海洋Penicillium屬化學成分與生物活性的研究
余 玥,林文翰
(1.哈爾濱商業大學生命科學與環境科學研究中心,哈爾濱 150076;2.國家教育部抗腫瘤天然藥物工程研究中心,哈爾濱 150076;3.北京大學天然藥物及仿生藥物國家重點實驗室,北京 100083)
海洋真菌是一類具有真核結構,能形成孢子,營腐生或寄生生活的海洋生物,包括來源于海洋的專性海洋真菌與來源于陸地或淡水,能在海洋生境中生長與繁殖的兼性海洋真菌.目前已發現的還具有產生理活性物質的海洋真菌有曲霉(Aspergillus)、青霉(Penicillium)、海殼霉(Corollospora)、長蠕孢屬(Helminthosporium)、莖點霉(Phoma)、光黑殼屬(Preussia)等.
自從青霉素問世以來,微生物產生的活性代謝產物就成為開發新藥的重要資源.對真菌Penicillium屬的研究較多,青霉屬在屬下分六個亞屬:雙輪亞屬、籃狀菌屬、類曲霉亞屬、正青霉屬、青霉亞屬、叉狀亞屬.其中,雙輪亞屬分14個種,籃狀菌屬分7個種,類曲霉亞屬分25個種,正青霉屬分9個種,青霉亞屬分19個種,叉狀亞屬分22個種[1].有性態的是籃狀菌屬和正青霉屬[2].發現其能夠代謝多種此生代謝產物,主要有生物堿、萜類及聚酮類,還有一些其他類別的化合物[3].其中一些化合物有細胞毒活性,還有很好的抗菌,殺蟲及抗蟲的功效[4].本文按結構類型與代謝產物活性對近年來國內外學者在海洋中分離得到的青霉屬化學成分有關研究進展進行介紹.
