豆皮水溶性多糖組分SHP-3的物化性質研究
賴富饒,吳 暉*,李曉鳳,任 堯,吳磊燕,,溫其標華南理工大學輕工與食品學院,廣州50640;江西農業大學食品科學與工程學院,南昌330045
豆皮水溶性多糖組分SHP-3的物化性質研究
賴富饒1,吳 暉1*,李曉鳳1,任 堯1,吳磊燕1,2,溫其標11華南理工大學輕工與食品學院,廣州510640;2江西農業大學食品科學與工程學院,南昌330045
對豆皮采用熱水浸提得到豆皮水溶性多糖,通過DEAE-cellulose離子交換柱洗脫,得到3個組分。本文主要研究了采用0.3 M NaOH溶液洗脫純化得到的組分SHP-3的物化性質。對SHP-3進行凝膠滲透色譜、氣相色譜分析、紫外光譜及高碘酸氧化-Smith降解分析,結果表明:SHP-3的分子量為45554,其糖醛酸含量為26171%(wt.%),單糖組成摩爾比為Rah∶Fuc∶Ara∶Xyl∶Man∶Gal∶Glu=3.55∶0.44∶11.58∶1∶7.45∶5.12∶1.12,紫外光譜在260~280 nm沒有吸收峰,表明沒有蛋白質或核酸等物質;高碘酸氧化-Smith降解結果表明SHP-3的連接結構以為(1→4)糖苷鍵為主,其摩爾比例占68.9%,(1→2)糖苷鍵占11.4%,(1→6)糖苷鍵占19.7%。
豆皮;水溶性多糖;純化;單糖組成;氣相色譜
Abstract:The water-soluble polysaccharide of soybean hull(SHP)was extracted by hot water and three fractions were obtained by using DEAE-cellulose anion exchange column.The physicochemical properties of the purified fractions SHP-3,eluted with 0.3M NaOH,were investigated.Gel permeation chromatography showed that the molecule weight of SHP-3 was 45554.GC an alysis of the monosaccharide composition showed that the molar ratio of Rah∶Fuc∶Ara∶Xyl∶Man∶Gal∶Glu was3.55∶0.44∶11.58∶1∶7.45∶5.12∶1.12 and the content of galacturonic acid was 26.71%(wt.%).The ultraviolet spectra indicated that there was no presence of protein in SHP-3.The molarpercentages of(1→2)glycosidic linkage,(1→4)glycosidic linkage and(1→6)glycosidic linkage were 11.4%,68.9%and 19.7%,respectively.
Key words:soybean hull;water-soluble polysaccharide;purification;monosaccharide composition;GC
Introduction
Soybean hull,the seed coat of soybean,is the major coproduct of soybean oil processing industry and is mainly used as animal feed.Soybean hull constitute about 8%of the whole seed and contains about86%complex carbohydrates thus making it a good source of dietary fiber.In some extent,the functions of water-soluble dietary fiber are better than insoluble dietary fiber such as reducing blood serum cholesterol[1].In previous papers,the researchers focused on the preparation of pectin[2],extraction conditions[3]of pectin and NMR analysis of chelating agent soluble solids fractions[4]about soybean hull.Very limited reports dealt with physicochemical properties of the water-soluble polysaccharide fraction extracted from soybean hull with hot water,although physicochemical properties such as molecular weight,shape or confor mation are primary factors controlling their functional properties,.In this study,a water-soluble polysaccharide fraction SHP-3 was isolated and purified by using DEAE-cellulose anion exchange column and Sephadex G-100 column,and then was characterized by GPC,UV spectra,IR spectra and GC.
Materials and Methods
Material
Soybean hull was supplied by Sinoglory Oil&Fat CO.,LTD.(Shandong,China).
