牛體外胚胎生產過程中培養液添加物研究進展

摘 要: 牛體外胚胎生產是一種廣泛應用于畜牧生產和生物研究的現代繁殖技術,在促進肉牛和奶牛品種改良以及高效繁育中發揮著關鍵作用。隨著分子生物學、生物化學和細胞生物學技術的不斷發展,牛體外胚胎生產技術已取得了長足進步。然而,當前牛體外胚胎生產技術仍存在胚胎生產效率低和胚胎發育潛能差等瓶頸問題,在很大程度上限制了該技術的規模化推廣應用。本文系統綜述了牛體外胚胎生產中經典添加物和最新添加物的研究進展,旨在為優化構建不同添加物組合的技術體系提供參考,以期更加高效地獲得牛體外優質胚胎。
關鍵詞: 牛;胚胎;培養液;受精;添加物
中圖分類號:S823.3
文獻標志碼:A
文章編號:0366-6964(2024)08-3309-12
收稿日期:2024-01-24
基金項目:湖北省重點研發計劃項目(2022BBA007);湖北省重點研發專項(2023BBB056);武漢輕工大學引進(培養)人才科研啟動項目(2023R2010)
作者簡介:戴舒穎(2001-),女,安徽滁州人,碩士,主要從事動物遺傳育種與繁殖研究,E-mail:dsy_0109@163.com
通信作者:余 博,主要從事家畜胚胎發育研究,E-mail:wonderfish@whpu.edu.cn;陳洪波,主要從事動物遺傳育種與繁殖研究,E-mail:chenhongbo@whpu.edu.cn
Research Progress on Culture Medium Additives in Bovine In Vitro Embryo Production
DAI" Shuying1, LIU" Qing1, LI" Aiguo2, YU" Bo1*, CHEN" Hongbo1*
(1.Laboratory of Genetic Breeding, Reproduction and Precision Livestock Farming, School of Animal
Science and Nutritional Engineering, Wuhan Polytechnic University, Wuhan 430023," China;
2.Shiyan
Centers for Disease Control and Prevention, Shiyan 442099," China)
Abstract:" Bovine embryo in vitro production (IVP) is a modern reproductive biotechnology, which is widely used in livestock production and biological research and plays an essential role in promoting breed improvement and reproductive efficiency for both beef and dairy cattle. As development of molecular biology, biochemistry and cell biology, a great progress has been made in bovine IVP. However, the current bovine IVP is still facing bottleneck problems, such as low efficiency of in intro embryo production and poor embryonic development potential, seriously hindering the further large-scale promotion and application of this technology. In this paper, the research progress of classical and latest additives in bovine IVP was systematically reviewed, intending to provide references for optimizing and constructing the technical system of different additive combinations, and aiming to more efficiently obtain high-quality bovine in vitro embryos.
Key words: bovine; embryo; culture solution; fertilization; additive
*Corresponding authors:" YU Bo, E-mail: wonderfish@whpu.edu.cn; CHEN Hongbo, E-mail: chenhongbo@whpu.edu.cn