1 生物堿
從海洋青霉菌中發現的眾多生物堿中,主要結構類型有吲哚、喹啉、喹唑啉、吡咯、哌啶等常見的雜環生物堿,還有一些其他生物堿類化合物,這些生物堿均具有重要生物活性.
1.1 吲哚類生物堿
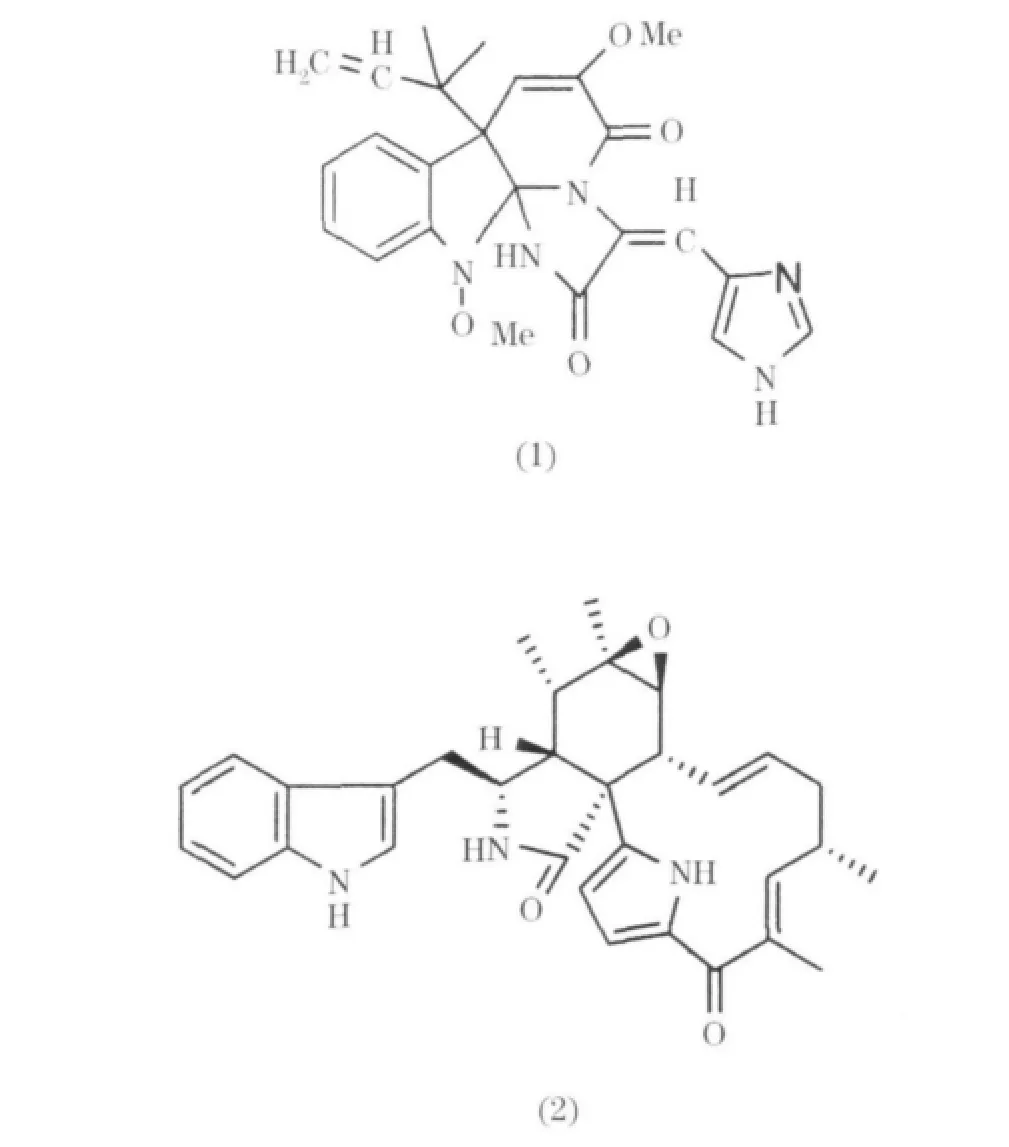
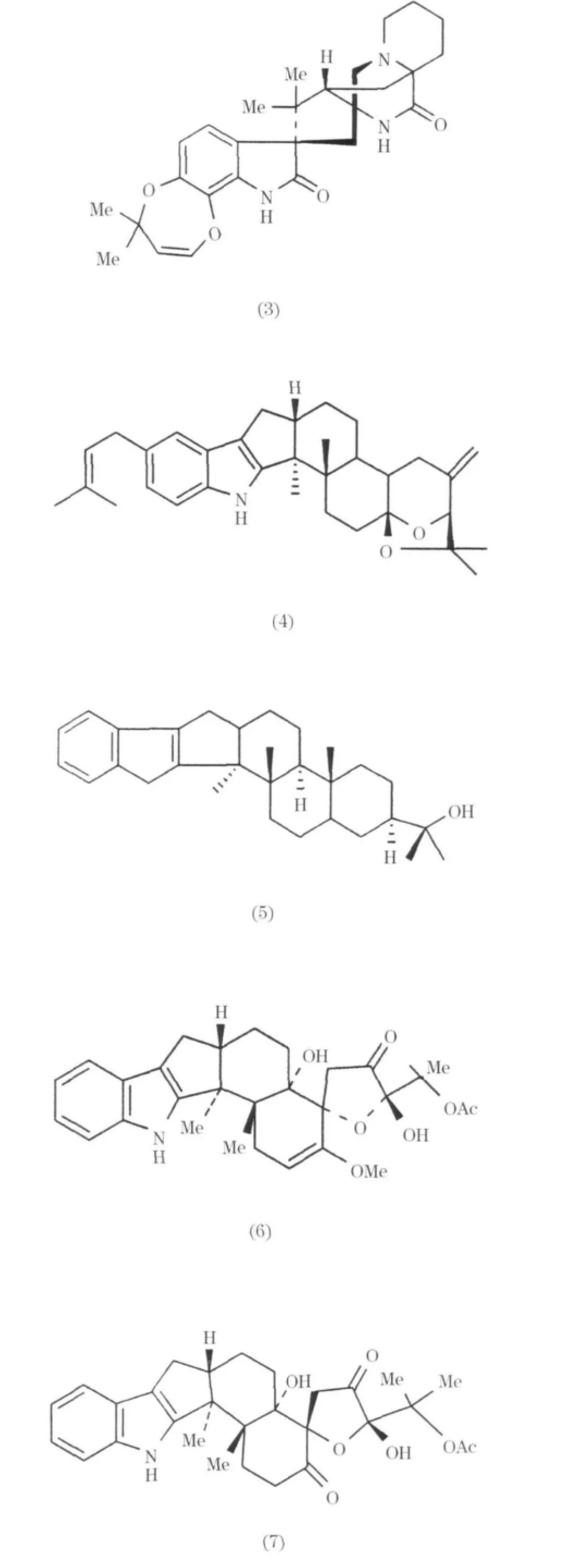
在海洋Penicillium屬產生的生物堿中,萜類吲哚生物堿數量很多,從Penicillium Oxaline(1)中分離得到[5];從一種海藻中分離出來的Penicillium屬真菌中分離出七個吲哚類生物堿,其中兩個為penochalasinA(2)和D(3),它們對細胞P388表現出明顯的細胞毒活性,ED50值為 3.2,2.1μg/mL[6].在海洋Penicillium屬產生的生物堿當中,萜類吲哚生物堿的數量很多.從寄生真菌Penicillium sp.中分離得到十個吲哚三萜生物堿,分別為shearinines A、D-K和paspalitrem A(4)以及一個吲哚二萜生物堿paspaline(5)shearinines,A-K在體外能阻斷電導Ca-K通道[7].從一種新的海洋 Penicillium霉菌中分離得到兩個吲哚二萜生物堿thiersinies A(6)和B(7),在抗黏蟲實驗中,表現出顯著活性,降低率為83%和84%[8].