Rhamnose,fucose,galactose,mannose,xylose,arabinose,galacturonic acid were purchased from Sigma Chemical Co.(StLouis,MO,USA).Glucose and inositol were obtained from Bo’ao Biological Technology Co. (Shanghai,China).DEAE-cellulose and Sephadex G-100 were obtained from Kayon Biological Technology Co.(Shanghai,China)and GE Healthcare Bio-Sciences Co.(Piscataway,NJ,USA),respectively.Phenol and sulfuric acid were from the Guangzhou Reagent Co.(Guangzhou,China).Trifluoroacetic acid and sodium borohydride were the products of Rich Joint Chemical Reagents Co.(Shanghai,China).The dextrans of varying molecular weigh tpurchased from Sigma (USA).All other chemicals were of the analytical grade available.
Portable autoclave(Boxun Apparatus,Shanghai);Rotary evaporator(Biochemical Equipment Co.,Ltd, Shanghai);High speed refrigerated centrifuge(Hitachi,Japan);Fraction collector(Huxi analysis instrument factory Co.,Ltd,Shanghai);Gas chromatograph (Sh imazu,Japan);Gel permeation chromatography (Waters,USA);717Plus autosampler(Waters, USA);UV-2300 double beam spectrophotometer (Pharmaceutical Machinery Co.,Ltd.Shanghai);Nicolet Fourier transform infrared(FT-IR)spectrometer (Thermo Nicolet Co.,USA);Ubbelohde capillary viscometer(Φ0.78,Glass Instrument Co.,Shanghai); TSK G-5000 PWxLgel column(Tosoh,Japan).
Isolation and purification of soybean hull water soluble polysaccharide
The water soluble polysaccharide was extracted from soybean(Glycine m ax)hull according to Wang Qiet al.[5]and Nakamuraet al.[6]with some modifications. Briefly,soybean hull was ground in a sample mill to pass a 0.25 mm screen.The powdered material was refluxed in 80%ethanol for 6 h.Then the cooled extract was discarded and the residue was washed with 95% ethanol,anhydrous ethyl alcohol,acetone and diethyl ether respectively.The residue was dried at room temperature for 24 h prior to extraction.Subsequently,the residue(30 g)was extracted with hot distilled water (750 mL)by heating at 120℃at pH 5.0 for 90 min with autoclave.The spent residue was centrifuged at 10000×g for 15 min,and the extract was concentrated to minimum volume using a rotary evaporator at 60℃under low pressure.After the protein in the concentrated solution were removed by Sevag reagent(chloroform and n-butanol in 4∶1 ratio)[7],the extract was dialyzed again tap water and deionized water for 24 h,respectively.In order to obtain the crude polysaccharide,the extract was precipitated with 4 volumes anhydrous ethanol at 4℃for overnight and the precipitation was centrifuged at 4500 g for 15 min,the precipitate was dissolved in distilled water,collected,frozen and freezedried,giving the crude polysaccharide SHP.The sample SHP(500 mg)was dissolved in distilled water,and purified on a column(2.6 cm×18 cm)of DEAE-cellulose equilibrated with distilled water at a flow rate of 1.0 mL/min.
After eluted with distilled water,the sample was eluted with 0.3 mol/L NaCl and 0.3 mol/L NaOH,respectively.The sugar contents were assayed by phenol-sulfuric acid method[8].Fractions of three peaks with positive response were pooled together separately,concentrated,dialyzed against running water for 3 days and deionized water for 2 days,and then freezing dried to give SHP1,HSP2 and SHP3,respectively.The fraction SHP3 was dissolved in phosphate buffer(pH 6.8)and then purified by gel–filtration column of Sephadex G-100(1.0 cm×45 cm).The content of total carbohydrate and uronic acid were determined by phenolsulfuric acid method and sulfuric acid-carbazole method[8]respectively,and obtained the purified fractions SHP-3.
Monosaccharide analysis
U ronic acids deter mination
Uronic acids was determined colorimetrically by mhydroxydiphenyl[9],and the galacturonic acid was used to make a standard curve.Absorbance of the sample wasmeasured at 525 nm using the standard curve to calculate the content of uronic acids of SHP-3.