牛體外胚胎生產(in vitro production,IVP)是指從不同年齡和生理狀態的供體卵巢上獲取未成熟的卵母細胞,經過體外培養成熟、受精并進一步培養發育,最終獲得可進行移植或冷凍保存的胚胎。卵母細胞體外成熟(in vitro maturation,IVM)、體外受精(in vitro fertilization,IVF)和體外培養(in vitro culture,IVC)是IVP的三個關鍵技術環節[1-3]。IVM作為IVP的第一步,對卵母細胞的質量將會產生重要影響并直接決定IVP的效率[4];IVF是將成熟后的卵母細胞和獲能后的精子在體外可控的環境中完成受精的技術;IVC則是將受精卵繼續培養至所需胚胎階段,它作為IVP的最后一步將直接關系到后續胚胎發育的質量[3]。
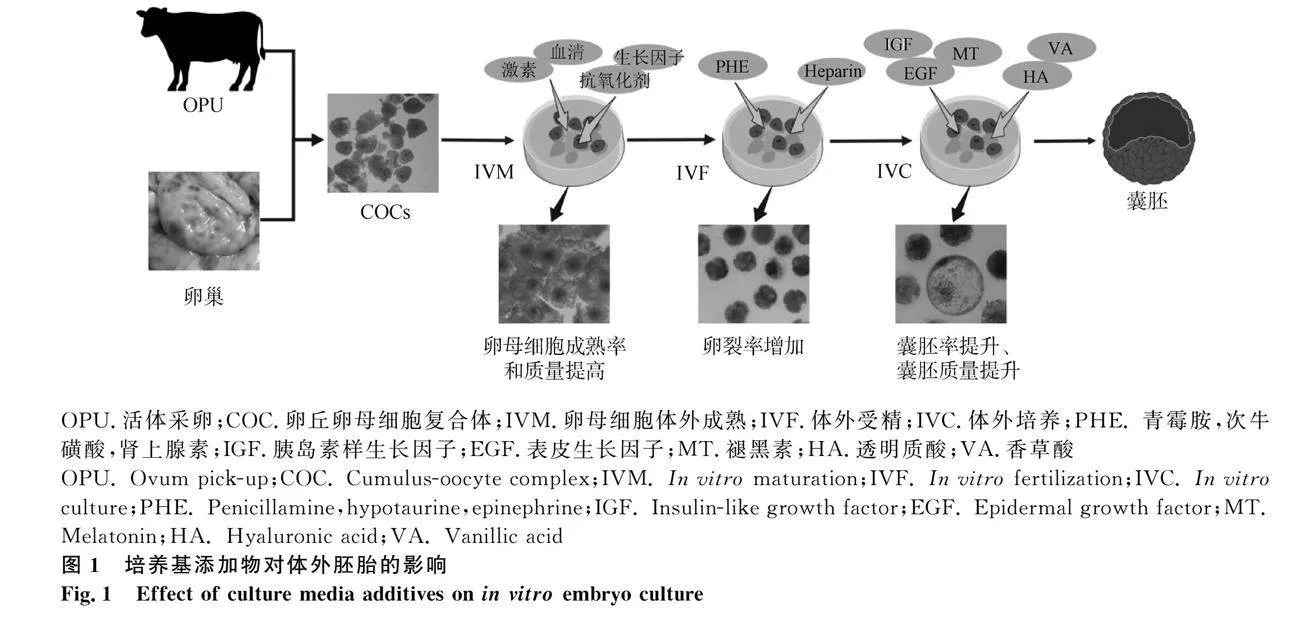
培養液是體外胚胎生長發育的外環境,直接為體外胚胎提供營養、能量等支持。近年來,研究人員通過向體外基礎培養液中添加不同物質以期能夠更加真實地模擬體內胚胎發育環境,從而獲得更為優質的囊胚(圖1)。卵母細胞的成熟品質和受精卵的質量直接關系到后續囊胚率[5]。研究表明,某些添加物如激素、肝素等對卵母細胞成熟和精子體外獲能必不可少[6]。此外,體外受精條件的優化,包括精子和卵子的質量控制、體外受精環境因素控制以及受精卵形成的時間窗口選擇等,能夠顯著提高受精率和受精卵品質[7]。再者,一些添加物對體外胚胎發育至關重要。例如,抗氧化應激類添加物褪黑素、維生素C等可以明顯提高發育胚胎的卵裂率和囊胚率[8-11];添加胰島素樣生長因子、表皮生長因子等則會顯著加快胚胎發育的速度并提高其發育質量。
目前,牛IVP技術的瓶頸問題在于體外胚胎的生產效率和發育潛能較低。據統計,牛體外囊胚率只有30%左右,這在很大程度上限制了牛體外胚胎生產技術的應用和推廣[12]。解決這一問題的關鍵在于持續優化體外培養液配方,尤其是需要針對減數分裂期、卵裂期、合子基因激活期等某些關鍵發育階段進行配方改良,以滿足不同發育階段胚胎的營養需求,進而顯著提高胚胎存活率和發育質量[13]。鑒于體外環境仿真模擬體內環境對牛IVP的至關重要性,本文重點綜述了胚胎體外生產過程中不同添加物的成分、作用及其應用價值,以期為優化構建不同添加物組合的技術體系并為高效獲取體外優質胚胎提供參考。
1 不同添加物對卵母細胞體外成熟的影響
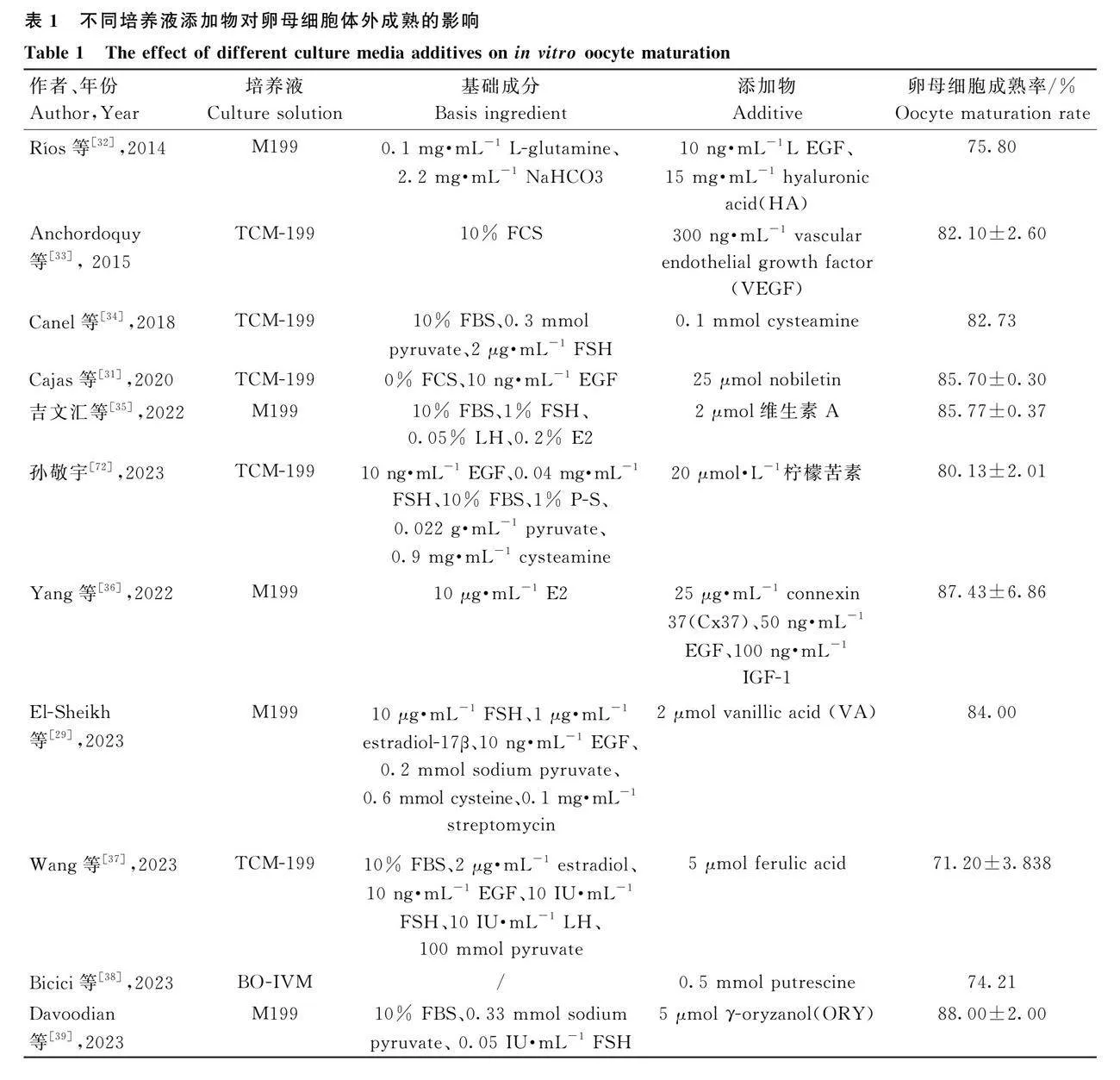
體外培養系統對于獲得高質量的胚胎具有關鍵作用,因此,IVM作為體外胚胎培養的第一步顯得尤為重要[14]。IVM是一個極為復雜的過程,細胞質和細胞核需要經過一系列的分子表達和結構變化才能最終實現生殖細胞完全成熟[15]。目前,對于體內卵母細胞所處的具體微環境以及卵泡液中的物質組成尚不完全清楚[16],牛卵母細胞體外成熟主要是以培養基199(tissue culture medium 199,TCM199)為基礎培養液,通過添加血清、激素、卵泡液和生長因子等物質盡可能地模擬卵母細胞體內成熟的環境(表1)。
1.1 血清
血清中含有豐富的氨基酸、維生素、激素和生長因子,對卵母細胞的成熟非常有益,在TCM199中補充血清是提高卵母細胞成熟率的有效方法之一。實際操作中常添加的血清包括胎牛血清(fetal bovine serum,FBS)、小牛血清(calf serum,CS)和發情母牛血清(estrus cow serum,ECS)等。在TCM199中補充10%的FBS后可促進成熟卵母細胞獲得更優的形態特征[17]。然而,血清來源和批次的不同可能會對培養效果產生影響,添加血清也可能導致污染問題。因此,近幾年無血清培養體系引起越來越多研究者們的關注[18],使用其他物質如牛血清白蛋白(bovine serum albumin,BSA)、聚乙烯醇(polyvinyl alcohol,PVA)和聚乙烯吡咯烷酮(polyvinyl pyrrolidone,PVP)等代替血清并取得了良好效果。此外,在探究不同濃度牛血清及BSA對水牛卵母細胞體外成熟的影響時發現,使用化學成分明確的無血清培養液可以實現與添加血清培養液同樣的效果[19]。值得一提的是,盡管BSA具有替代血清的效果,但IVM環節使用BSA的具體益處及其作用機制尚未明晰。
1.2 激素
激素是卵母細胞成熟必不可少的成分,常用來添加的激素主要包括促卵泡素(follicle-stimulating hormone,FSH)、促黃體生成素(luteinizing hormone,LH)、雌二醇(estradiol,E2)、孕馬血清促性腺激素(pregnant mare serum gonadotropin,PMSG)以及重組人卵泡刺激素(human follicle stimulating hormone,hFSH)等。培養液中添加激素的種類、配方及用量尚無統一的標準。Mihoko等[20]發現體外培養系統中添加FSH可促進COCs發育及后續卵母細胞的生長。丁麗艷等[21]研究表明,添加FSH對卵母細胞成熟有促進作用。研究發現,添加0.075 IU·mL-1促性腺激素(重組卵泡刺激素和尿促性素)改善促進了卵母細胞細胞核成熟,進而促進改善了牛卵母細胞的體外受精效果[22]。絨毛膜促性腺激素在促進卵母細胞體外成熟方面具有與FSH類似的效果,且絨毛膜促性腺激素對卵母細胞的核成熟至關重要[23]。10 ng·mL-1的17β-雌二醇和10 ng·mL-1雄烯二酮組合使用也可以促進50%以上的卵母細胞完全生長并充分具有減數分裂的能力[24]。激素的添加可有效促進卵母細胞成熟,但各種激素促進卵母細胞成熟的具體作用機制有待進一步解析。
