Oxidation of p/o-Cresols to p/o-Hydroxybenzaldehydes Catalyzed by Metalloporphyrins with Molecular Oxygen*
SHE Yuanbin (佘遠斌)**, WANG Weijie (王維潔) and LI Guojun (李國君)
Institute of Green Chemistry and Fine Chemicals, Beijing University of Technology, Beijing 100124, China
Oxidation of p/o-Cresols to p/o-Hydroxybenzaldehydes Catalyzed by Metalloporphyrins with Molecular Oxygen*
SHE Yuanbin (佘遠斌)**, WANG Weijie (王維潔) and LI Guojun (李國君)
Institute of Green Chemistry and Fine Chemicals, Beijing University of Technology, Beijing 100124, China
The two novel green oxidation processes of p/o-cresols to p/o-hydroxybenzaldehydes catalyzed by metalloporphyrins in the presence of molecular oxygen were developed in this work. Among the metalloporphyrins with different central ions and substituents studied, T(p-NO2)PPCoCl and T(p-OCH3)PPFeCl presented the highest activities for p-cresol and o-cresol oxidation reactions respectively. The molar ratio of sodium hydroxide to cresols and different reaction parameters including reaction temperature, reaction time and reaction pressure have been investigated, and 69.8%/50.4% conversions of p/o-cresol and 86.6%/26.6% selectivities for p/o-hydroxybenzaldehydes were reached under optimized conditions.
metalloporphyrin, p/o-cresol, p/o-hydroxybenzaldehyde, biomimetic catalytic oxidation, oxygen
1 INTRODUCTION
Hydroxybenzaldehydes are important chemical intermediates for preparing pharmaceuticals, pesticides, fragrance, petrochemicals and electroplating chemicals. Recently the formation of hydroxybenzaldehydes is increasingly gathering much attention. The classical methods for synthesizing p- and o-hydroxybenzaldehydes are the Reimer-Tiemann reactions from phenols with chloroform [1-3].p-Hydroxybenzaldehyde can also be synthesized from phenol and acetonitrile through the Gattermann reaction;o-hydroxybenzaldehyde is also produced from o-hydroxy-benzylalcohol and o-hydroxybenzoic acid.Industrially, p/o-hydroxybenzaldehydes are synthesized by the side-chain chlorination of p/o-cresols and resulting dichloromethyl groups by subsequent saponification [4]. However, conventional methods have several disadvantages such as consuming high energy,using heavy metal catalysts [5], chloroform and large amounts of inorganic salts [6], which may cause serious effluent problems. Therefore, it is very important to develop new processes under mild and environmentalbenign reaction conditions with green oxidants for synthesizing p- and o-hydroxybenzaldehydes.
Metalloporphyrins, as biomimetic catalysts in the chemical industry [7, 8], have attracted more and more interest for their low costs and eco-benign features in oxidation reactions. They have been used in alkane oxidation as efficient catalysts under mild conditions [9-11].The patent of preparing p-hydroxybenzaldehyde through oxidation of p-cresol over metalloporphyrins has been applied by our group. In this paper, two green oxidation processes of p/o-cresols to p/o-hydroxybenzaldehydes catalyzed by metalloporphyrins in the presence of molecular oxygen were further studied.
2 EXPERIMENTAL
2.1 Reagents and instruments
p/o-Cresols, sodium hydroxide and methanol were analytical grade, purchased from commercial sources.Oxygen was supplied in a high-pressure cylinder and used without further treatment. Metalloporphyrins were prepared according to our published procedures [12].
Products analysis were performed on an Agilent 1200 HPLC (high performance liquid chromatography),equipped with a HC-C18 column (250 mm×4.6 mm,f5 μm), and acetonitrile-water (60︰40) with a flow rate of 1.0 ml·min-1were used. IR (infra-red) spectra were obtained with a Nicolet Avatar 360 E.S.P.
2.2 Catalytic oxidation of p/o-cresols
The oxidation reactions of p/o-cresols were carried out in a 100 ml boiling flask-3-neck with a condensing tube under atmospheric pressure or a 100 ml autoclave under higher oxygen pressure conditions. In a typical oxidation experiment, p/o-cresols, sodium hydroxide, a metalloporphyrin and methanol were placed in the preheated reactor and mixed through magnetic stirring. Then oxygen was bubbled into the boiling 3-neck flask or aerated into the autoclave, and reaction timer was started. After the reaction was stopped, the liquid samples were analyzed by HPLC.