Neutral sugars com position
The sample(5 mg)were treated with 2 mol/L trifluoroacetic acid(TFA)at 120℃for 6 h to release themonosaccharide,removed TFA by evaporating under reduced pressure.Methanol(1.5 mL)was added,evaporated to dryness,the process was repeated four times. The hydrolyzed polysaccharide sample and rhamnose (Rha),fucose(Fuc),arabinose(Ara),xylose (Xyl),mannose(Man),galactose(Gal),glucose (Glu)as monosaccharide standard and inositol(5 mg)as internal standard were dissolved in distilled water(0.5 mL),reduced with sodium borohydride (NaBH4,~20 mg,2 h,with oscillation by each 30 min),treated with glacial acetic acid(AcOH),dried. Methanol(1-2 mL)was added to vessel,dried,and repeated fifth to avoid the influence of AcOH,then dried at 100℃for 15 min in the drying cabinet.After derivatization[10,11]with pyridine and acetic anhydride(the ratio was 1∶1,reacted sealedly at 100℃for 2 h)and filtration with 0.22μm filtering membrane,the derivatives were analyzed by gas chromatograph(GC)equipped with flame ionization detector(F ID)on a capillary column(30 m×0.22 mm×0.25μm).1μL samples injected into GC analyzer and the column temperature programmed as follows:the initial temperature was 180℃,hold for 2 min,then from 180℃to 230℃at rate of 5℃/min with a 3 min hold at 230℃.Injector and detector temperature were 230℃and 250℃, respectively.Nitrogen was used as the carrier gas with a flow rate at 30 mL/min,and the peak areas of monosaccharide was compared with inositol peak area(internal standard)to computer the content of neutral sugars.
Molecular weight distribution
The purified polysaccharides were dissolved in 0.02 mol/L phosphate solution(KH2PO4,pH 6.0)to from the concentration about 2 mg/mL,characterized by gel permeation chromatography(GPC)on TSK G-5000 PWxLgel column and a detectoron Refractive index Detector.The dextrans of varying molecular weight (5200,11600,23800,48600,148000,273000, 410000,668000,1482000)were used as standard to calibrate the column and fit the regression curve.20μL sample swith drew by autosampler to load on the gel column,eluted with 0.02 mol/L KH2PO4at 35℃and a flow rate of 0.6 mL/min;the weight average molecular weight(Mw),number average molecular weight(Mn) and polydispersity index(Mw/Mn)were obtained.All samples were filtrated on a 0.22μm pore diameter membrane(Millipore)prior to analysis.
UV spectroscopy
UV spectrophotometric analysis was conducted according to the method of Yanget al.[12]with a minormodification.SHP-3(10 mg)was dissolved in 10 mL of phosphate buffer(0.05 mol/L,pH 6.8).The absorbance of SHP-3 was read over the range of 190-400 nm,using a UV-2300 double beam spectrophotometer.
Infrared spectroscopy
I R spectra of the samples were obtained by using the KBr-disk method with a Nicolet Fourier transform infrared(FT-I R)spectrometer in the range of 400-4000 cm-1.
Periodate oxidation-Smith degradation
The purified polysaccharide SHP-3 was dissolved in 50 mmol/L sodium periodate(Na IO4)(40 mL),oxidized in the dark at 4℃.0.1 mL solutions were put in the volumetric flask,diluted to 25 mL with distilled water at 12 h intervals and read the value in an ultraviolet spectrophotometer at 223 nm till the value was constant,and then stopped the reaction by adding glycol (4 mL).2 mL oxidized product as sample titrated by 0.009 mol/L sodium hydroxide(NaOH)to calculate the content of methanoic acid yielded during titration, and the rest were dialyzed against tap water and distilled water for 48 h,respectively.The internal portion was concentrated and reduced with NaBH4(~30 mg,2 h),neutralized to pH 5.5 with AcOH.The derivate were hydrolyzed with TFA(2 mol/L)at 100℃for 2 h,evaporated to dryness.After removal of TFA,the final productswere dissolved in distilled water,reduced, and the derivatization was performed as foresaid for GC analysis.Phycite,glycol,glycerol and glucose were analyzed as contrast with GC.