1.3 生長因子
已有大量研究證明,在IVM培養液中添加生長因子有助于卵母細胞成熟以及早期胚胎發育。主要用于添加的生長因子包括:表皮生長因子(epidermal growth factor,EGF)、胰島素樣生長因子(insulin-like growth factor,IGF)、白血病抑制因子(leukemia inhibitory factor,LIF)、轉化生長因子(transforming growth factor,TGF)、血管內皮生長因子(vascular endothelial growth factor,VEGF)等。EGF已被證明可以促進顆粒細胞的增殖和卵母細胞成熟,并通過與表皮生長因子受體結合誘導排卵過程[25]。IVM培養液中補充EGF可提高卵母細胞的減數分裂能力,并促進胚胎更好地發育到胚泡階段[26]。IGF-1是顆粒細胞有絲分裂原,它能促進牛卵母細胞核成熟,IVM階段添加50 ng·mL-1 EGF和100 ng·mL-1 IGF-1可促進體外受精后卵母細胞的核成熟和胚泡發育;IVM階段添加50 ng·mL-1 EGF、100 ng·mL-1 IGF-1和25 μg·mL-1連接蛋白37(Cx37)組合可增加線粒體功能和谷胱甘肽(glutathione,GSH)水平,顯著改善牛胚胎發育質量[27]。
1.4 其他添加物
在卵母細胞體外成熟過程中,研究人員也在嘗試在培養基中添加其他物質從而提高卵母細胞體外成熟率(表1)。例如,體外成熟培養基中添加微量礦物質混合物可促進卵母細胞體外成熟進而改善后續的胚胎發育質量[28]。近些年,研究還發現許多其他化合物如香草酸、檸檬苦素、抗氧化劑nobiletin等可以通過抑制氧化應激提升卵母細胞體外成熟質量[29-31]。
2 不同添加物對體外受精的影響
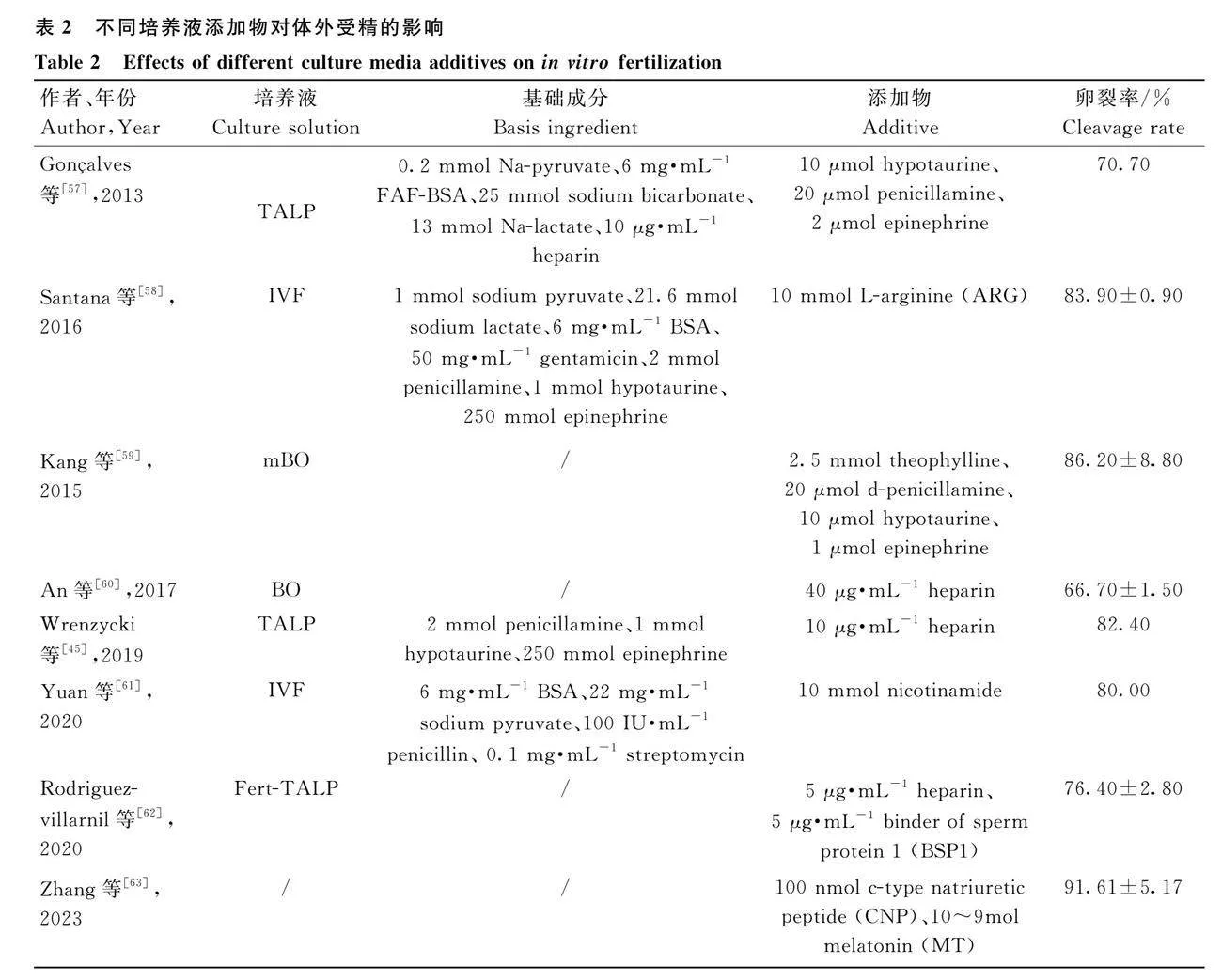
哺乳動物的精子在曲精小管中生成,并在母畜的生殖道中經歷一系列反應過程獲能。隨后精子會經歷一系列變化,包括腺苷酸環化酶被激活、磷脂酶被激活、蛋白磷酸化加劇、精子膜流動性增強以及膜電位的改變[40-41]。IVF是一項復雜的過程,它的成功與否離不開卵母細胞的成熟、精子的分選、精子的獲能以及適宜的培養條件[42]。培養液在IVF過程中起著至關重要的作用,對IVF的成功以及后續胚胎的發育和品質有著直接影響。研究人員通過調整培養液的營養物質,如氨基酸、糖類、脂類和添加物的比例和配方,致力于優化培養基的組成,以提高后續胚胎的存活率和發育能力。
2.1 肝素
肝素(heparin)在牛體外受精研究中扮演著重要的角色,近年來對其在該領域的應用進行了廣泛的研究。作為一種多糖類藥物,肝素具有抗凝血和抗炎作用,同時在體外受精過程中展現出多種潛在的益處[43]。為了在無生殖液的條件下誘導精子的獲能,研究人員使用了一些化合物,如鈣離子載體或黃體酮。肝素作為一種常見的誘導劑,能有效地誘導精子獲能[44]。研究發現,肝素作為牛精液的獲能劑,可加速受精過程,影響后續的胚胎發育[45]。此外,在體外受精培養基中添加肝素,對于精子獲能、原核形成和體外早期胚胎發育起著重要作用。
2.2 PHE(penicillamine,hypotaurine,epinephrine)
青霉胺(penicillamine)是一種含有巰基的氨基酸衍生物,被廣泛應用于治療類風濕性關節炎和銅代謝障礙等不同疾病[46]。除了臨床應用外,青霉胺在生殖生物研究領域也扮演著重要的作用,其中包括牛的體外受精。多個試驗結果表明,適宜濃度的青霉胺可以提高受精率,但過高的濃度可能會阻礙受精[47]。次牛磺酸(hypotaurine)是一種硫氨基酸衍生物,在多種生物過程中發揮著細胞保護和抗氧化應激的重要作用[48]。次牛磺酸已被證明可以提高受精率,可能是由于其促進了精子的活力和卵子的受精能力。Seify等[49]發現,次牛磺酸作為一種抗氧化劑,可以在一定程度上減少冷凍保存對精子形態和頂體完整的影響。腎上腺素(epinephrine)是一種兒茶酚胺,通過刺激可溶性腺苷酸環化酶,導致精細胞內cAMP濃度增加[50],在加速鞭毛跳動、促進精子運動方面具有重要作用[51]。PHE混合物常與肝素一同添加至IVF培養液中,研究發現,與不添加PHE的對照組相比,PHE的添加能夠顯著提高卵裂率[52]。此外,在體外受精培養基中添加PHE可以改善公牛精子相關參數。Kang等[53]研究發現,在IVF培養液中添加PHE混合物(表2)可以保持牛精子的活力和壽命,并保證體外胚泡的穩定生產;同時,PHE可能對受精率、胚胎發育和生殖細胞質量產生多方面的影響。然而,目前其確切的作用機制尚不清楚,尚有待深入研究,以期豐富家畜受精過程基礎理論。