1.2 喹啉類生物堿
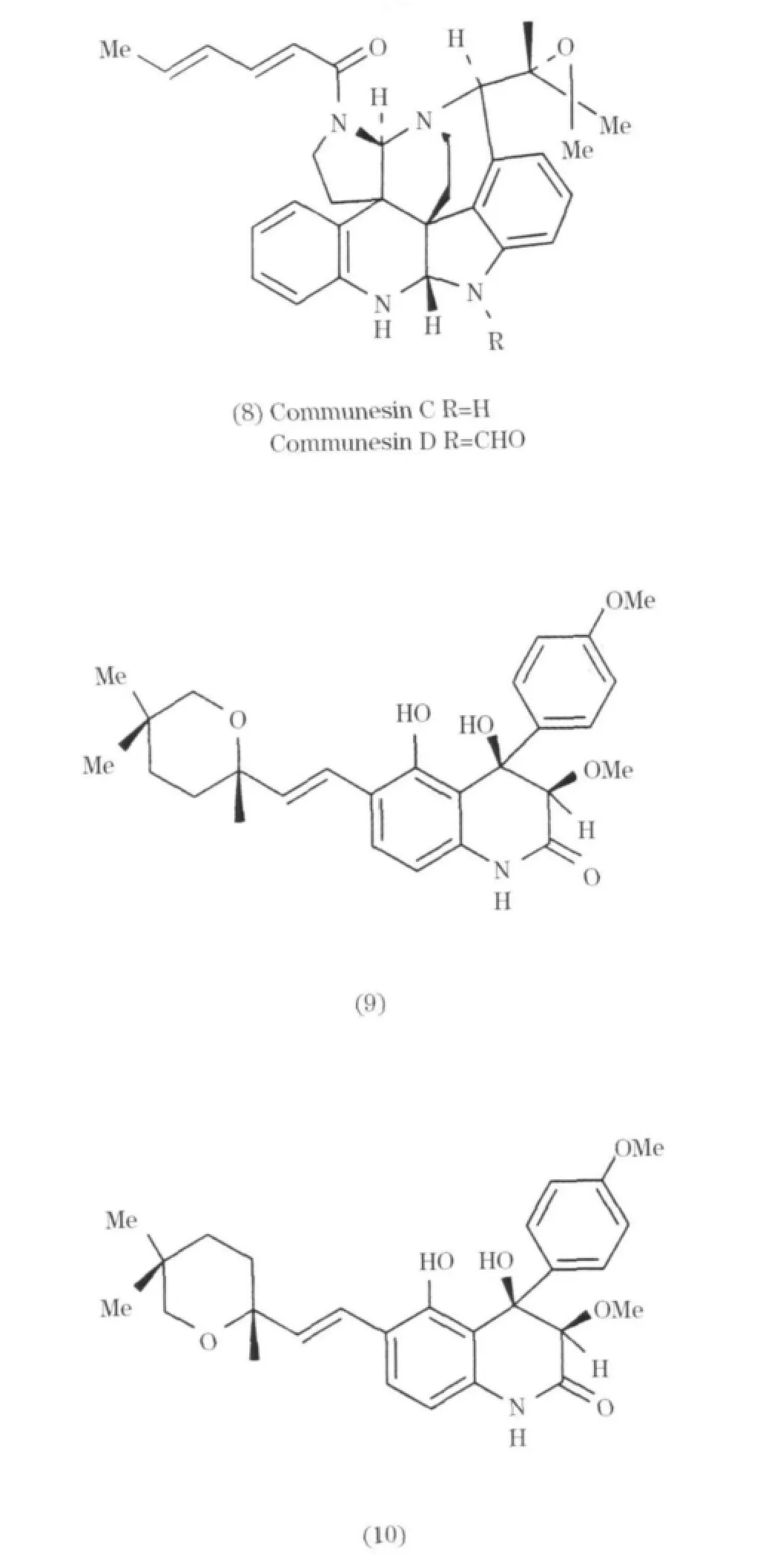
Communesin C和 D(8)是一種附生于海綿Axinella verrucosa的真菌,從 Penicillium sp.中首次分離得到[9];Penigequinolones A 和 B(9~10)也是從真菌 Penicillium sp.中分離所得[10].
