Evaluation of treatment response for breast cancer: are we entering the era of “biological complete remission”?
Li Bian, Tao Wang, Yi Liu, Hui-Qiang Zhang, Jin-Jie Song, Shao-Hua Zhang, Shi-Kai Wu, San-Tai Song, Ze-Fei Jiang
1Department of Breast Cancer, Affiliated Hospital of Academy of Military Medical Sciences, Beijing, China; 2Laboratory of Oncology, Affiliated Hospital of Academy of Military Medical Sciences, Beijing, China
Introduction
Breast cancer is one of the most common malignancies in women.Its incidence has continuously increased, with 1 million new cases annually worldwide.The breast cancer cases accounted for 25-30% of all malignancies in European and American women (1).The incidence of breast cancer grew rapidly in China in the past years.It ranks first in urban areas and fifth in rural areas, and the mortality of breast cancer ranks fourth or fifth among cancer-related mortalities in metropolitan areas.The post-operative recurrence and metastasis are the leading causes of breast cancer-related mortality.Surgery for early breast cancer usually can achieve satisfactory results.The 5-year survival rate for stage I breast cancer can reach 92%, and the 5-year survival rate for stage II breast cancer can be 81% (2).However, most breast cancer cases were identified and confirmed at their late stages and have became surgically unresectable.In some other patients, although their tumors are confirmed at the early stage and show no symptoms of metastasis, tumor metastasis and/or relapse do occur after the resection of the tumor.These results are close related with the occult micrometastases of tumors and the circulating tumor cell (CTC) (3).
The micrometastases originate from the seeding of tumor cells in circulation.Therefore, CTC may be a marker of distant metastasis.In recent years, the development of sensitive molecular biological techniques have made the isolation and counting of peripheral CTC possible.Traditionally, the efficacy of breast cancer treatment is evaluated via imageological methods; however, along with the development of molecular biological technology, the biological efficacy evaluation has shown certain advantages.As shown by research, in the early stage, CTC can be used for the differentiation of benign and malignant tumors and for predicting and judging the risk of metastasis; during the baseline phase before treatment and immediately after treatment, determination of CTC can be used for the early prediction of the treatment efficacy (4,5).Also in recent years, the advances in PET/CT technology has made it possible to identify the residual disease and relapse after treatment, monitor the existence of distant metastasis, and evaluate the treatment response, moreover, PET/CT can detect changes in the tumor metabolism; thus, compared with the conventional imageological techniques, it can reflect the efficacy in an earlier and more accurate manner (5-7).
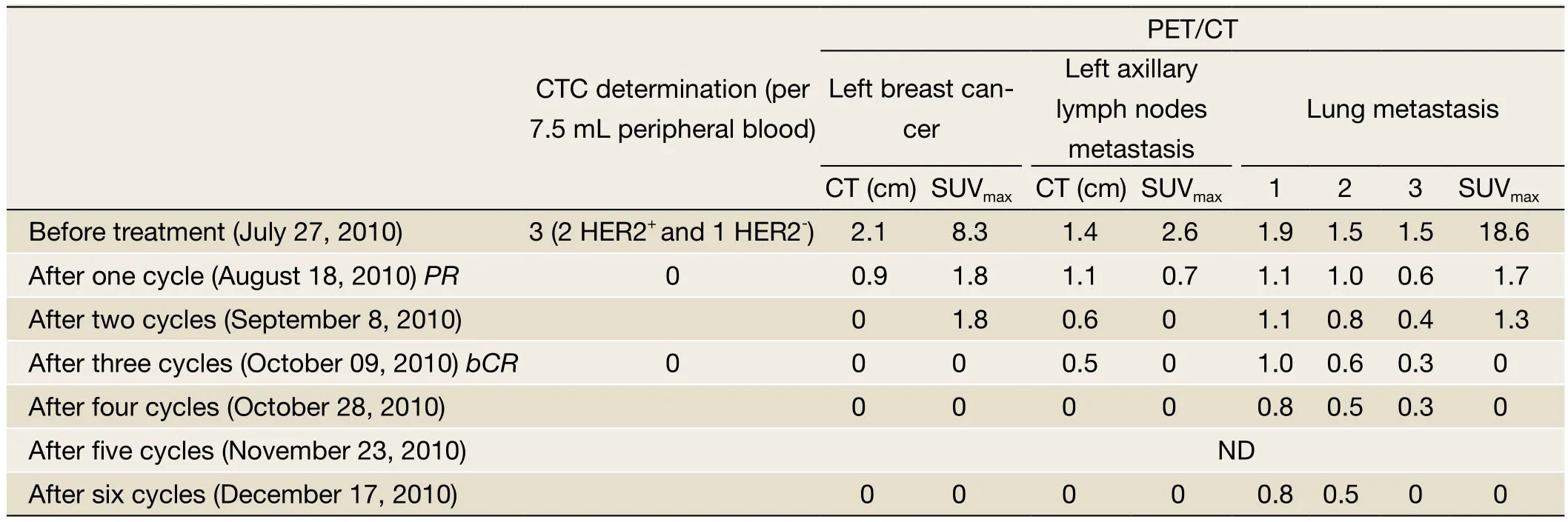
Table 1 Circulating tumor cell (CTC) detection combination PET/CT technology evaluating the treatment response
The combined application of CTC technology and PET/CT technology can realize the “evaluation of biological efficacy” for breast cancer.When evaluating the efficacy of treatment for breast cancer, the concept of “complete remission” (CR) initially includes clinical imaging complete remission and pathological complete remission (pCR).However, along with the optimization of CTC and PET/CT technology, a new concept, biological complete remission(bCR) has emerged in clinical practices.In our depart, the efficacy of treatment for a patient with stage IV breast cancer was evaluated with CTC technology and PET/CT.The data are reported as below.
