127 (20), 6075.doi: 10.1002/ange.201500590
(46) Li, H.; Zhou, C.; Wang, E.; Kang, X.; Xu, W.; Zhu, M. Chem.Commun. 2022, 58 (33), 5092. doi: 10.1039/D2CC00987K
(47) Yang, Y.; Chen, C.; X, G.-Y.; Yuan, J.; Ye, S.-F.; Chen, L.; Lv, Q.-L.; Luo, G.; Yang, J.; Li, M.-B.; et al. J. Catal. 2021, 401, 206.doi: 10.1016/j.jcat2021.07.023
(48) Zhu, M.; Wang, P.; Yan, N.; Chai, X.; He, L.; Zhao, Y.; Xia, N.; Yao,C.; Li, J.; Deng, H.; et al. Angew. Chem. Int. Ed. 2018, 57 (17), 4500.doi: 10.1002/anie.201800877
(49) Yi, H.; Han, S. M.; Song, S.; Kim, M.; Sim, E.; Lee, D. L. Angew.Chem. Int. Ed. 2021, 60 (41), 22293. doi: 10.1002/anie.202106311
(50) Das, A.; Li, T.; Nobusada, K.; Zeng, C.; Rosi, N. L.; Jin, R. J. Am.Chem. Soc. 2013, 135 (49), 18264. doi: 10.1021/ja409177s
(51) Zhu, M.; Lanni, E.; Garg, N.; Bier, M. E.; Jin, R. J. Am. Chem. Soc.2008, 130 (4), 1138. doi: 10.1021/ja0782448
(52) Zhu, M.; Aikens, C. M.; Hollander, F. J.; Schatz, G. C.; Jin, R. J. Am.Chem. Soc. 2008, 130 (18), 5883. doi: 10.1021/ja801173r
(53) Fan, W.; Yang, Y.; You, Q.; Li, J.; Deng, H.; Yan, N.; Wu, Z.J. Phys. Chem. C 2023, 127 (1), 816. doi: 10.1021/acs.jpcc.2c07678
(54) Zhuang, B.; Jin, Z.; Tian, D.; Zhu, L.; Zeng, L.; Fan, J.; Lou, Z.; Li,W. Acta Phys. -Chim. Sin. 2023, 39 (1), 2209007. [莊必浩, 靳子驄,田德華, 朱遂意, 曾琳茜, 范建東, 婁在祝, 李聞哲. 物理化學學報, 2023, 39 (1), 2209007.] doi: 10.3866/PKU.WHXB202209007
國家自然科學基金(21925303, 21771186, 21829501, 21222301, 21528303, 21171170, 92061110), 中國科學院合肥研究院院長基金(BJPY2019A02), 中國科學院合肥研究院十三五重點計劃(KP-2017-16), 中國科學院合肥科學中心協同創新項目(2020HSC-CIP005, 2022HSC-CIP018), 安徽省自然科學基金(2108085Y05), 合肥微尺度物理科學國家實驗室(KF2020102)及安徽大學啟動經費(S020318006/037)資助