Intrinsic viscosity measurement
SHP-3 was dispersed in phosphate buffer(pH 6.8), heated at 90℃for 10 min,and then cooled down to room temperature.For the measurement of intrinsic viscosity,the sample solution were loaded into a Ubbelohde capillary viscometer which was immersed in awater bath at(25±0.1)℃.The measured viscosity was plotted against concentrations and the intrinsic viscosity was determined by extrapolating the viscosity to zero concentration according to the Huggins equation (Eq.1):
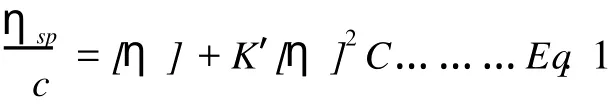
Whereηspis the specific viscosity,[η]the intrinsic viscosity,C the concentration,and K′is the Huggins constant.
Results and Discussion
Three acid fractions,SHP1,SHP2,and SHP3(Fig.1) were purified from the crude polysaccharide from soybean hull on a DEAE-cellulose anion-exchange column.SHP3 was collected and submitted to gel filtration chromatography,yielding a major part named SHP-3 (Fig.2)which was collected for further identification. Intrinsic viscosity is a fundamental rheological parameter of macromolecules which is obtained by extrapolating the specific viscosity/concentration curves to zero concentration.As a direct index of the flow behavior of macromolecules and an indirect index of their size and shape,it indicates the hydrodynamic volume of individual polymer molecules[13,14].In addition,the intrinsic viscosity is a practical way to determine the molecular weight of polymers as suggested by the Mark-Houwink relation([η]=K Ma,[η]is the intrinsic viscosity and M is the molecularweight).
The intrinsic viscosity value of SHP-3,calculated by the Huggins equation(Eq.1),was found to be 0.21 dL/g.It was reported that polysaccharides have typical intrinsic viscosity ranging from 1 dL/g for compact coil or flexible chains to 20 dL/g for extended chains. Therefore,the relatively low intrinsic viscosity observed in this study indicates a decrease in the hydrodynamic volume of the SHP-3, implying their compact and spherical shape[15].
By ploting elution time(t)with the logarithm of molecule weight of dextrans standard(Mw),the fitted regression equation,log Mw=6.15-1.68t+1.69t2-0105t3,was observed with R2and standard error being of 0.999324 and 0.02764,respectively.According the equation,the average molecular weight(Mw)of SHP-3 was 45554 and the polydispersity index(Mw/Mn)was 1.18.
UV absorbance of sample is an effective tool for structure characterization.Absorbance of UV radiation by a molecule is related to excitation of the outer electrons[16].As can be seen from the UV spectra of SHP-3 presented in Fig.3,there was a predominant peak at~200 nm while no obvious peak at 250-290 nm,indicating the absence of proteins.
FT-IR spectroscopy(Fig.4)revealed the characteristic absorptions of SHP-3,such as the broad and strong absorption at 3440 cm-1and 2930 cm-1(O-H stretching and C-H antisymmetrical stretching vibrations,respectively)and the weak absorption around 2335 cm-1indicated existence(aliphatic C-H bonds).The bands around 1645cm-1corresponding to the carboxy late (COO-)stretching band indicated that there were esterified and free carboxyl groups existed in the samples from soybean hull.The peak absorption around 1420 cm-1was due to carboxylate groups symmetric stretching.The weak bending at 1330 cm-1showed the presence of C-H bonds.The absorption band from 1300 cm-1to 800 cm-1were called“finger print”region related to conformation and surface structure of molecule. Although they were hard to be analyzed[17],the peak between 1000 cm-1and 1100 cm-1were determined due to C-O bonds and C=O stretching;and absorption at 895 cm-1was characteristic of β-D-mannose[18,19].The broad band around 640 cm-1indicated plane oscillate vibration of carbonic chain.
Comparision of the GC chromatograms of monosaccharide composition of SHP-3(Fig.6)and monosaccharide standards(Fig.5)indicated that the arabinose was the predominant monosaccharide of SHP-3.Its neutral saccharides molar ratio of Rah∶Fuc∶Ara∶Xyl∶Man∶Gal∶Glu was of 3.55∶0.45∶11.59∶1∶7.45∶5.13∶1.12 and the content of galacturonic acid was 26.71% (wt.%)(Table 1).