3 RESULTS AND DISCUSSION
3.1 Aerobic oxidation of p/o-cresols with metalloporphyrins
First of all, preliminary tests of the p/o-cresolsoxidation reactions were carried out in the absence of metalloporphyrins, and the results were poor. And metalloporphyrins containing different central metal ions and substituents were generally researched in this section.
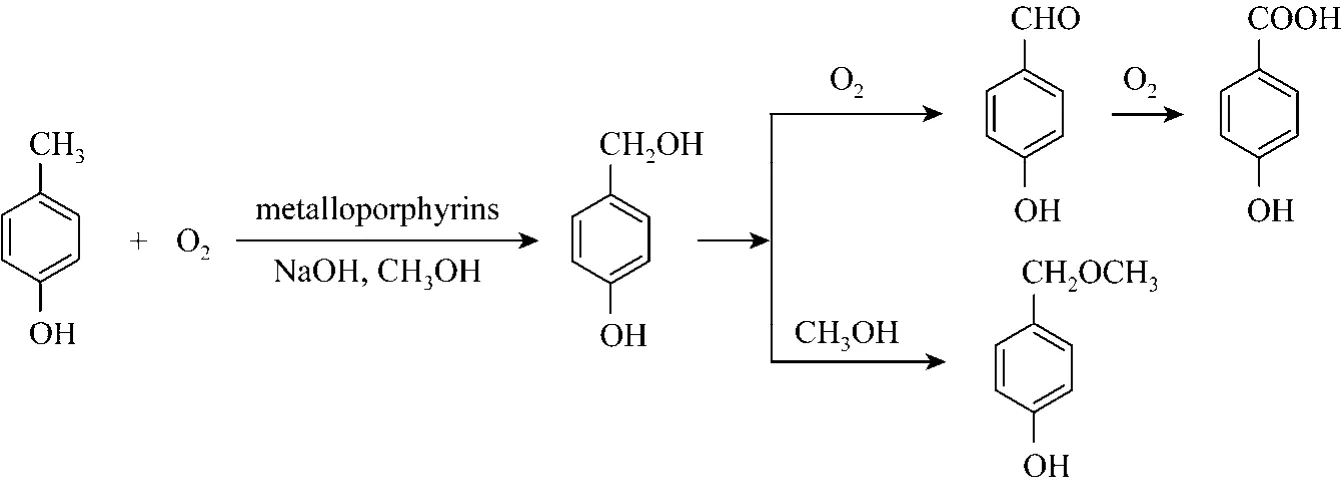
Figure 1 p-Cresol oxidation reaction
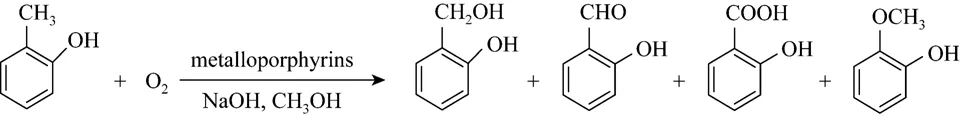
Figure 2 o-Cresol oxidation reaction
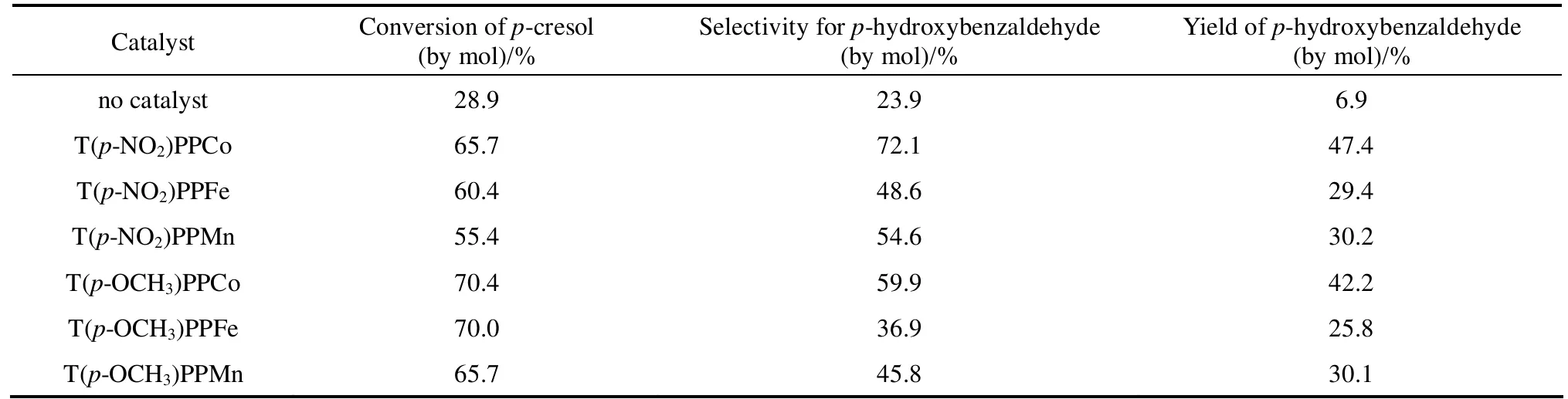
Table 1 Effect of metalloporphyrins with different central ions on the conversion of p-cresol,the selectivity and yield of p-hydroxybenzaldehyde
In the p-cresol oxidation reaction, besides the desired product p-hydroxybenzaldehyde, byproducts such as p-hydroxybenzoic acid, p-hydroxybenzylalcohol and p-hydroxybenzylmethyl ether were also observed(Fig. 1). And for the o-cresol oxidation reaction,o-hydroxybenzoic acid, o-hydroxybenzylalcohol and o-hydroxyanisole were observed as byproducts,o-hydroxybenzylmethyl ether and o-hydroxybenzoic acid methyl ester were not detected in this method(Fig. 2).
The catalytic performances of metalloporphyrins containing different central ions: T(p-NO2)PPM and T(p-OCH3)PPM (M Co, Fe, Mn), in the p-cresol oxidation reactions were showed in Table 1. And the effects of cobalt porphyrins with different substituents were investigated for their better performances comparing with manganese and iron porphyrins, the results were showed in Table 2. The experimental results in the tables were taken under the optimum conditions according to the researches in the following sections.
As shown in Table 1, metalloporphyrins exhibited good activities and selectivities in the p-cresol oxidation reaction system. Both the selectivity and yield of p-hydroxybenzaldehyde followed a sequence Co > Mn > Fe, while the conversion of p-cresol over these metalloporphyrins had an order Co > Fe > Mn.In Table 2, cobalt porphyrins with different peripheral substituents around porphyrin rings were used to investigate their effect on p-hydroxybenzaldehyde selectivity. Those cobalt porphyrins, either with or without an axial ligand, which had electron-withdrawing groups such as NO2and Cl exhibited higher selectivities than those had electron-donating groups such as CH3and OCH3. Among the catalysts studied, T(p-NO2)PPCoCl was the most effective one by which the highest p-cresol conversion and yield of p-hydroxybenzaldehyde were obtained.
Similar researches of catalytic performances of metalloporphyrins were applied on o-cresol oxidation reactions, and iron porphyrins exhibited higher catalytic performances in Fe/Mn/Co metalloporphyrins.The effects of the five iron porphyrins with different peripheral substituents around porphyrin rings were showed in Table 3, in which iron porphyrins with electron-donating group performed better on both the conversion of o-cresol and selectivity for o-hydroxybenzaldehyde.