2.3 其他
近年來研究者也在不斷嘗試不同的IVF培養液添加物(表2),進而提高卵裂率及后續的胚胎發育品質。例如,研究發現,在無血清條件下,IVF過程中添加4 μL的外源性黃體酮可以改善卵丘剝落的卵母細胞的發育能力[54]。Peixoto De Souza等[55]發現在培養液中增加50 ng·mL-1 維生素D可以顯著提高體外受精率。最新研究還發現Rho激酶抑制劑可以改善解凍后公牛精子的品質和受精能力[56]。
3 不同添加物對胚胎體外培養的影響
胚胎體外培養是決定體外胚胎發育成功的最后一步,其中存在許多影響因素。為了提升囊胚率和胚胎質量,需要在培養液中添加適當的營養成分,如氨基酸、糖類、脂質、維生素和礦物質。此外,添加生長因子(如IGF和EGF)也可對胚胎發育產生積極影響。通過在培養基中添加上述物質,可以更好地模擬體內胚胎生長環境,從而獲得更多優質的囊胚。
3.1 胰島素樣生長因子(IGF)
IGF能夠促進牛胚胎細胞的增殖和生長,提高胚胎的發育速度和質量。IGF-1由輸卵管和子宮在早期產生,植入前的牛胚胎中含有IGF-1因子以及IGF-1受體的轉錄物。近十年,IGF-1對牛體外胚胎培養的影響得到了廣泛地研究。Wang等[64]發現,敲除IGF-1受體會抑制胚泡發育,表明IGF-1對提高牛胚胎體外培養具有重要作用。Nogueira等[65]發現,在牛胚胎體外生產過程中補充IGF-1、LIF和FGF2可以改善植入前胚胎的質量。還有相關研究表明,IGF-1在牛體外胚胎培養中起到抗凋亡和存活因子的作用,并且可以減輕牛胚泡中的活性氧損傷[66]。
3.2 表皮生長因子(EGF)
EGF是一種由53個氨基酸殘基構成的耐熱單鏈低分子多肽,通過與EGF受體特異性識別結合后發揮作用。在牛的輸卵管、子宮、卵母細胞和早期胎盤中均檢測到EGF的轉錄產物。Mesalam等[67]發現,在培養基中添加EGF可以改善胚胎質量,其作用機制可能是通過調節DNA甲基化和局點黏附通路的表達模式來調控胚胎質量。然而,多項研究表明,補充EGF后并不能增加達到囊胚階段的總細胞數[68]。
3.3 抗氧化應激添加物
氧化應激是引起胚胎發育異常的重要因素之一,胚胎內的活性氧(reactive oxygen species,ROS)來源于胚胎發育的各種代謝過程,生理含量的ROS可作為胚胎發育過程中的信號轉化分子,但過量的ROS會引起胚胎內線粒體損傷、DNA損傷、脂質過氧化甚至細胞凋亡,進而導致胚胎發育阻滯[69-70]。因此在體外培養時,經常通過添加外源性抗氧化劑清除ROS,減少其危害,以提高胚胎發育率[70]。最常用的抗氧化劑如GSH,研究表明添加3mmol的外源性GSH可以顯著提升囊胚發育率[71]。此外,孫敬宇[72]發現,20 μmol·L-1檸檬苦素能提高GSH水平,降低ROS水平,提高早期胚胎的發育能力和囊胚質量。
3.4 其他
褪黑素(melatonin,MT)是一種抗衰老藥物,近年研究發現,補充MT可以增加IVP牛囊胚的形成[73-74]。當胚胎在高氧條件下培養時,MT增加了囊胚的形成,但在低氧條件下,補充MT則減少了囊胚的發育。MT的補充對IVP過程中胚胎的具體影響機制仍需進一步探索。溶血磷脂酸作為卵母細胞成熟、受精和胚胎培養基的潛在補充劑在早期胚胎發育中發揮一定作用。Yu等[75]發現,補充溶血磷脂酸加速了IVP囊胚形成。近年來研究人員不斷探索培養基添加物對體外胚胎培養的影響(表3),以期提高囊胚的數量和質量。
4 IVM-IVF-IVC最佳添加物體外培養體系的構建探討
與普通的細胞培養類似,體外胚胎培養基礎培養基中往往需添加糖類、氨基酸、BSA等用于維持正常的養分供給,同時還需注意培養液pH、滲透壓以及培養環境[80]。但胚胎在不同的發育階段往往需要添加一些特殊的物質,達到最終提升囊胚率、加速胚胎生長、優化囊胚質量的目的。
卵母細胞的成熟受激素調控,尤其是促性腺激素對卵母細胞成熟的啟動具有重要作用。因此在IVM期間激素的添加必不可少,如FSH、LH、E2等。近年的研究發現,添加生長因子和抗氧化應激物質能極大地提高卵母細胞成熟率和質量,如EGF、IGF-1、維生素A、檸檬苦素等[35]。通過在培養液中添加激素、生長因子、抗氧化應激物質,卵母細胞的成熟率可達85.00%及以上。
精子獲能是IVF的關鍵,heparin作為促進精子獲能的物質常被添加,添加量為10 μg·mL-1時,卵裂率可達82.40%。青霉胺、次牛磺酸和腎上腺素也常被添加用于提高受精率。ARG作為一種潛在的促能劑,研究發現添加10mmol ARG卵裂率為83.90%[58]。近年來,抗氧化應激物質也被添加在受精培養基中,以期提升受精卵的質量。