1.3 喹唑啉類生物堿
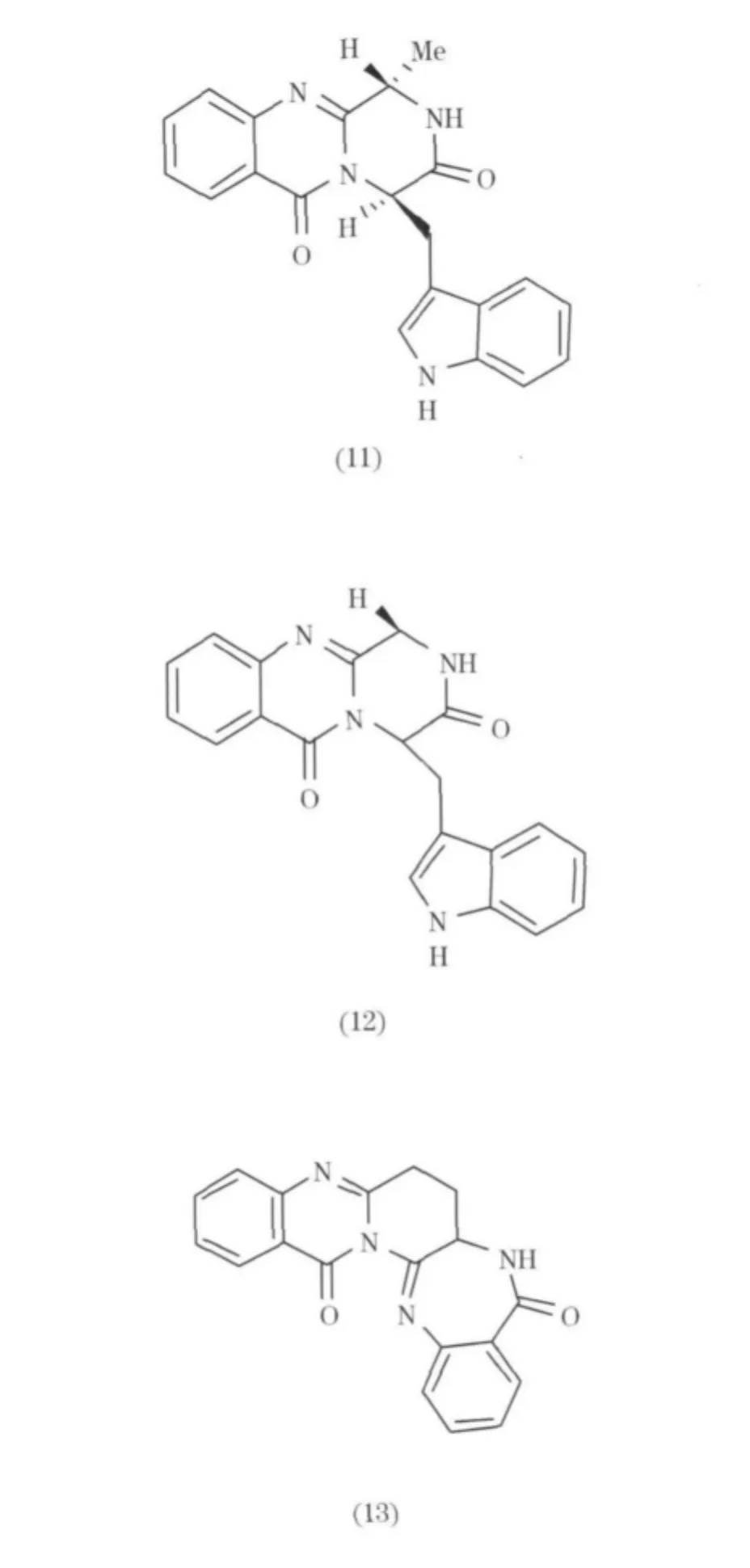
從Penicillium thymicola中分離得到幾個喹唑啉類生物堿(11 ~13)[11].