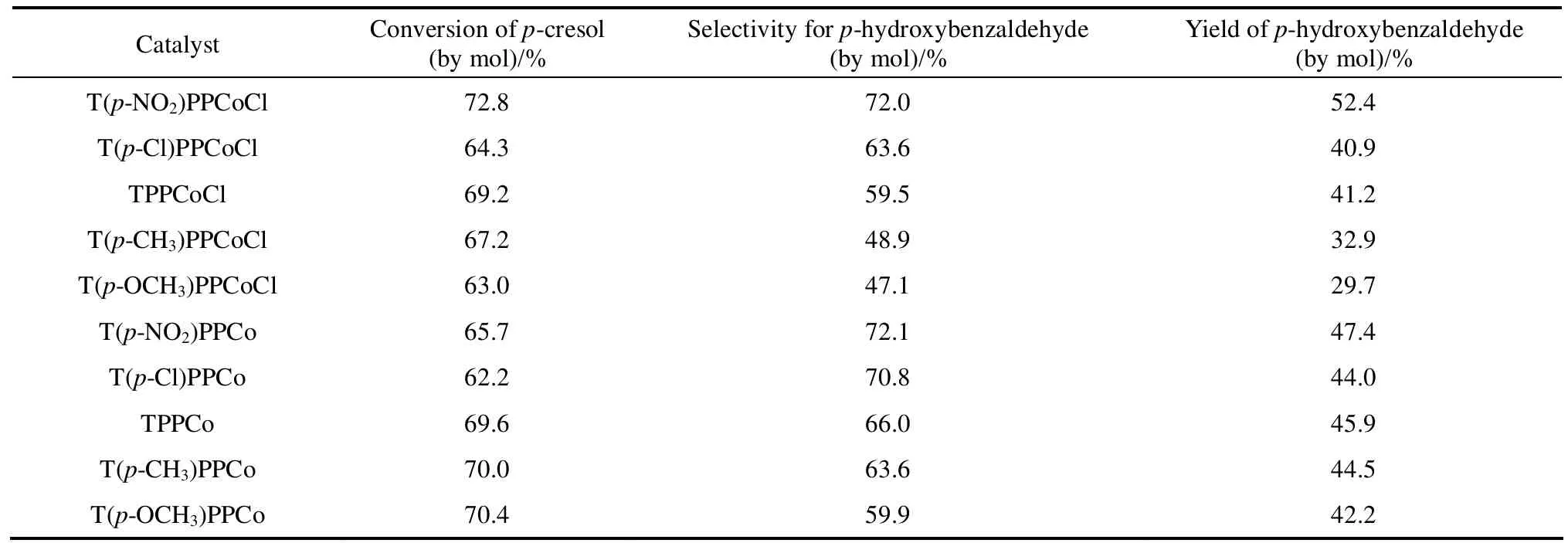
Table 2 Effect of cobalt porphyrins with different substituents on the conversion of p-cresol,the selectivity and yield of p-hydroxybenzaldehyde
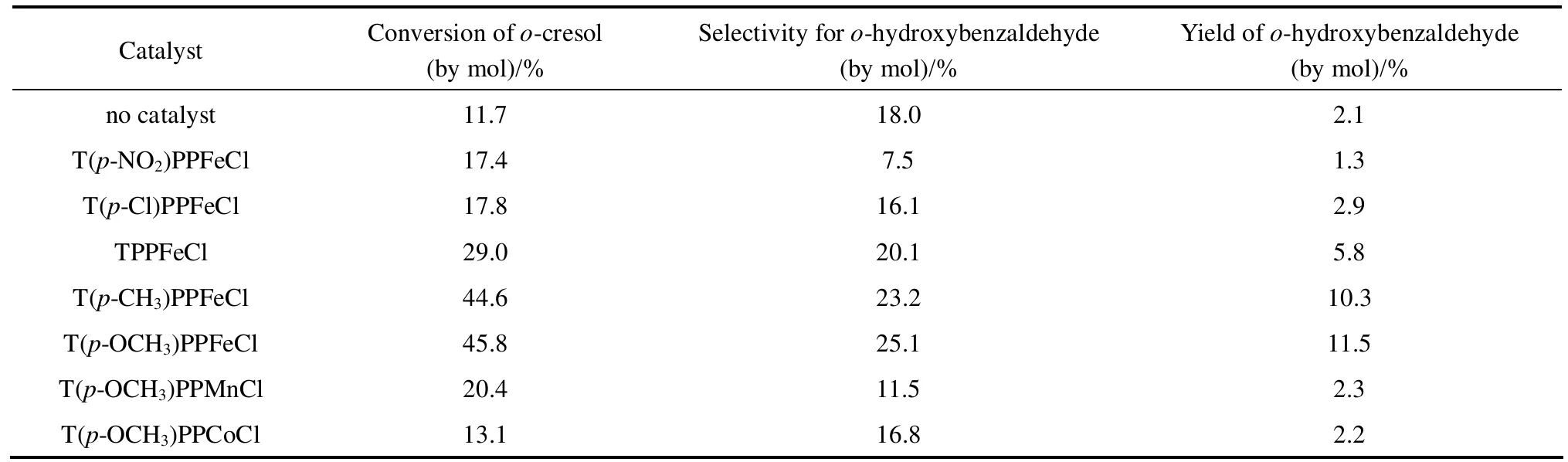
Table 3 Effect of iron porphyrins on the conversion of o-cresol, the selectivity and yield of o-hydroxybenzaldehyde
In summary, various metalloporphyrins presented different catalytic activities in both reaction systems.The different catalytic activities of metalloporphyrins might be explained by the stability of different valences [13], outer electron structure and atomic size of central metal atoms, and the influences of substituent electronic properties. Also the reaction mechanisms for different raw materials might not be the same.Therefore, T(p-NO2)PPCoCl and T(p-OCH3)PPFeCl were used in the optimization of the reaction conditions in the following sections.