Case presentation
A 56-year-old woman was presented in our department in July 2010.On physical examination, a 3.5-cm mass was detected in the lower outer quadrant of the left breast.Ultrasound-guided biopsy showed infiltrating ductal carcinoma of the left breast.Immunohistochemistry showed ER(-), PR(-), and HER2(+++).PET/CT indicated left breast cancer accompanied with metastasis to axillary lymph nodes and both lungs.The final diagnosis was T2N1M1, stage IV left breast cancer, with metastasis to axillary lymph nodes and lungs.After the diagnosis was confirmed, chemotherapy was used in combination with Her2 targeted therapy.Since weekly albumin-bound paclitaxel (ABP) has shown higher efficacy in treating metastatic breast cancer than docetaxel,let alone its good safety and patient adherence.The final treatment protocol was ABP (200 mg, 126.6 mg/m2; d1 and d8) in combination with capecitabine (3,000 mg, 1898.7 mg/m2, d1-14)and trastuzumab (440 mg, d1).Before treatment and in each 3-week cycle, CTC was determined using CellSearch System(Veridex LLC, Raritan, NJ); meanwhile, the treatment response was evaluated with PET/CT (General Electric Medical Systems, Waukesha, WI).The results are shown inTable 1.
Discussion
Albumin-bound paclitaxel (Abraxane) is a new, solvent-free paclitaxel chemotherapy drug.Its outer layer is wrapped in albumin, and its core is paclitaxel nanoparticles that do not dissolve in water.One albumin molecule can be bound with seven paclitaxel molecules.Mediated by the albumin receptor Gp60 and caveolae on the cell membrane, the nanoparticles enter the tumor tissue via the gap junctions in vascular endothelial cells and bind to the secreted protein acidic and rich in cysteine (SPARC) in the tumor interstitium.Furthermore, upon the effect of Gp60-Caveolin-1 on the cell membrane, the drug moves via a membrane transport protein into the tumor cells.As a result, the local drug concentration inside the tumor cells is dramatically increased, realizing the efficacy of targeted treatment (8,9).Talbotet al.(10) found that the overall response rate of capecitabine monotherapy ranged 20-40% in breast cancer patients who either had failed or were resistant to treatment with an anthracyclineor paclitaxel-containing regimen.Xeloda (capecitabine) is a new generation of oral cytotoxic drugs.Following oral administration, capecitabine passes intact through the intestinal mucosa, is rapidly and extensively metabolised via a sequential triple enzyme pathway, finally converts to 5-fluorouracil (5-FU) by thymidine phosphorylase (TP).The activity of TP is remarkably higher in breast cancer tissue than in the healthy tissues, and therefore the cytotoxic effect of capecitabine is highly selective (11).Herceptin(trastuzumab) is the first drug that successfully targeted the HER2 pathway.It can bind specifically to the HER2 protein on protein on the breast cancer cell surface and inhibit cellular signal transduction pathways.Meanwhile,it can accelerate the degration of HER2 receptor, downregulating the expression of HER2 receptor and supressing the growth of breast cancer cells.In the existence of human peripheral mononuclear cells (PMC), it exerts effect on tumor cell-mediated antibody dependent cellular cytotoxicity(ADCC) and kills the target cells.Also, it can suppress the production of vascular endothelial growth factor and block the growth of blood vessels within the tumor tissue.Thus,it induces the apoptosis of tumor cells and ultimately kills the tumor cells (12-15).The patient in our study received 6 cycles of salvage treatment with albumin-bound paclitaxel plus capecitabine and trastuzumab.Then, she underwent CTC detection and PET/CT-based examinations for efficacy evaluation, which showed “biological complete remission”(bCR).