1.4 其他類型生物堿
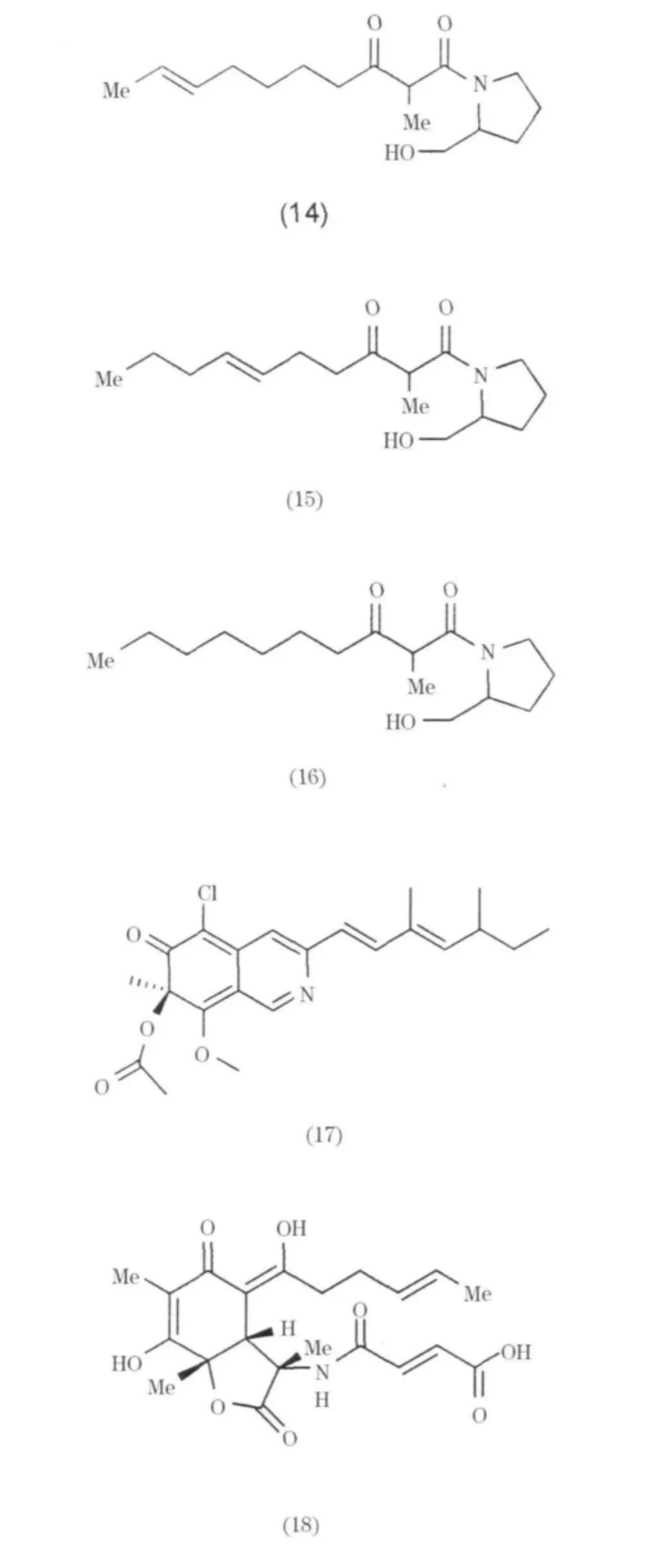
自Penicillium citrinum分到吡咯類生物堿Scalusamide A-C(14~16),Scalusamide A有較弱的抗菌活性,對Cryptococcus nepformans和Micrococcus luteus的 MIC 值為 16.7 和 33.37μg/mL[12];從Penicillium 屬分離生物堿 herquline A(17)[13];從Penicillium aurantiogriseum中分離得到一個具有苯并二氮結構的生物堿anacine1 A(18),具有抗蟲活性,在抑制 H.zea幼蟲蛻變實驗中,抑制率為42%[14];自寄生菌Penicillium sp分離得到 Penicildones A-C(19~20)[15];分離自 Penicillium citrinum的生物堿Perinadine A具有較弱的細胞毒活性,對鼠白細胞 L1210 的 IC50值為 20μg/mL[16].
2 萜類
2.1 倍半萜
Shim等人從Penicillium griseofulvum中分到五個倍半萜烯Penifulvins A-E(21~25),其具有一個新穎的二氧[5-6]壬烷四環結構[17-18].Penifulvin A在抗黏蟲Spodopetera frugiperda的試驗中表現出強抗蟲活性,降低了74%的抑制率.

2.2 二萜
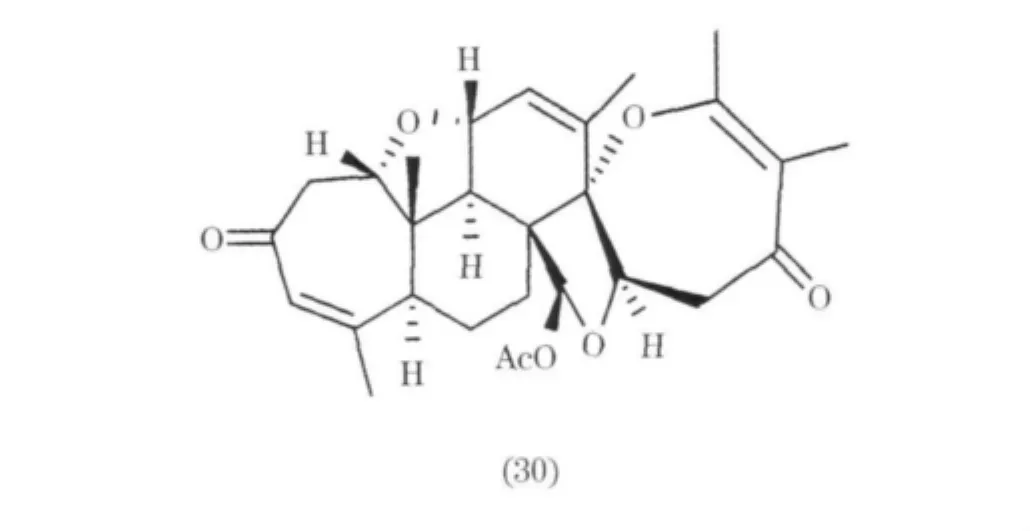
從Penicillium sp.中分離得到一個二萜Brevione A(26)[19],另外還有四個二萜 Brevione B ~ E(27 ~30)[20].