Until the photodensity value were not changed anymore,the periodate oxidation was completed.For fractions SHP-3,there was 1.092 mol periodate consumed and 0.197 molmethanoic acid generated per sugar res-idue,which meant the molar percentage of(1→6)glycosidic linkage was 19.7%.The results of Smith degradation showed the molar percentages of glycerol,erythritol,rhamnose,arabinose,mannose and galactose were 31.1%,21.3%,3.4%,37.6%,3.6%and 3.0%,respectively.No(1→3)glycosidic linkage was found in SHP-3.The molar percentages of(1→2)glycosidic linkage,(1→4)glycosidic linkage and(1→6)glycosidic linkage were 11.4%,68.9%and 19.7%,respectively.The arabinose in branch chain was almost hydrolyzed and other monosaccharide linked with 1→4 glycosidic linkage.
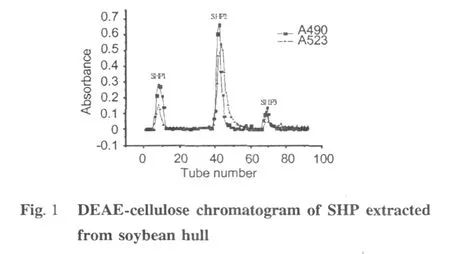
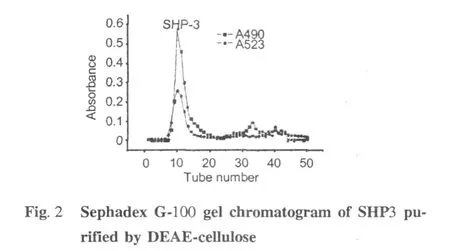
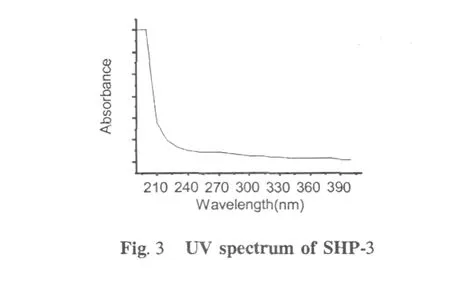
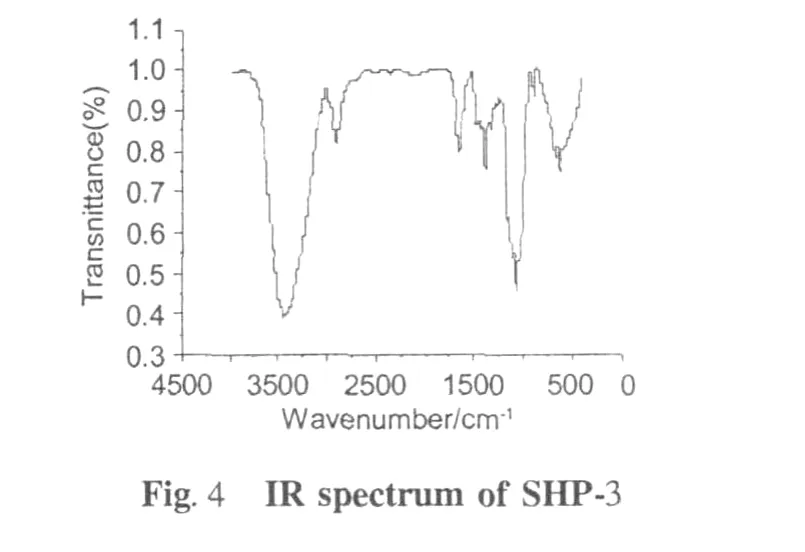