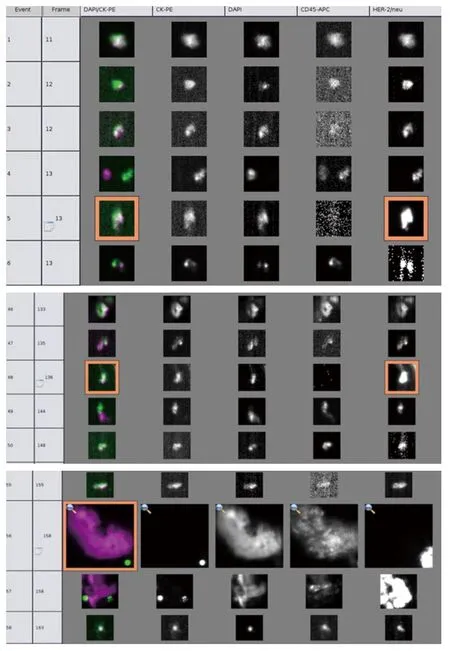
Figure 1 Two HER2-positive CTCs and one HER2-negative CTC isolated from peripheral blood before treatment by immunomagnetic capture and analyzed by a fluorescent microscope.HER2-positive CTCs are positive for cytokeratin,DAPI and HER2 and negative for CD45 markers and HER2-negative CTC is positive for cytokeratin and DAPI and negative for CD45 markers
According to Cristofanilliet al.(4), in patients with metastatic breast cancer, the pre-treatment threshold level of CTCs equal to 5 per 7.5 mL of whole blood can be used for predicting the survival.Patients in a training set with levels of CTCs equal to or higher than 5 per 7.5 mL of whole blood, as compared with the group with fewer than 5 CTCs per 7.5 mL, had a shorter median progression-free survival and shorter overall survival.Furthermore, the levels of CTC declined within 4 weeks after treatment, which was associated with longer progression-free survival and overall survival (16),and therefore can also be used for predicting survival.In our study, after the patient received 1 cycle (3 weeks) of salvage treatment with albumin-bound paclitaxel plus capecitabine and trastuzumab, the level of CTC decreased from 3 to 0(Figure 1).The decline of HER2-positive cancer cell was relatively more obvious, which may be explained by the targeting effect of trastuzumab.The real-time detection of the genetic expression/mutation of CTC can also facilitate the individualized medications for tumor patients, and the observation of the change in CTC amount can also be used to predict the prognosis and efficacy.Also in our study,hematological CTC determination can reveal biological therapeutic effect within 4 weeks after the initiation of treatment.Compare with conventional imageological evaluation, PET/CT can be used to detect the metabolism of the lesions and explore the possible decline of SUVmax value,so it can be used accurately for monitoring the treatment response.Our study showed that SUVmax value of PET/CT decreased to 0 after the patient received 3 cycles (9 weeks) of salvage treatment (Figure 2), at that moment the patient got“biological complete remission” (bCR), but imageological CR was not reached, while the conventional CT still displays residue diseases until 6 cycles treatments (4.5 months).In addition, patients with imageology diagnosed advanced breast cancer but with smaller CTC amount tends to have longer survival than those with larger CTC amount.Therefore,compared with imaging examinations, CTC determination can provide additional diagnostic information.The patient in our study underwent left total mastectomy and left axillary lymph node dissection on December 30, 2010.The postsurgical pathology showed: lobular and ductal structures were seen in the left breast.The breast stroma had became fibrous connective tissue showing hyaline degeneration.Infiltration of inflammatory cell (mainly a few lymphocytes)but no tumor cell was observed.The post-chemotherapy change was judged as histopathological grade 5 (i.e., pCR),The lymph node metastasis was 0/18 (Figure 3), suggesting that bCR was consistent with histological (pathological) CR(pCR).
