氧化鐵薄膜的水熱合成及其光電轉換性能
萬麗娟 王治強 楊再三 羅文俊 李朝升*,,2,3 鄒志剛,2,3
(1南京大學環境材料與再生能源研究中心,南京大學物理系,南京 210093)
(2南京大學材料科學與工程系,南京 210093)(3南京大學固微結構物理國家重點實驗室,南京 210093)
氧化鐵薄膜的水熱合成及其光電轉換性能
萬麗娟1,3王治強1楊再三1羅文俊1,3李朝升*,1,2,3鄒志剛1,2,3
(1南京大學環境材料與再生能源研究中心,南京大學物理系,南京 210093)
(2南京大學材料科學與工程系,南京 210093)(3南京大學固微結構物理國家重點實驗室,南京 210093)
通過水熱方法在摻雜氟的SnO2(FTO)導電玻璃上制備了不同形貌的氧化鐵薄膜。利用無機鐵鹽浸漬法在FTO玻璃上進行氧化鐵晶種的預處理使得所制備的氧化鐵薄膜更致密且均一。研究了表面活性劑對氧化鐵晶體形貌的影響。使用十二烷基苯磺酸鈉(SDBS)和三嵌段聚合物P123做為形貌導向劑分別得到棒狀和四方體形貌的氧化鐵薄膜。氧化鐵薄膜可調的形貌可能是由于表面活性劑和鐵氧團簇的組裝或者某些晶面吸附了陰離子而改變了生長速率引起的。同時,研究了其光電性能,具有四面體形貌的氧化鐵薄膜可以產生較大的光電流,這是由于其縮短了光生空穴的擴散距離。
氧化鐵;水熱;薄膜;光電化學
Since the hydrogen production from the direct photo electrolysis of water was first demonstrated by Fujishima and Honda[1],conversion of solar energy to hydrogen as a clean and renewable energy source hasreceived more attention.The classical work shows that it is possible to induce the water-splitting by light,using TiO2semiconductor as photoanode.However,TiO2has a wide band gap(3.2 eV),and only a small fraction of the solar spectrum (light below 420 nm)can be utilized.Therefore the main interest of the present study is focused on the shift of activity of photoanode materials to the visible region of sunlight[2-4].
Iron oxide(α-Fe2O3,or hematite)with a favorable band gap of 2.0~2.2 eV remains a promising material,due to its chemical stability in aqueous environments and matchless abundance[5].Thin-films Fe2O3photoanodes has been reported fabrication through several methods,including spin coating[6];DC magnetron sputtering[7];atmospheric pressure chemical vapor deposition (APCVD)[8];spray pyrolysis of Fe(III)-containing solutions[9]etc.Hydrothermal route has been widely used in the synthesis of semiconductor thin films on different substrates[10-12],which is a facile method and the products morphologies may be controlled by additives,e.g.,anions or surfactant.To the best of our knowledge,there have been no reports on α-Fe2O3thin films prepared through hydrothermal route.
We report here the synthesis of different morphologies of α-Fe2O3thin films by hydrothermal route,using ferric chloride as inorganic precursor,dodecylbenzensulfonate (SDBS) and triblock copolymer P123 as morphology directing-agent,respectively.In the hydrothermal system,crystalline seeds of iron oxides on FTO (F-doped tin oxide)substrates make the as-prepared α-Fe2O3thin films more uniform and compact,compared with those films on FTO substrates without crystalline seeds treatment.The photoelectrochemical properties of α-Fe2O3thin films with different morphologies are also reported.
1 Experimental
1.1 Preparation of Fe2O3films
All reagents were of analytical grade and used without further purification.Manipulations and reactions were carried out in air without the protection of nitrogen or inert gas.FTO glass substrates were washed with ethanol and water several times before use.TogettheFTO covered with iron oxides crystalline seeds,the substrates were dipped in the ferric nitride ethanol solution for several times,then dried and calcined at 500 ℃ for 4 h.In a typical run,1.621 7 g of FeCl3·6H2O was added into 35 mL of deionized water and stirred until totally dissolved.The reaction solution was then transferred into a 50 mL Teflon-lined stainless steelautoclave.The FTO substrates (1.5 cm×2 cm)were put on the bottom of Teflon-lined stainless steel autoclave.The autoclave was sealed and heated to 200℃and kept for 2 h(FeCl3+H2O→Fe2O3+HCl).After being cooled down to room temperature naturally,the as-prepared iron oxides thin films/FTO were washed with deionized water and absolute ethanol several times.In order to study the effects of surfactants,0.2 g dodecylbenzensulfonate(SDBS)or 0.5 g(EO)20(PO)70(EO)20(P123)was added into the reaction solution with otherconditions unchanged.