3.2 Effect of NaOH
Sodium hydroxide was essential for oxidation cresols [14], because it was essential material in oxidizing phenol benzyl position to protect OH group of cresols. So there were no hydroxybenzaldehydes observed in cresols oxidation experiments without sodium hydroxide. Figs. 3 and 4 showed the effect of the molar ratio of NaOH to p/o-cresols on conversion and selectivity. Both the conversion of p-cresol and selectivity for p-hydroxybenzaldehyde increased to the maximum at 2︰1 molar ratio of NaOH to p-cresol and then began to decrease. However, the conversion of o-cresol increased with the increasing molar ratio of NaOH to o-cresol, but the selectivity for o-hydroxybenzaldehyde peaked at 3.5︰1.
3.3 Effects of various reaction factors on p/o-cresol oxidation reactions
3.3.1 Effect of reaction temperature
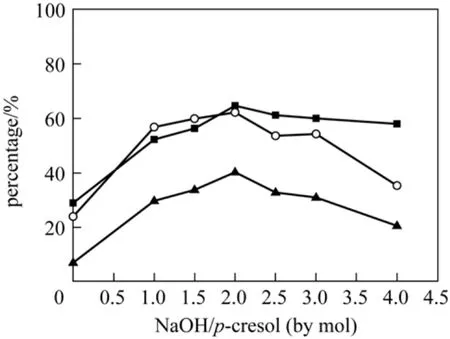
Figure 3 Effect of the molar ratio of NaOH to p-cresol on oxidation reaction (p-Cresol, 3.75 mol·L-1; T(p-NO2)PPCoCl,7.5×10-5 mol·L-1; MeOH 30 ml; flow amount of oxygen under atmospheric pressure, 20 ml·min-1; temperature, 343 K; reaction time, 10 h)■ conversion of p-cresol; ○ selectivity for p-hydroxybenzaldehyde;▲ yield of p-hydroxybenzaldehyde
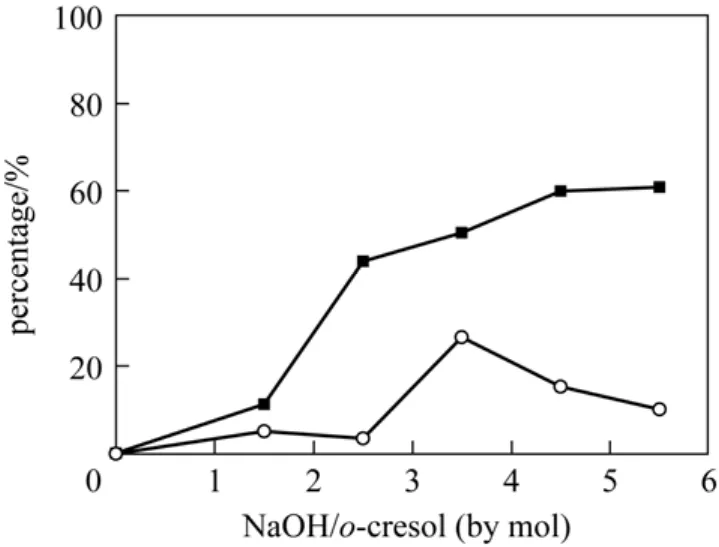
Figure 4 Effect of the molar ratio of NaOH to o-cresol on oxidation reaction (o-Cresol, 2.50 mol·L-1; T(p-OCH3)PPFeCl,5×10-4 mol·L-1; MeOH 30 ml; flow amount of oxygen under atmospheric pressure, 20 ml·min-1; temperature, 343 K; reaction time, 8 h)■ conversion of o-cresol; ○ selectivity for o-hydroxybenzaldehyde
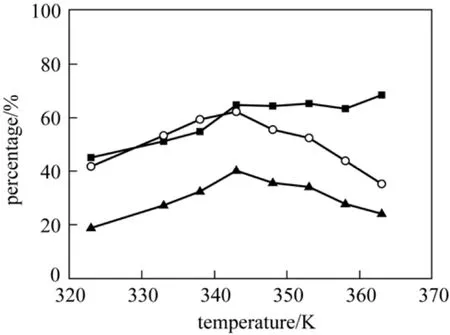
Figure 5 Effect of reaction temperature on the oxidation of p-cresol (p-Cresol, 3.75 mol·L-1; NaOH, 8.75 mol·L-1;T(p-NO2)PPCoCl, 7.5×10-5 mol·L-1; MeOH 30 ml; flow amount of oxygen under atmospheric pressure, 20 ml·min-1; reaction time, 10 h)■ conversion of p-cresol; ○ selectivity for p-hydroxybenzaldehyde;▲ yield of p-hydroxybenzaldehyde