2 .3 二萜半萜
從該屬中分離得到的二萜半萜均為混合聚酮-萜類(meroterpenoid)結構,是分離得到萜類的主要成分.從Penicillium sp.中分離得到Peaustinoid A(31),具有四個六元環結構,Peaustinoid A對Escherichia coli和 Bacilus sp.有中等抑菌活性[21].

3 大環內酯類
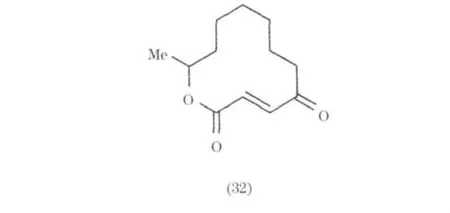
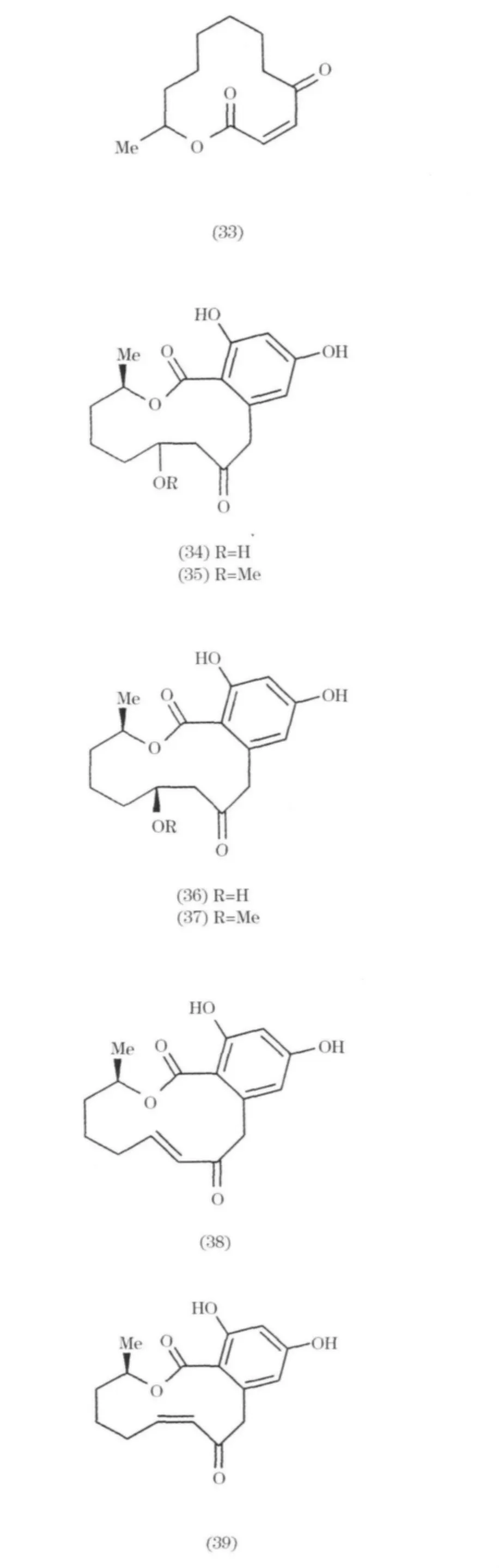
來源于海洋微生物的大環內酯和聚醚類化合物一般都具有特殊結構和顯著的生物活性,如抗腫瘤、抗菌、抗病毒等.目前發現的大環內酯和聚醚類化合物主要是從海綿和軟體動物中分離的.但隨著現代分離技術的提高,近來從深海極端微生物中也分離得到有意義的化合物.Patulolide A(32)和B(33)是從Penicillium urticae mutants中分離得到兩個科單大環內酯[22-23];從 Penicillium 屬中分離得到一系列含苯環的大環內酯(34~39).
4 聚酮類
聚酮類是由C2單位形成的脂肪酸、酚類、苯醌等化合物.海洋青霉屬產生的此種化合物較多,而且結構多樣,并沒有固定的骨架.分離自Penicillium sp.的兩個聚酮-萜preaustinoid A和B對 Escherichia coil,Bacillus sp.及 Staphylococcus aureus均有中等抑菌活性[24].在Penicillium expansum中分離出三個苯并吡喃化合物,具有抑菌作用,100μg/mL時對Lasiodiplodia theobromae菌體生長抑制 率 為 76%,74%和69%[25].分離自Penicillium sp.JP-1的 Penicillienone及 leptosphaerone C具有細胞毒活性,Penicillienone對細胞P388 的 IC50值為 1.38μg/mL,leptosphaerone C 對細胞 A-549 的 IC50值為 1.45μg/mL[26].