1.2 Characterization
X-raydiffraction (XRD)measurementswere performed on a Rigaku D/MAX-Ultima III X-ray diffractometer with Cu Kα radiation (λ=0.15418 nm,40 kV, 40 mA), flash detector, graphite monochromator and a scan rate of 10°·min-1(scan range:20°~60°,CPS type of X-ray recording).UV-Vis transmission spectra were performed at Varian Cary 50 Probe UV-Visible Spectrophotometer.Morphologies of the as-prepared samples at different experimental conditions were characterized by scanning electron microscopy (SEM)Philips XL30 with an electron accelerating voltage of 10 kV.
The photoelectrochemical properties were characterized by linear scanning voltampere(LSV)technique performed on a CHI633C electrochemical workstation system with 500W xenon lamp(USHIO Optical Module X500) illumination.The LSV measurements were performed in a 1 mol·L-1NaOH aqueous solution from-0.30 to 0.40 V with a scan rate of 20 mV·s-1in a standard three-electrode configuration coupled with the sample films(working electrode),an Ag/AgCl electrode (reference electrode)and a high purity platinum(counter electrode).
2 Results and discussion
Wide-angle XRD pattern of the as-prepared samples isshown in Fig.1.As seen from Fig.1,with the exception of the marked FTO glass substrate peaks,the XRD data is consistent with the rhombohedral symmetry of Fe2O3(space group:R3c(167),a=0.503 5 nm,b=0.503 5 nm and c=1.3748 nm;PDF card No.33-0664)indicating the presence of α-Fe2O3structure and absence of impurity phases.The inset digital photograph in Fig.1 shows the as-prepared hematite films is uniform and transparent in a large scale,suggesting that α-Fe2O3films can be synthesized on the crystalline seed-treated FTO glass substrates successfully through hydrothermal treatment in a simple inorganic iron salt system.

Fig.1 XRD pattern of iron oxide/FTO films
The morphologies of the as-prepared α-Fe2O3films are characterized by SEM observation in Fig.2.Fig.2(a)and(b)show the relatively uniform iron oxide films,and there are no obvious cracks.When the reaction solution was added with surfactant SDBS,the morphology of asprepared iron oxide thin films appears different from that without surfactant.It seems more uniform with rodlike morphology (Fig.3a,inset is the magnified SEM image).When triblock copolymer P123 was used as morphology-directing agent,the iron oxide thin films with connected tetrahedral morphology was observed(Fig.3b,inset is the magnified SEM image).The modulated morphologies of iron oxide thin films may be attributed to the assembly between surfactant and ferric oxo-clusters.For the samples produced from SDBS,some crystalline facets may be adsorbed by the anions during the crystal growth of iron oxides.While for the samples from P123,ferric oxo-clusters may be interacted with the copolymer to form iron oxide with tetrahedral morphology.