IVC是牛體外胚胎生產中耗時最長的培養過程,受精卵在此過程經歷卵裂、合子基因組激活、第一次譜系分化等一系列復雜過程。培養液是胚胎生長發育的直接場所,其添加成分尤為重要。除基礎成分外,常用添加物促生長因子不僅可以加速囊胚的形成,更能提升囊胚的質量。與卵母細胞的體外成熟類似,氧化應激也是胚胎發育的重要影響因素,值得一提的是,胚胎通常在低氧條件下進行培養。在IVC培養液中添加抗氧化物質,如2 μmol VA可使囊胚率達38.75%。目前,與小鼠相比,牛體外胚胎培養仍存在囊胚率較低,胚胎發育遲緩,移植體外胚胎妊娠率低等問題。因此,未來還需通過探尋不同添加物來優化培養液,以期提升囊胚率,加速囊胚形成,改善胚胎質量。本課題組研究發現,在IVC培養液中添加10 μmol的溶血磷脂酸(LPA)可加速囊胚的形成,最早可在第5天出現未擴張囊胚(未發表數據)。
根據最新的研究成果綜合分析,我們需要綜合考慮添加物對卵母細胞成熟、卵母細胞受精和胚胎發育的影響。然而,值得注意的是,文獻中所使用的基礎培養液各有不同,添加物的具體作用還需進一步的對比試驗論證。
5 小結與展望
牛體外胚胎培養受到多種因素的影響。作為卵母細胞體外成熟、精子獲能、體外受精和胚胎發育的直接場所,培養液的選擇和添加物的優化對于牛體外胚胎培養至關重要。為了獲得更多高質量囊胚,研究人員精心調整培養基配方。從IVM開始,通過添加激素、血清、生長因子等物質,可以獲得更多成熟的卵母細胞,為后續IVF奠定基礎。在IVF過程中,添加肝素促進精子獲能,添加PHE提高體外受精率。最后核心環節IVC通過添加生長因子、抗氧化應激物、LPA等,最大程度地提升囊胚率、加速囊胚形成、優化囊胚質量。
我國是牛肉產品和牛乳制品最具潛力的消費市場,牛產品供給能力不足導致我國需要大量進口填補其空缺。IVP結合胚胎移植可加快我國良種牛培育和擴繁速度,顯著提升能繁母牛繁殖力,是增加我國當前牛產業產能的重要途徑。然而,相對于歐美發達國家,IVP技術在我國牛產業上的應用僅處于起步階段,具有廣闊的發展前景。結合前沿的高通量深度測序和代謝組學技術,探索并完善添加物應用于體外胚胎培養的方案,將有效提高體外高質量胚胎生產、能繁母牛的生產效能,這對于進一步優化完善我國優良種畜繁育技術體系,提升我國牛產品的綜合產能具有重要意義。
參考文獻(References):
[1] 陳超磊, 王利娟, 胡新宇, 等. 牛體外受精早期胚胎培養體系優化的研究[J].畜牧獸醫雜志, 2019, 38(2):7-12.
CHEN C L, WANG L J, HU X Y, et al. Optimization study of the early embryo culture system of bovine in vitro fertilization[J].Journal of Animal Science and Veterinary Medicine,2019, 38(2): 7-12. (in Chinese)
[2] EZZ M A, TAKAHASHI M, RIVERA R M, et al. Cathepsin L regulates oocyte meiosis and preimplantation embryo development[J].Cell Prolif, 2023, 57(1): e13526.
[3] FERRE L B, KJELLAND M E, STROBECH L B, et al. Review: Recent advances in bovine in vitro embryo production: reproductive biotechnology history and methods[J].Animal, 2020, 14(5) : 991-1004.
[4] PYTEL A T, Z·YZ·YN'SKA-GALEN'SKA K, GAJEWSKI Z, et al. Factors defining developmental competence of bovine oocytes collected for in vitro embryo production[J].Biol Reprod, 2024, 4(25): ioae065.
[5] PASQUARIELLO R, PENNAROSSA G, ARCURI S, et al. Sperm fertilizing ability in vitro influences bovine blastocyst miRNA content[J].Theriogenology, 2024, 222(1) : 1-9.
[6] 徐 華, 邢寶奎, 朱 捷. 牛卵母細胞體外成熟存在的問題及可能的解決方法[J].黑龍江動物繁殖, 2022, 30(6): 16-22.
XU H, XING B K, ZHU J. The issues of in vitro maturation in bovine oocytes and possible solution[J].Heilongjiang Journal of Animal Reproduction, 2022, 30(6): 16-22. (in Chinese)
[7] 王騰飛, 張 燕, 王彥平, 等. 奶牛活體采卵-體外受精效率的影響因素研究[J].中國畜牧獸醫, 2021, 48(2): 574-580.