Figure 5 showed the effect of reaction temperature raging from 323 K to 363 K on the reaction. The conversion of p-cresol increased with reaction temperature increased but the increasing speed was rather slow when temperature was higher than 348 K, whereas the selectivity for p-hydroxybenzaldehyde peaked at 348 K. When the reaction temperature was lower than 348 K, the selectivities for p-hydroxybenzaldehyde were lowered as a result of the formation of p-hydroxybenzyl methyl ether. On the other hand,p-hydroxybenzaldehyde as an unstable product tended to be further oxidized to p-hydroxybenzoic acid at higher temperature to cause the reduction of p-hydroxybenzaldehyde. Furthermore, increasing the reaction temperature might also result the side reactions such as polymerization. So 348 K was indicated to be appropriate. And for the o-cresol oxidation reaction, the conversion increased with the rising reaction temperature, while the selectivity for o-hydroxybenzaldehyde also peaked at 348 K.
3.3.2 Effect of reaction time
The effect of reaction time in the presence of T(p-NO2)PPCoCl on oxidation of p-cresol was investigated and the results were shown in Fig. 6. The conversion of p-cresol increased with the time increased, while the yield of p-hydroxybenzaldehyde peaked at 9 h of reaction time. Because p-hydroxybenzaldehyde was the intermediate between p-hydroxybenzenemethanol and p-hydroxybenzoic acid, so it could be deeply oxidized to p-hydroxybenzoic acid as the time elapsed,which might be the reason for the decreasing of the selectivity for p-hydroxybenzaldehyde. And T(p-OCH3)PPFeCl performed in the same trend of o-cresol oxidation, but reached a plateau after 8 h.
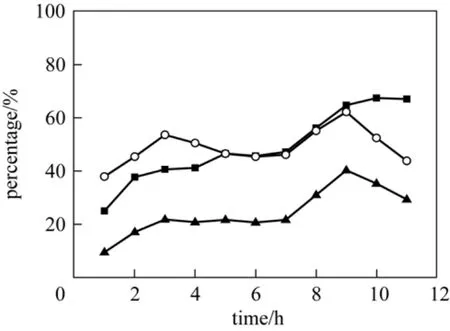
Figure 6 Effect of reaction time on the oxidation of p-cresol(p-Cresol, 3.75 mol·L-1; NaOH, 8.75 mol·L-1; T(p-NO2)PPCoCl,7.5×10-5 mol·L-1; MeOH 30 ml; flow amount of oxygen under atmospheric pressure, 20 ml·min-1; temperature, 343 K)■ conversion of p-cresol; ○ selectivity for p-hydroxybenzenemethanol; ▲ yield of p-hydroxybenzenemethanol
3.3.3 Effect of reaction pressure
Table 4 showed the effect of reaction pressure on the catalytic performance. As seen, the conversion increased clearly comparing with the result under atmospheric pressure while the selectivity for p-hydroxybenzaldehyde peaked at 86.6% with the conversion of 69.8% under 0.4 MPa. According to Henry’s law, higher pressure increase the oxygen solubility and excess oxygen can oxidize p-hydroxybenzaldehyde to p-hydroxybenzoic acid. Therefore, the reaction pressureat 0.4 MPa had been demonstrated to be the suitable value.