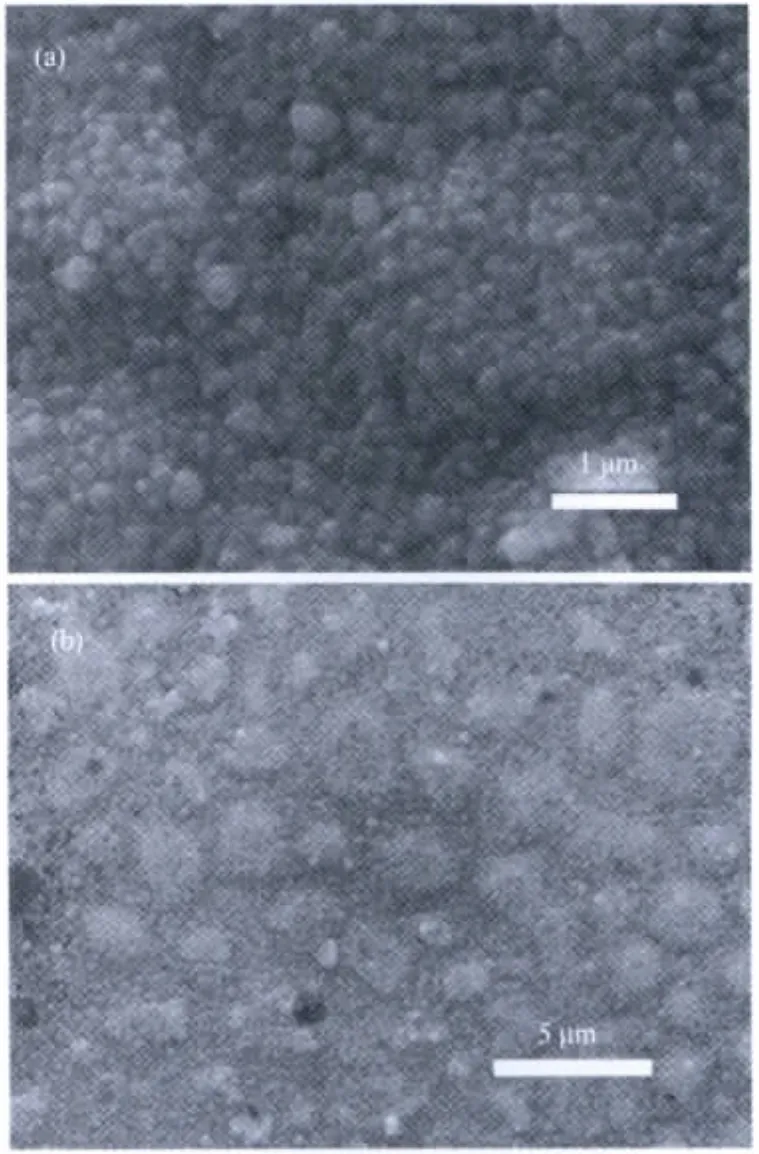
Fig.2 SEM image of iron oxide/FTO films prepared in ferric chloride
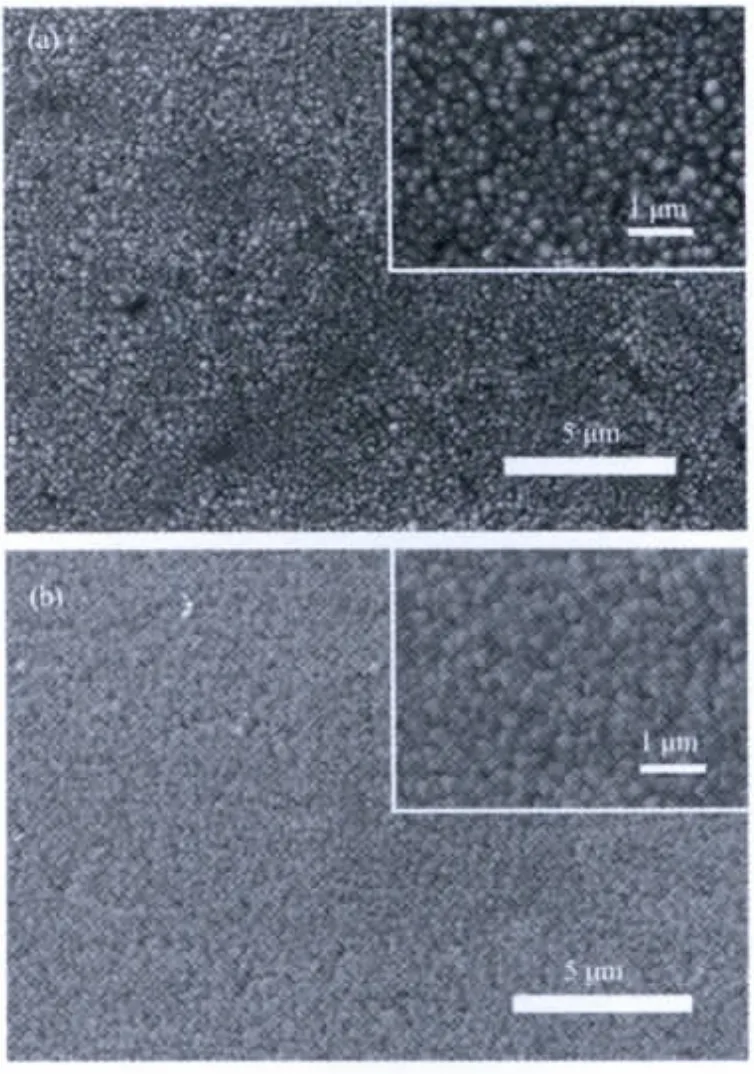
Fig.3 SEM image of iron oxide/FTO films prepared in ferric chloride with surfactant
Fig.4 shows transmittance spectra for the asprepared Fe2O3films with different morphologies.The optical band gap of films can be estimated by calculating the intercept of the extrapolated linear fit to the experimental data of a plot of(-lnT)n(T is the transmittance value,n is 1/2 for hematite,as hematite is known to be indirect band gap semiconductor)versus incident photon energy(hν),which can be seen from the insets of Fig.4.The insets show the indirect band gap values of all films are about 2.0 eV,which is consistent with the values of the samples prepared through atmospheric pressure chemical vapor deposition and USP (Ultrasonic Spray Pyrolysis).reported in the literature[5,13].

Fig.4 Transmittance spectra of the as-prepared samples
Fig.5 shows the photocurrent curves of the iron oxide thin films with different morphologies.The photocurrent of Fe2O3thin films with tetrahedral morphologies is larger than others,which may be in part attributed to the nanostructure.The morphologies of iron oxide thin films may influence the photoelectrochemical