WANG T F, ZHANG Y. WANG Y P, et al. Study on the influencing factors in vitro fertilization efficiency in dairy cows[J].China Animal Husbandry amp; Veterinary Medicine, 2021, 48(2): 574-580. (in Chinese)
[8] 閻來慶, 劉雪凝, 劉巧香, 等. 褪黑素在牛體外胚胎生產中的應用研究進展[J].中國畜牧雜志, 2022, 58(2): 22-25.
YAN L Q, LIU X N, LIU Q X, et al. Advance in application of melatonin in bovine embryo production in vitro[J].Chinese Journal of Animal Science, 2022, 58(2): 22-25. (in Chinese)
[9] KEANE J A, EALY A D. An Overview of Reactive Oxygen Species Damage Occurring during In Vitro Bovine Oocyte and Embryo Development and the Efficacy of Antioxidant Use to Limit These Adverse Effects[J].Animals (Basel), 2024, 14(2): 330.
[10] YIN Y J, ZHANG H H, WANG Y, et al. Ferulic acid ameliorates the quality of in vitro-aged bovine oocytes by suppressinng oxiadative stress and apoptosis.[J].Aging (Albany NY), 2023, 15(21): 12497-12512.
[11] TUTT D A R, GUVEN-ATES G, KWONG W Y, et al. Developmental, cytogenetic and epigenetic consequences of removing complex proteins and adding melatonin during in vitro maturation of bovine oocytes[J].Front Endocrinol (Lausanne), 2023, 14:1280847.
[12] 劉自營, 鄭 卓, 高漢婷, 等. 牛體外胚胎生產技術研究進展[J].中國畜禽種業, 2023, 19(7): 170-175.
LIU Z Y, ZHENG Z, GAO H T, et al. Research progress in bovine in vitro embryo production technology[J].The Chinese Livestock and Poultry Breeding, 2023, 19(7): 170-175. (in Chinese)
[13] HANSEN P J. Review: Some challenges and unrealized opportunities toward widespread use of the in vitro-produced embryo in cattle production[J].Animal, 2023, 17(1): 100745.
[14] 張培培, 郝海生, 杜衛華, 等. OPU卵母細胞體外成熟體系的優化研究進展[J].畜牧獸醫學報, 2023, 54(4): 1359-1369.
ZHANG P P, HAO H S, DU W H, et al. A review of optimization of in vitro maturation of opu oocytes[J].Chinese Journal of Animal and Veterinary Sciences, Acta Veterinaria et Zootechnica Sinica, 2023, 54(4): 1359-1369. (in Chinese)
[15] DE VOS M, GRYNBERG M, HO T M, et al. Perspectives on the development and future of oocyte IVM in clinical practice[J].J Assist Reprod Genet, 2021, 38(6): 1265-1280.
[16] MOOREY S E, HESSOCK E A, EDWARDS J L. Preovulatory follicle contributions to oocyte competence in cattle: importance of the ever-evolving intrafollicular environment leading up to the luteinizing hormone surge[J].J Anim Sci, 2022, 100(7): 153.
[17] RUSSELL D F, BAQIR S, BORDIGNON J, et al. The impact of oocyte maturation media on early bovine embryonic development[J].Mol Reprod Dev, 2006, 73(10): 1255-1270.
[18] SHAHZAD Q, XU H Y, PU L, et al. Developmental potential of buffalo embryos cultured in serum free culture system[J].Theriogenology, 2020, 149(2): 38-45.
[19] 李曉霞, 曹平華, 鄧 星, 等. 不同濃度的牛血清及牛血清白蛋白對水牛卵母細胞體外成熟的影響[J].河南農業科學, 2008, 37(7): 108-116.
LI X X, CAO H P, DENG X, et al. In vitro maturation of buffalo oocytes with different concentrations of bovine serum or BSA[J].Journal of Henan Agricultural Sciences, 2008, 37(7): 108-116. (in Chinese)
[20] MIHOKO F, YAMADA R, MIYANO T. In vitro growth of bovine oocytes in oocyte-cumulus cell complexes and the effect of follicle stimulating hormone on the growth of oocytes[J].J Reprod Dev, 2021, 76(1) : 5-13.
[21] 丁麗艷, 韓永勝, 丁得利, 等.培養液中添加物對牛卵母細胞體外成熟、受精和胚胎早期發育的影響[J].畜牧獸醫科技信息, 2015, 31(01): 26-27.
DING Y L, HAN Y S, DING D L, et al. The effects of additives in culture medium on in vitro maturation, fertilization, and early embryonic development of bovine oocytes[J].Chinese Journal of Animal Husbandry and Veterinary Medicine, 2015, 31(01): 26-27. (in Chinese)
[22] LU C L, WANG T R, YAN L Y, et al. Gonadotropin-mediated dynamic alterations during bovine oocyte maturation in vitro[J].Biol Reprod, 2014, 91(2): 44.
[23] 王雪梅, 馮懷亮, 張 展, 等. 絨毛膜促性腺激素與卵泡刺激素對牛未成熟卵母細胞體外發育的影響[J].河南醫學研究, 2018, 27(18): 3267-3270.
WANG X M, FENG H L, ZHANG Z,et al. Effect of CG and FSH on maturation of bovine immature oocytes in vitro[J].Henan Medical Research, 2018, 27(18): 3267-3270. (in Chinese)
[24] MAKITA M, MIYANO T. Steroid hormones promote bovine oocyte growth and connection with granulosa cells[J].Theriogenology, 2014, 82(4): 605-612.
[25] ARIAS M E, VARGAS T, GALLARDO V, et al. Simple and Efficient Chemically Defined In Vitro Maturation and Embryo Culture System for Bovine Embryos[J].Animals (Basel), 2022, 12(21): 3057.
[26] RIOS G L, BUSCHIAZZO J, MUCCI N C, et al. Combined epidermal growth factor and hyaluronic acid supplementation of in vitro maturation medium and its impact on bovine oocyte proteome and competence[J].Theriogenology, 2015, 83(5): 874-880.
[27] YANG S, YANG Y, HAO H, et al. Supplementation of EGF, IGF-1, and Connexin 37 in IVM Medium Significantly Improved the Maturation of Bovine Oocytes and Vitrification of Their IVF Blastocysts[J].Genes (Basel), 2022, 13(5): 805.
[28] ANCHORDOQUY J P, BALBI M, FARNETANO N A, et al. Trace mineral mixture supplemented to in vitro maturation medium improves subsequent embryo development and embryo quality in cattle[J].Vet Res Commun, 2022, 46(4): 1111-1119.
[29] EL-SHEIKH M, MESALAM A, JOO M D, et al. Attenuation of Oxidative Stress and Regulation of AKT Signaling by Vanillic Acid during Bovine Pre-Implantation Embryo Development[J].Nutrients, 2023, 15(10): 2257.
[30] JIAO A, SUN J, SUN Z, et al. Effects of limonin on oxidative stress and early apoptosis in oocytes during in vitro maturation[J].Theriogenology, 2024, 218(1): 8-15.