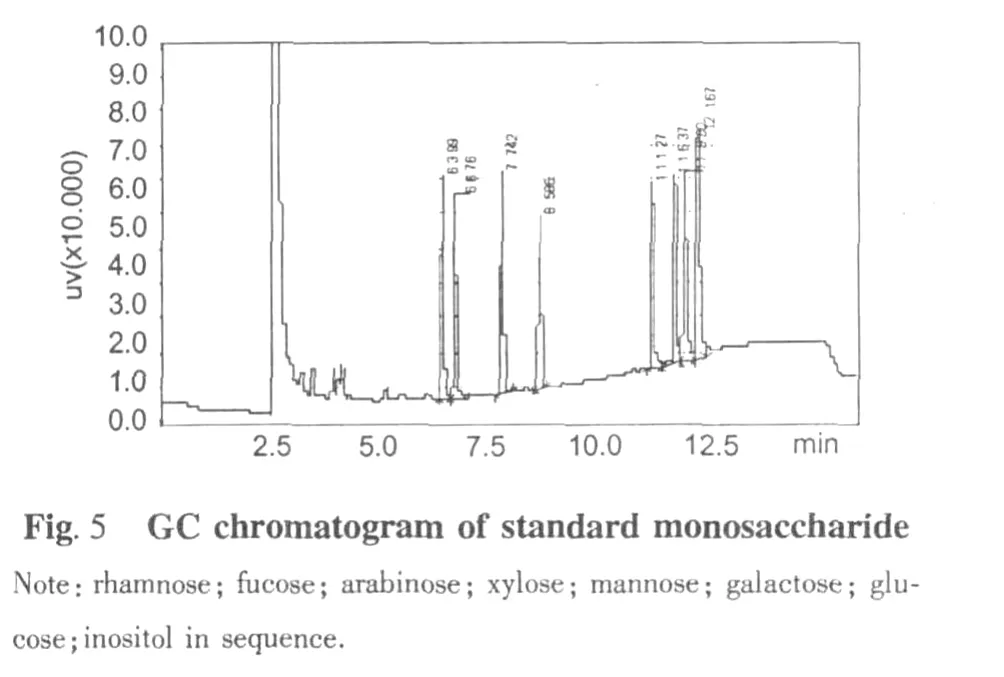
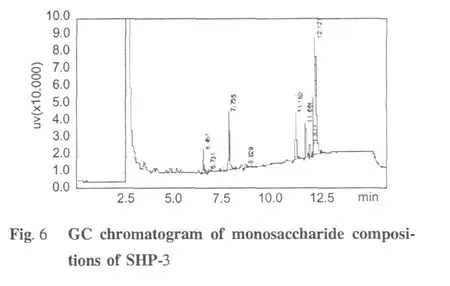
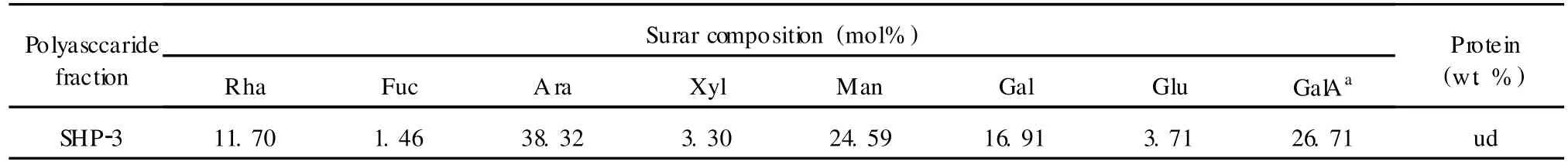
Table 1 M olar percentage of monosaccharide compositions of SHP-3
Conclusion
A purified polysaccharide fraction,SHP-3,extracted from soybean hull by hot water,isolated by DEAE-cellulose anion exchange column and gel filtration column.The physicochemical properties of SHP-3 were investigated by chromatography and spectrometric method.The molecule weight of SHP-3 is 45554,the neutral saccharides molar ratio of Rah∶Fuc∶Ara∶Xyl∶Man∶Gal∶Glu=3.55∶0.44∶11.58∶1∶7.45∶5.12∶1.12 and the content of galacturonic acid is 26.71%(wt.%). There is no presence of protein and the result of periodate oxidation-smith degradation indicated that the molar percentages of(1→2)glycosidic linkage,(1→4) glycosidic linkage and(1→6)glycosidic linkage were 11.4%,68.9%and 19.7%,respectively.