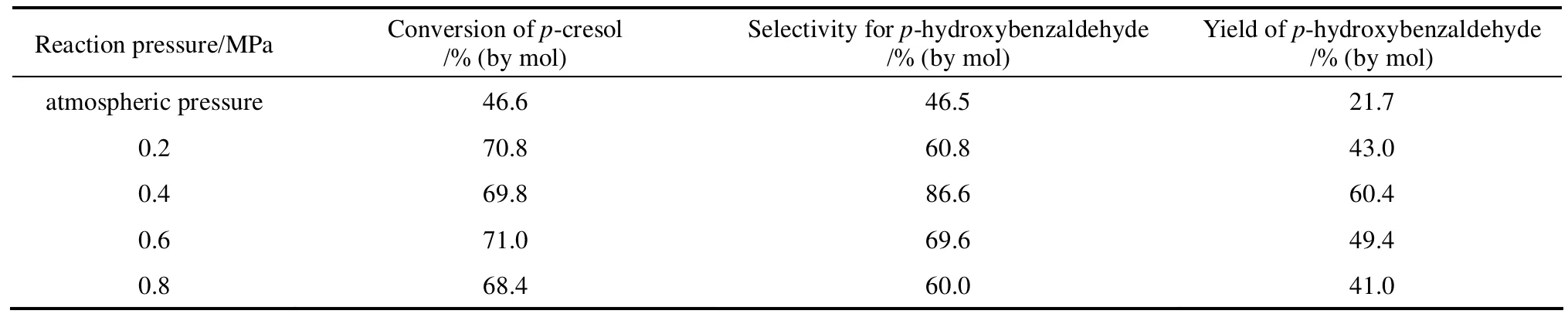
Table 4 Effect of reaction pressure on p-cresol oxidation
4 CONCLUSIONS
Metalloporphyrins presented good catalytic performances for oxidations of p/o-cresols to p/ohydroxybenzaldehydes in the presence of molecular oxygen under mild conditions. The metalloporphyrin catalysts with different central ions exhibited different catalytic activities, and the selectivities for p-hydroxybenzaldehydes in p-cresol oxidation performed in the order of Co > Mn > Fe, while Fe porphyrins performed better in o-cresol oxidation. As for the effects of peripheral substituents around porphyrin rings on the selectivities for p/o-hydroxybenzaldehydes,the electron-withdrawing and electron-donating groups could improve the selectivities for p-hydroxybenzaldehydes and o-hydroxybenzaldehydes respectively. Under the following optimal p-cresol reaction conditions: 3.75 mol·L-1p-Cresol, 7.50 mol·L-1NaOH, 7.5×10-5mol·L-1T(p-NO2)PPCoCl,and 20 ml·min-1of oxygen flow under atmospheric pressure at 343 K for 10 h, the selectivity for p-hydroxybenzaldehyde reached 86.6%. And under the following optimal o-cresol reaction conditions:2.50 mol·L-1o-Cresol, 8.75 mol·L-1NaOH, 5×10-4mol·L-1T(p-OCH3)PPFeCl, and 20 ml·min-1of oxygen flow under atmospheric pressure at 343 K for 8 h,the selectivity for o-hydroxybenzaldehyde reached 26.6%.
1 Smith, W.E., (Dow Chemical Corporation), “Reimer-Tiemann aldehyde synthesis process”, US Pat., 4324922 (1982).
2 Thoer, A., Denis, G., “The Reimer-Tiemann reaction in slightly hydrated solid-liquid medium: A new method for the synthesis of formyl and diformyl phenols”, Synth. Commun., 18, 2095-2101 (1988).
3 Neumann, R., Sasson, Y., “Increased para selectivity in the Reimer-Tiemann reaction by use of polyethylene glycol as complexing agent”, Synthesis, 7, 569-571 (1986).