[31] CAJAS Y N, CAóN-BELTRáN K, LADRóN DE GUEVARA M, et al. Antioxidant Nobiletin Enhances Oocyte Maturation and Subsequent Embryo Development and Quality[J].Int Mol Sci, 2020, 21(15): 5340.
[32] RíOS G L, BUSCHIAZZO J, MUCCI N C, et al. Combined epidermal growth factor and hyaluronic acid supplementation of in vitro maturation medium and its impact on bovine oocyte proteome and competence[J].Theriogenology, 2015, 83(5): 874-80.
[33] ANCHORDOQUY J M, ANCHORDOQUY J P, TESTA J A, et al. Influence of vascular endothelial growth factor and Cysteamine on in vitro bovine oocyte maturation and subsequent embryo development[J].Cell Biol Int, 2015, 39(10): 1090-1098.
[34] CANEL N G, SUVá M, BEVACQUA R J, et al. Improved embryo development using high cysteamine concentration during IVM and sperm co-culture with COCs previous to ICSI in bovine[J].Theriogenology, 2018, 117(1): 26-33.
[35] 吉文匯, 王玉玲, 何翃閎, 等. 維生素A對牦牛卵母細胞體外成熟及后續胚胎發育能力的影響[J].中國畜牧獸醫, 2022, 49(12): 4707-4714.
JI W H, WANG Y L, HE H H, et al. Effect of vitamin A on the maturation and subsequent development of yak oocytes in vitro[J].China Animal Husbandry amp; Veterinary Medicine, 2022, 49(12): 4707-4714. (in Chinese)
[36] YANG S, YANG Y, HAO H S, et al. Supplementation of EGF, IGF-1, and Connexin 37 in IVM Medium Significantly Improved the Maturation of Bovine Oocytes and Vitrification of Their IVF Blastocysts[J].Genes(Basel), 2022, 13(5): 805.
[37] WANG Y, QI J J, YIN Y J, et al. Ferulic Acid Enhances Oocyte Maturation and the Subsequent Development of Bovine Oocytes[J].Int J Mol Sci, 2023, 24(19): 14804.
[38] BICICI E, SATILMIS F, BODU M, et al. Effect of putrescine supplementation to in vitro maturation medium on embryo development and quality in cattle[J].Anim Biotechnol, 2023, 34(8): 3887-3896.
[39] DAVOODIAN N, KADIVAR A, MEHRBAN H. Supplementation of media with gamma-oryzanol as a novel antioxidant to overcome redox imbalance during bovine oocyte maturation in vitro[J].Reprod Domest Anim, 2023, 59(1):e14503.
[40] GADELLA B M. The assembly of a zona pellucida binding protein complex in sperm[J].Reprod Domest Anim, 2008, 43(5): 12-19.
[41] O’CALLAGHAN E, NAVARRETE-LOPEZ P, ?TIAVNICKá M, et al. Adenylate kinase 9 is essential for sperm function and male fertility in mammals[J].Proc Natl Acad Sci U S A, 2023, 120(42): e2305712120.
[42] MENDES J O JR, BURNS P D, TORRE-SANCHEZ J F, et al. Effect of heparin on cleavage rates and embryo production with four bovine sperm preparation protocols[J].Theriogenology, 2003, 60(2): 331-340.
[43] KAMATH M S, MASCARENHAS M, FRANIK S, et al. Clinical adjuncts in in vitro fertilization: a growing list[J].Fertil Steril, 2019, 112(6): 978-986.
[44] LI-YOU, SANJEEV A" Y, et al. Significant heparin effect on bovine embryo development during sexed in vitro fertilization[J].J Reprod Dev, 2017, 63(2): 175-183.
[45] WRENZYCKI C, CUNHA A T M, CARVALHO J O, et al. Bovine epididymal spermatozoa treatment for in vitro fertilization: Heparin accelerates fertilization and enables a reduction in coincubation time[J].PloS One, 2019, 14(1): e0209692.
[46] TESCHKE R, EICKHOFF A. Wilson Disease: Copper-Mediated Cuproptosis, Iron-Related Ferroptosis, and Clinical Highlights, with Comprehensive and Critical Analysis Update[J].Int J Mol Sci, 2024, 25(9): 4753.
[47] PAVLOK A. D-penicillamine and granulosa cells can effectively extend the fertile life span of bovine frozen-thawed spermatozoa in vitro effect on fertilization and polyspermy.[J].Theriogenology, 2000, 53(5): 1135-1146.
[48] BASEGGIO CONRADO A, D’ANGELANTONIO M, D’ERME M, et al. The Interaction of Hypotaurine and Other Sulfinates with Reactive Oxygen and Nitrogen Species: A Survey of Reaction Mechanisms[M].Adv Exp Med Biol, 2017,975(1): 573-83.
[49] SEIFY M, ZARABADIPOUR M, GHALENO L R, et al. The anti-oxidant roles of Taurine and Hypotaurine on acrosome integrity, HBA and HSPA2 of the human sperm during vitrification and post warming in two different temperature[J].Cryobiology, 2019, 90: 89-95.
[50] SCHUH SM, HILLE B, BABCOCK D F. Adenosine and catecholamine agonists speed the flagellar beat of mammalian sperm by a non-receptor-mediated mechanism[J].Biol Reprod, 2007, 77(6): 960-969.
[51] SCHUH SM, CARLSON AE, MCKNIGHT GS, et al. Signaling pathways for modulation of mouse sperm motility by adenosine and catecholamine agonists[J].Biol Reprod, 2006, 74(3): 492-500.
[52] HASLER J F, STOKES J E. Effect of the presence or absence of percoll centrifugation; penicillamine, hypotaurine, and epinephrine; and heparin on in vitro production of bovine embryos[J].Reprod Fertil Dev, 2013, 25(1): 255-259.
[53] KANG S S, KOYAMA K, HUANG W P, et al. Addition of D-penicillamine, hypotaurine, and epinephrine (PHE) mixture to IVF medium maintains motility and longevity of bovine sperm and enhances stable production of blastocysts in vitro[J].J Reprod Dev, 2015, 61(2): 99-105.
[54] GARCíA M F S, GABBANELLI N, RíOS G L. Exogenous progesterone during in vitro fertilization improves developmental competence of partially cumulus-denuded bovine oocytes[J].Theriogenology, 2023, 211(7): 11-18.
[55] PEIXOTO DE, SOUZA V, JENSEN J, WHITLER W, et al. Increasing vitamin D levels to improve fertilization rates in cattle[J].J Anim Sci, 2022, 100(7): 1-12.
[56] BEHNAM M, ASADPOUR R, TOPRAGGALEH T R, et al. Improvement of post-thaw quality and fertilizing ability of bull spermatozoa using Rho kinase inhibitor in freezing extender[J].Front Vet Sci, 2023, 10: 1155048.
[57] GON?ALVES F S, BARRETTO L S S, ARRUDA R P, et al. Heparin and penicillamine-hypotaurine-epinephrine (PHE) solution during bovine in vitro fertilization procedures impair the quality of spermatozoa but improve normal oocyte fecundation and early embryonic development[J].In Vitro Cell Dev Biol Anim, 2013, 50(1): 39-47.