properties,e.g.the improvement of photocurrent of the iron oxide films may be attributed to the change of nanostructures(smaller grains),which improved photocurrent is in part attributed to the dendritic nanostructure which minimizes the distance photogenerated holes have to diffuse to reach the Fe2O3/electrolyte interface while still allowing efficient light absorption[7-8].The improved photocurrentofasprepared iron oxide thin films with tetrahedral morphology may be attributed to minimize the distance photogenerated holes of diffusion.
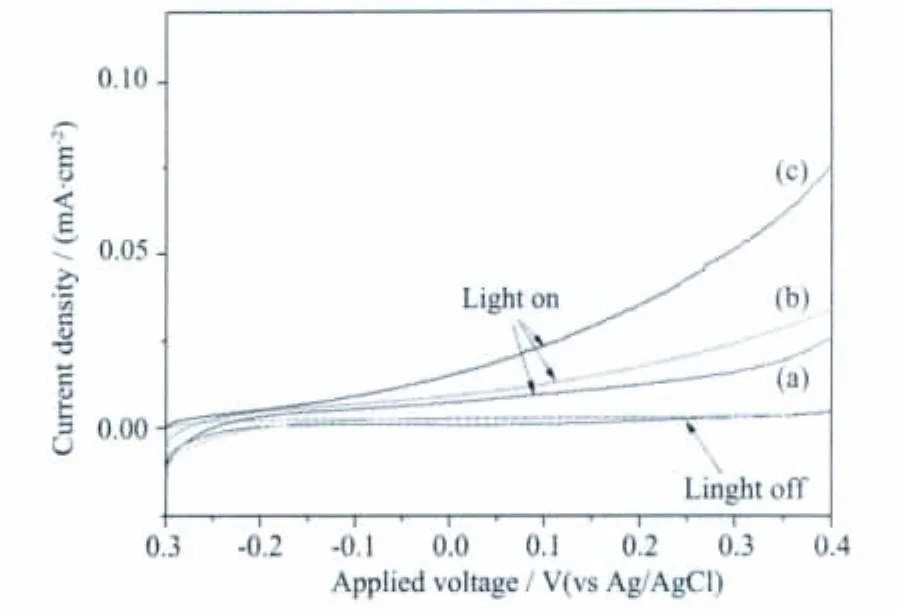
Fig.5 Photocurrent-voltage curves for iron oxide electrodes in darkness and under illumination at a scan rate of 20 mV·s-1
3 Conclusions
Different morphologies of α-Fe2O3thin films were synthesized by hydrothermal route,using ferric chloride as inorganic precursor,SDBS and P123 as morphology directing-agent. The rod-like and tetrahedral morphologies were obtained.Crystalline seed of iron oxide on FTO substrate make the as-prepared α-Fe2O3thin films more uniform and compact compared with those FTO without pretreatment.The photocurrent of Fe2O3thin films with tetrahedral morphologies is larger than others,which may be attributed to the tetrahedral morphology in minimization of the diffuse distance of photogenerated holes.