從Penicillium citrinum F5中分離得一個具有苯并二氫呋喃結構的化合物(40),是氧化劑[27];從Penicillium citrinum和Penicillium steckii中分離得到兩個化合物(41~42);從一株源自深水沉積物的Penicillium真菌中分離出一個苯醌類化合物(43).

5 其他類型
海洋Penicillium屬還產生了許多其他類型的代謝物,如:內酯類、環肽類、甾體、吡喃酮類等.
5.1 環肽類
從Penicillium brevicompactum中分離出環四肽brevigellin(44).

5.2 吡喃酮類
該類化合物結構簡單,分子質量小.一般有兩種,一種為 2-酮,如:citreopyrone(45)[28];一種為4-酮,如 citreo-γ-pyrone(46)[29].在 Penicillium expansum中分離出三個苯并吡喃化合物,具有抑菌作用,100μg/mL 時對 Lasiodiplodia theobromae 菌體生長抑制率為76%,74%和69%[30].

5.3 其他
此外,從該屬真菌中還分離出一個硫醇以及一些酰胺、胺類、酮等化合物.Ravenic Acid有抗真菌活性,在25μg/mL時能抑制青霉素及多種藥物生長;7-deacetoxyyanuthone A對該菌也有中等抑制作用,MIC 值為 50μg/mL[31-36].
6 結語
綜上所述,青霉菌屬在高壓、高鹽度、低養分、低溫這樣所謂生命的極限環境中,發展出了獨特的代謝方式,這不僅保證了其可以在極端環境下生存,也提供了產生新型代謝產物的潛力.迄今為止,人們從海洋青霉菌中發現了許多具有藥用價值的活性化合物,有力地推動了海洋天然產物化學的發展.
[1]孔華忠.中國真菌志(第三十五卷):青霉屬及其相關性型屬[M].北京:科學出版社,2007.
[2]宋愛環,李紅葉,劉小紅,等.指狀青霉(Penicillium digitatum)原生質體制備和再生條件[J].農業生物技術學報,2004,12(2):197-201.
[3]梁宗琦.真菌次生代謝產物多樣性及其潛在應用價值[J].生物多樣性,1999,7(2):145-150.
[4]姜 健,楊寶靈.海洋微生物生物活性物質的研究[J].云南大學學報,2004,26(6A):91-95.
[5]韓妍妍,張亞娟,王維娜,等.海洋微生物是開發海洋藥物的重要資源[J].海洋化學,2000,26(9):7-12.
[6]XU M J,GESSNER,GUIDO,et al.Shearinines D-K,new indole triterpenoids from an endophytic Penicillium sp.(strain HKI0459)with blocking activity on large-conductance calcium- activated potassium channaels[J].Tetrahedron,2007,63(2):435-444.
[7]LI C,GLOER,JAMES B,et al.Thiersinines A and B:novel antiinsectan indole diterpenoids from a new fungicolous Penicillium species(NRRL 28147)[J].Organic Letters,2002,4(18):3095-3098.
[8]JADULCO,RAQUEL,EDRADA,et al.New communesin derivatives from the fungus Penicillium sp.derived from the Mediterranean sponge Axinella verrucosa[J].Journal of Natural Products,2004,67(1):78-81.
[9]KIMURA,YASUL,KUSANO,et al.Penigequinolones A and B,pollen-growth inhibitors produced by Penigequinolone A and B,pollen-growth inhibitors produces by Penicillium sp[J].Tetrahedron Letters,1996,37(28):4961-4964.
[10]LARSEN,THOMAS O,FTYDENVANG,et al.UV-Guided I-solation of Alantrypinone,a Novel Penicillium Alkaloid[J].Journal of Natural Products,1998,61(9):1154-1157.
[11]TSUDA,MASASHI,SASAKI,et al.Scalusamides A-C,new pyrrolidine alkaloids from the marine-derived fungus Penicillium citrinum[J].Journal of Natural Products,2005,68(2):273-276.
[12]KAGATA,TOSHINORI,SHIGEMORI,et al.Coruscol A,a new metabolite from the marine-derived fungus Penicillium species[J].Journal of Natural Products,2000,63(6):886-887.
[13]BOYES K,JANE M,GURNEY,et al.Anacine,a new benzodiazepine metabolite of Penicillium aurantiogriseum produced with other alkaloids in submerged fermentation[J].Journal of Natural Products,1993,56(10):1707-17.
[14]GE H M,SHEN,YAO,et al.Penicidones A-C,three cytotoxic alkaloidal metabolites of an endophytic Penicillium sp[J].Phytochemistry,2008,69(2):571-576.
[15]SHIM,SANG H,SWENSON,et al.Penifulvin A,a sesquiterpenoid-derived metabolite containing a novel dioxa[5,5,5,6]fenestranering system from a fungicolous isolate of Penicillium griseofulvum[J].Organic Letters,2006,8(6):1225-1228.