Reference
1 Mahalko JR,Sandsteas HH,Johnson LK,et al.Effect of consuming fiber from corn bran,soy hulls,or apple poweron glucose tolerance and plasma lipids in type Ⅱ diabetes.Am J Clin Nutr,1984,39:25.
2 Ravin G,Proctor A.Preparation of soy hull pectin.Food Chem,1999,65:461-467.
3 Kalapathy U,Proctor A.Effect of acid extraction and alcohol precipitation conditions on the yield and purity of soy hull pectin.Food Chem,2001,73:393-396.
4 Fransen Carel T M,Laarvan H,Kamerling JP,et al.CP-NMR analysis of carbohydrate fractions of soybean hulls and endosperm.Carbohydr Res,2000,328:549-559.
5 WangQ,Huang XQ,Nakamura A,et al.Molecular characterization of soybean polysaccharides:an approach by size exclusion chromatography,dynamic and static light scattering methods.Carbohydr Res,2005,340:2637-2644.
6 Akihiro N,Taro T,Ryuji Y,et al.Emulsifying properties of soybean soluble polysaccharide.Food Hydrocoll,2004,18: 795-803.
7 Staub AM.Removeal of protein-Sevag method.M ethods Carbohydr Chem,1965,5:5-6.
8 DuboisM,Gilles KA,Hamilton JK,et al.Color imetric method for determination of sugars and related substances.Anal Chem,1956,28:350-356.
9 Blunenkrantz N,AsbobHansen G.New method for quantitative determination of uronic adids.Anal Biochem,1973,54: 484-489.
10 Borchardt LG,Piper CV.(1970).Tappi,53(2):257.
11 Zhang WJ.(2001)Technology of biochemical research on compound polysaccharide(second edition).Hangzhou:Zhejiang University Press,2001.108-111.
12 Yang B,Jiang Y M,Zhao MM,et al.Effects of ultrasonic extraction on the physical and chemical properties of polysaccharides from longan fruit pericarp.Polym Degra Stab,2008, 93:268-272.
13 Kar F,Arslan N.Effect of temperature and concentration on viscosity of orange peel pectin solutions and intrinsic viscosity-molecular weight relationship.Carbohydr Polym,1999,40: 277-284.
14 Lazaridou A,Biliaderis CG,Izydorczyk MS.Molecular size effects on rheological properties of oatβ-glucans in solution and gels.Food Hydrocolloid,2003,17:693-712.
15 Arvidson SaraA,Rinehart TB,Gadala-Maria F.Concentration regimes of solutions of levan polysaccharide from Bacillus sp.Carbohydrate Polym er,2006,65:144-149.
16 Pons MN,Le Bonte S,Potier O.Spectral analysis and fingerprinting for biomedia characterization.J B iotechnol,2004, 113:211-230.
17 Gnanasambanda R,ProctorA.Determinations of pectin fegree of esterification by diffuse reflectance Fourier transform infrared spectroscopy.Food Chem,2000,68:327-332.
18 Coimbra MA,Goncalves F,Barros AS,et al.Fourier trans for m infrared spectroscopy and chemometric analysis of white wine polysaccharide extracts.J Agric Food Chem,2002,50:3405-3411.
19 Yang B,Zhao MM,Liu Y,et al.Characterization of litchipericarp polysaccharide.Nat Prod Res Dev(天然產物研究與開發),2005,17:685-687.
Physicochemical Properties of a Water-soluble Polysaccharide Fraction SHP-3 Purified from Soybean Hull
LAI Fu-rao1,WU Hui1*,L IXiao-feng1,REN Yao1,WU Lei-yan1,2,WEN Qi-biao11College of Light Industry and Food Sciences,South China University of Technology,Guangzhou 510640, China;2College of Food Science and Engineering,Jiangxi Agricultural University,Nanchang 330045,China
Q946.91;R285.1;O629.12
A
1001-6880(2011)01-0076-06
Received May 4,2009;Acceptd September 27,2009
Foundation Item:This project was supported by Program for New Century Excellent Talents in University(NCET-06-0746).
*Corresponding author Tel:86-20-87112853;E-mail:fehwu@scut.edu. cn