[1]Fujishima A,Honda K.Nature,1972,238:37-38
[2]Park J H,Kim S,Bard A.J.Nano Lett.,2006,6:24-28
[3]Matsuoka M,Kitano M,Takeuchi M,et al.Catal.Today,2007,122:51-61
[4]Mor K G,Prakasam H E,Varghese O K,et al.Nano Lett.,2007,7:2356-2364
[5]Cesar I,Sivula K,Kay A,et al.J.Phys.Chem.C,2009,113:772-782
[6]Souza F L,Lopes K P,Longo E,et al.Phys.Chem.Chem.Phys.,2009,11:1215-1219
[7]Glasscock J A,Barnes P R F,Plumb I C,et al.J.Phys.Chem.C,2007,111:16477-16488
[8]Kay A,Cesar I,Gr?tzel M.J.Am.Chem.Soc.,2006,128:15714-15721
[9]Sartoretti C J,Alexander B D,Solarska R,et al.J.Phys.Chem.B,2005,109:13685-13692
[10]Chen J,Huang K L,Liu S Q.Electrochimica Acta,2009,55:1-5
[11]Na J S,Gong B,Scarel G,et al.ACS Nano,2009,3:3191-3199
[12]Zhao H J,Shen Y M,Zhang S Q,et al.Langmuir,2009,25:11032-11037
[13]Duret A,Gr?tzel M.J.Phys.Chem.B,2005,109:17184-17191
Seed-Mediated Hydrothermal Synthesis and Photoelectrochemical Properties of Hematite Thin Films
WAN Li-Juan1,3WANG Zhi-Qiang1YANG Zai-San1LUO Wen-Jun1,3LI Zhao-Sheng*,1,2,3ZOU Zhi-Gang1,2,3
(1Eco-Materials and Renewable Energy Research Center(ERERC),Department of Physics,Nanjing University,Nanjing 210093,china)
(2Department of Materials Science and Engineering,Nanjing University,Nanjing 210093,china)(3National Laboratory of Solid State Microstructures,Nanjing University,Nanjing 210093,china)
Iron oxides with different morphologies on F-doped tin oxide (FTO)covered glass substrates were synthesized through hydrothermal route.The pretreatment of iron oxide crystalline seeds on FTO glass through inorganic iron salts solution dipping method makes the as-prepared iron oxide thin films more uniform and compact.The effects of surfactants on the crystal morphologies of iron oxides were studied.Uniform iron oxides thin films with rod-like and tetrahedral morphologies could be obtained through this route by using dodecylbenzensulfonate(SDBS)and triblock copolymer P123 as morphology directing-agent,respectively.The modulated morphologies of iron oxide thin films may be attributed to the assembly between surfactant and ferric oxo-clusters or different velocity of crystalline facets growth induced by adsorbed anions.Photoelectrochemical properties of iron oxide thin films with different morphologies were studied.The larger values of produced photocurrents of iron oxide thin films with tetrahedral morphology may be attributed to the minimization of the diffuse distance for photogenerated holes.
iron oxide;hydrothermal;thin films;photoelectrochemical properties
O614.24
A
1001-4861(2011)04-0747-05
2010-09-15。收修改稿日期:2010-11-01。
973(No.2007CB613305),國家自然科學基金(No.50732004),江蘇省自然科學基金(No.BK2008028)資助項目。*
。 E-mail:zsli@nju.edu.cn