[16]SASAKI,MAI,TSUDA,et al.Perinadine A,a Novel Tetracyclic Alkaioid from Marine-Derived Fungus Pencicllium citrinum[J].Organic Letters,2005,19(7):4261-4264.
[17]SHIM,SANG H,GLOER,et al.Penifulvins B-E and a Silphinene Analogue:Sesquiterpenoids from a Fungicolous Isolate of Penicillium griseofulvum[J].Journal of Natural Products,2006,69(11):1601-1605.
[18]DIERCKX.MACIAS,FRANCISCO A,et al.Allelochemicals from New Zealand fungi.(+)-Brevione A.The first member of a novel family of bioactive spiroditerpenoids isolated from Penicillium brecicompactum[J].Tetrahedron Letters(2000),41(15),2683-2686.
[19]TAKIKAWA,HIROSATO,IMAMURA,et al.Synthesis and absolute configuration of brevione B,an allelochemical isolate from Penicillium sp[J].Tetrahedron,2006,62(1):39-48.
[20]GERIS S,REGINA M,RODRIGUES F,et al.Meroterpenes from Penicillium sp found in association with Melia azedarach[J].Phytochemistry,2002,61(8):907-912.
[21]SEKIGUCHI,AugICHIET.Structure of patulolide A,a new macrolide from Penicillium urticae mtants[J].Tetrahedron Letters,1985,26(19):2341-2.
[22]KALITA,DIPAK,KHAN A T,et al.Total synthesis of(R)-(+)-Patulolide A and(R)-(-)-Patulolide B:the macrolides isolated from Penicillium urticae mutant[J].Tetrahedron,1999,55(16):5177-5184.
[23]CHEN,CHEN H,SHAW C Y.2,3,4-Trimethy1-5,7-dihydroxy-2,3-dihydroben-zofuran,a novel antioxidant,from Penicillium citrinum F5[J].Journal of Natural Products,2002,65(5):740-741.
[24]GERIS S,REGINA M,REDRAGUES F,et al.Meroterpenes from Pencicllium sp.found in association with Melia azedarach[J].Phytochemistry,2002,61(8):907-912.
[25]SASAKI M,TSUDA,MASASHI,et al.Perinadine A,a Novel Tetracyclic Alkaioid from Marine-Derived Fungus Pencicllium citrinum[J].Organic Letters(2005),7(19),4261-4264.
[26]LIN Z J,ZHU T X.Polyketides from Pencicllium sp.JP-1,anendophytic fungus associated with the mangrove plant Aegiceras corniculatim[J].Phytochemistry,2008,69(5):1273-1278.
[27]NIWA,MASATAKE.Isolation and structure of citreopyone,a metabolite of Penicillium citreo-viride Biourge[J].Tetrahedron Letters,1980,46(21):4481-2.
[28]NAKADA,TAKASHI,SUDO,et al.Two new metabolites of hybrid strains KO 0201 and 0211 derived from penicillium citreo- viride B.IFO 6200 and 4692[J].Tetrahedron Letters,1999,40(37):6831-6834.
[29]STIEILE,DONALD B,STIERLE,et al.New phomopsolides from a Penicillium sp[J].Journal of Natual Products,1995,41(36):7481-7485.
[30]HE Guochun.A new antifungal metabolite from Pencicllium expansum[J].Journal of Natural Products,2004,67(7):1084-1087.
[31]HENSENS,OTTO D.Structure elucidation of restricticin,a novel antifungal agent from Penicillium restrictixt[J].Tetrahdron,1991,47(24):3915-24.
[32]IWAMOTO,CHIKA,MINOURA,et al.Absolute stereostuctures of novel cytotoxic metabolites,penostatins A-E,from a Penicillium species separated from an Enteromorpha alga[J].Tetrahedron,1999,55(50):14353-14368.
[33]MICHAEL,ADAM P,GRACE,et al.Ravenic Acid,a New Tetra Acid Isolated from a Cultured Microfungus,Penicillium sp[J].Journal of Natural Products,2002,65(9):1360-1362.
[34]KIMURA,YASUO,MIZUNO,et al.Penienone and penihydrone,new plant growth regulators produced by he fungus,Penicillium sp.NO.13[J].Tetrahedron Letters,1997,38(3):469-472.
[35]LI,X F,CHOI,HONG D,et al.New polyoxygenated farnesylcyclohexenones,deacetoxyyanuthone A and its hydro derivative from the marine-derived fungus Penicillium sp[J].Journal of Natural Products,2003,66(11):1499-1500.
[36]TAKAHASHI,CHIKA.Penostains,novel cytotoxic metabolites from a Pencicllium sp.seprarted from a green alga[J].Tetrahedron Letters,1996,37(5):655-658.