[58] SANTANA P P B, DA SILVA B B, SILVA T V G, et al. Addition of L-arginine to the fertilization medium enhances subsequent bovine embryo development rates[J].Theriogenology, 2016, 85(6): 1132-1138.
[59] KANG S S, KOYAMA K, HUANG W P, et al. Addition of D-penicillamine, hypotaurine, and epinephrine (PHE) mixture to IVF medium maintains motility and longevity of bovine sperm and enhances stable production of blastocysts in vitro[J].J Reprod Dev, 2015, 61(2): 99-105.
[60] AN L Y, SANJEEV A" Y, et al. Significant heparin effect on bovine embryo development during sexed in vitro fertilization[J].J Reprod Dev, 2017, 63(2): 175-183.
[61] YUAN Y G, MESALAM A, SONG S H, et al. Effect of nicotinamide supplementation in in vitro fertilization medium on bovine embryo development[J].Mol Reprod Dev, 2020, 87(10): 1070-1081.
[62] RODRíGUEZ-VILLAMIL P, MENTZ D, ONGARATTO F L, et al. Assessment of binder of sperm protein 1 (BSP1) and heparin effects on in vitro capacitation and fertilization of bovine ejaculated and epididymal sperm[J].Zygote, 2020, 28(6): 489-494.
[63] ZHANG P P, YANG B G, XU X, et al. Combination of CNP, MT and FLI during IVM Significantly Improved the Quality and Development Abilities of Bovine Oocytes and IVF-Derived Embryos[J].Antioxidants(Basel), 2023, 12(4): 897.
[64] WANG L M, FENG H L, MA Y Z, et al. Expression of IGF receptors and its ligands in bovine oocytes and preimplantation embryos[J].Anim Reprod Sci, 2009, 114(1/3): 99-108.
[65] NOGUEIRA M F G, STOECKLEIN K S, ORTEGA M S, et al. Improved cryopreservation of in vitro produced bovine embryos using FGF2, LIF, and IGF1[J].PloS One, 2021, 16(2): e0243727.
[66] MOSS J I, PONTES E, HANSEN P J. Insulin-like growth factor-1 protects preimplantation embryos from anti-developmental actions of menadione[J].Arch Toxicol, 2009, 83(11): 1001-1007.
[67] MESALAM A, LEE K-L, KHAN I, et al. A combination of bovine serum albumin with insulin-transferrin-sodium selenite and/or epidermal growth factor as alternatives to fetal bovine serum in culture medium improves bovine embryo quality and trophoblast invasion by induction of matrix metalloproteinases[J].Reprod Fertil Dev, 2019, 31(2): 333-346.
[68] AHUMADA C J, SALVADOR I, CEBRIAN-SERRANO A, et al. Effect of supplementation of different growth factors in embryo culture medium with a small number of bovine embryos on in vitro embryo development and quality[J].Animal, 2013, 7(3): 455-462.
[69] NASPINSKA R, MOREIRA DA SILVA M H, MOREIRA DA SILVA F. Current Advances in Bovine In Vitro Maturation and Embryo Production Using Different Antioxidants: A Review[J].J Dev Biol, 2023, 11(3): 36.
[70] BáEZ F, DE BRUN V, RODRíGUEZ-OSORIO N, et al. Low oxygen tension during in vitro embryo production improves the yield, quality, and cryotolerance of bovine blastocysts[J].Anim Sci J, 2024, 95(1): e13941.
[71] 孫尉峻. 外源谷胱甘肽對牛體外受精胚胎發育的影響[D].北京: 中國農業科學院, 2015.
SUN W J. Exogenous glutathione supplementation in culture medium improves the bovine embryo development after in vitro fertilization[D].Beijing: Chinese Academy of Agricultural Sciences, 2015. (in Chinese)
[72] 孫敬宇. 檸檬苦素對牛卵母細胞體外成熟及早期胚胎發育的影響[D].延吉: 延邊大學, 2023.
SUN J Y. Effect of Limonin on vitro maturation and early embryonic development of bovine oocytes[D].Yanji: Yanbian University, 2023. (in Chinese)
[73] 佟桂芝, 宋 斌, 王洪寶, 等. 褪黑素對牛體外胚胎生產效果的影響[J].現代畜牧科技, 2017, 45(12): 1-2.
TONG J Z, SONG B, WANG H B, et al. Effect of melatonin on cow embryo production in vitro[J].Modern Animal Husbandry Science amp; Technology, 2017, 45(12): 1-2. (in Chinese)
[74] MARQUES T C, DA SILVA SANTOS E C, DIESEL T O, et al. Melatonin reduces apoptotic cells, SOD2 and HSPB1 and improves the in vitro production and quality of bovine blastocysts[J].Reprod Domest Anim, 2017, 53(1): 226-236.
[75] YU B, VAN TOL H T A, OEI C H Y, et al. Lysophosphatidic Acid Accelerates Bovine In Vitro-Produced Blastocyst Formation through the Hippo/YAP Pathway[J].Int J Mol Sci, 2021, 22(11): 5915.
[76] CARRILLO-GONZáLEZ D F, HERNáNDEZ-HERRERA D Y, MALDONADO-ESTRADA J G. The role of L-carnitine in bovine embryo metabolism. A review of the effect of supplementation with a metabolic modulator on in vitro embryo production[J].Anim Biotechnol, 2021, 34(2): 413-423.ZOLINI A M, CARRASCAL-TRIANA E, RUIZ DE KING A, et al. Effect of addition of l-carnitine to media for oocyte maturation and embryo culture on development and cryotolerance of bovine embryos produced in vitro[J].Theriogenology,2019,133:135-143.
[77] EL-MAARRI O, SAEED-ZIDANE M, TESFAYE D, et al. Hyaluronic acid and epidermal growth factor improved the bovine embryo quality by regulating the DNA methylation and expression patterns of the focal adhesion pathway[J].PloS One, 2019, 14(10): e0223753.
[78] FABRA M C, ANCHORDOQUY J P, CARRANZA-MARTíN A C, et al. Alpha-lipoic acid improves bovine preimplantation blastocyst quality and cryotolerance[J].Theriogenology, 2023, 198: 61-68.
[79] MIGLIO L, NOVAES M A S, DE LIMA L F, et al. Zinc oxide nanoparticles functionalized with curcumin supplementation during in vitro embryo culture impaired in a concentration-dependent-manner blastocyst production in cattle[J].Reprod Domes Anim, 2023, 58(8): 1172-1175.
[80] 徐 華, 車瑞香, 朱 捷. 牛OPU-IVF技術發展現狀和趨勢[J].中國奶牛, 2023, (02): 19-24.
XU H, CHE R X, ZHU J. The development status and trend of bovine OPU-IVF technology[J].China Dairy Cattle, 2023, (02): 19-24. (in Chinese)
(編輯 郭云雁)