4 Sparfel, D., Baranne-Lafont, J., Nguyen, K.C., Capdevielle, P.,Maumy, M., “Catalytic-oxidation of 2-hydroxymethyl phenols by MnCln/M0/O2(M Cu, Fe)”, Tetrahedron, 46, 793-802 (1990).
5 Formanek, K., “o-Hydroxybenzaldehydes”, Eur. Pat., EP 77279 A1(1983).
6 Qiao, Y.H., Teng, J.J., “The synthesis of o-hydroxybenzaldehyde by oxidating o-cresol with oxygen”, Mao Ming Xue Bao, 15, 26-29(2005). (in Chinese)
7 She, Y.B., Wang, L.Z., Song, X.F., Zhang, Y.H., Chen, Y.X., “Synthesis and QSAR of metal porphyrin biomimetic catalysts and their application in oxidation of hydrocarbons”, Fine Chemicals, 22,401-408 (2005). (in Chinese)
8 Poltowicz, J., Haber, J., “The oxyfunctionalization of cycloalkanes with dioxygen catalyzed by soluble and supported metalloporphyrins”, J. Mol. Catal., 220, 43-51 (2004).
9 Meunier, B., “Metalloporphyrins as versatile catalysts for oxidation reactions and oxidative DNA cleavage”, Chem. Rev., 92 (6),1411-1456 (1992).
10 Groves, J. T., Bonchio, M., Carofiglio, T., Shalyaev, K., “Rapid catalytic oxygenation of hydrocarbons by ruthenium pentafluorophenylporphyrin complexes: Evidence for the involvement of a Ru(III) intermediate”, J. Am. Chem. Soc., 118, 8961-8963 (1996).
11 She, Y.B., Fan, L.L., Zhang, Y.H., Song, X.F., “Novel technology for green synthesis of p-nitrobenzaldehyde with metalloporphyrins as biomimetic catalysts”, J. Chem. Ind. Eng. (China), 55 (12),2032-2037 (2004). (in Chinese)
12 Wang, L.Z., She, Y.B., Zhong, R.G., Ji, H.B., Zhang, Y.H., Song, X.F.,“A green process for oxidation of p-nitrotoluene catalyzed by metalloporphyrins under mild conditions”, Org. Process Res. Dev., 10 (4),757-761 (2006).
13 Bottomley, L.A., Kadish, K.M., “Counterion and solvent effects on the electrode-reactions of iron porphyrins”, Inorg. Chem., 20 (5),1348-1357 (1981).
14 Liu, Y.M., Liu, S.T., Zhu, K.Z, Ye, X.K., Wu, Y., “Catalysis of hydrotalcite-like compounds in liquid phase oxidation: (II) Oxidation of p-cresol to p-hydroxybenzaldehyde”, Appl. Catal. A Gen., 169,127-135 (1998).
2011-03-07, accepted 2011-12-18.
* Supported by the Key Project of National Natural Science Foundation of China (21036009, 20776003), the Key Project of Natural Science Foundation of Beijing (2061001), the Funding Project for Academic Human Resources Development in Institutions of Higher Learning under the Jurisdiction of the Beijing Municipality (PHR 200907105, PHR 201107104).
** To whom correspondence should be addressed. E-mail: sheyb@bjut.edu.cn
 Chinese Journal of Chemical Engineering2012年2期
Chinese Journal of Chemical Engineering2012年2期
- Chinese Journal of Chemical Engineering的其它文章
- Optimization for Production of Intracellular Polysaccharide from Cordyceps ophioglossoides L2 in Submerged Culture and Its Antioxidant Activities in vitro*
- A Pilot-scale Demonstration of Reverse Osmosis Unit for Treatment of Coal-bed Methane Co-produced Water and Its Modeling*
- ECT Image Analysis Methods for Shear Zone Measurements during Silo Discharging Process*
- Temperature-triggered Protein Adsorption and Desorption on Temperature-responsive PNIPAAm-grafted-silica: Molecular Dynamics Simulation and Experimental Validation*
- Adsorptive Thermodynamic Properties and Kinetics of trans-1,2-Cyclohexandiol onto AB-8 Resin
- Tracking Submicron Particles in Microchannel Flow by Microscopic Holography*