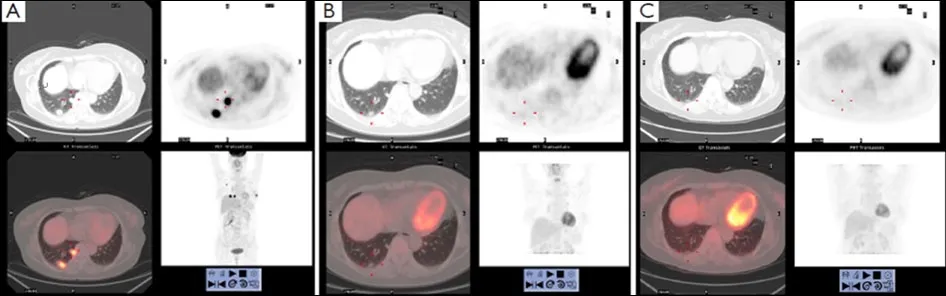
Figure 2 The SUVmax value of lung metastasis decreased from 18.6 (A) to 1.7 (B) after one cycle treatment and the SUV value declined to 0 (C) after three cycles treatment on PET-CT
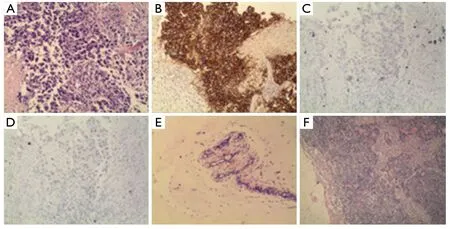
Figure 3 Ultrasound-guided biopsy showed infiltrating ductal carcinoma of the left breast (A) and immunohistochemistry showed HER2(+++) (B), ER(-) (C) and PR (-) (D).The post-surgical pathology showed the post-chemotherapy change was judged as histopathological grade 5: left breast (E) and left axillary lymph node (F)
Conclusions
A better understanding of the biological features of breast cancer will facilitate the early, accurate evaluation of the prognosis and treatment response of breast cancer.In this study, we evaluated the treatment response using CTC detection in combination with PET/CT technology, which realized the evaluation of biological complete remission(bCR) in patients with breast cancer.The bCR of the patient appeared earlier than the conventional clinical imaging CR and promised the histological (pathological)CR.The integrated application of the concepts including bCR, imageological CR and histological CR can achieve the early and accurate evaluation of biological therapeutic reponse and prognosis of breast cancer.
Acknowledgements
This work was supported by the department of pathology and the PET/CT center of Affiliated Hospital of Academy of Military Medical Sciences.
Disclosure:All authors have no conflict of interest and no financial relationship with the organization that sponsored the research.
1.Campbell JB.Breast cancer-race, ethnicity, and survival: a literature review.Breast Cancer Res Treat 2002;74:187-92.
2.Veronesi U, Salvadori B, Luini A, et al.Conservative treatment of early breast cancer.Long-term results of 1232 cases treated with quadrantectomy, axillary dissection, and radiotherapy.Ann Surg 1990;211:250-9.
3.Mocellin S, Keilholz U, Rossi CR, et al.Circulating tumor cells: the ‘leukemic phase’ of solid cancers.Trends Mol Med 2006;12:130-9.
4.Cristofanilli M, Budd GT, Ellis MJ, et al.Circulating tumor cells, disease progression, and survival in metastatic breast cancer.N Engl J Med 2004;351:781-91.
5.De Giorgi U, Valero V, Rohren E, et al.Circulating tumor cells and [18F]fluorodeoxyglucose positron emission tomography/computed tomography for outcome prediction in metastatic breast cancer.J Clin Oncol 2009;27:3303-11.
6.Giannopoulou C.The role of SPET and PET in monitoring tumour response to therapy.Eur J Nucl Med Mol Imaging 2003;30:1173-200.
7.Dose-Schwarz J, Tiling R, Avril-Sassen S, et al.Assessment of residual tumour by FDG-PET: conventional imaging and clinical examination following primary chemotherapy of large and locally advanced breast cancer.Br J Cancer 2010;102:35-41.
8.Tiruppathi C, Finnegan A, Malik AB.Isolation and characterization of a cell surface albumin-binding protein from vascular endothelial cells.Proc Natl Acad Sci U S A 1996;93:250-4.
9.Desai NP, Trieu V, Hwang LY, et al.Improved effectiveness of nanoparticle albumin-bound (nab)paclitaxel versus polysorbate-based docetaxel in multiple xenografts as a function of HER2 and SPARC status.Anticancer Drugs 2008;19:899-909.
10.Talbot DC, Moiseyenko V, Van Belle S, et al.Randomised,phase II trial comparing oral capecitabine (Xeloda) with paclitaxel in patients with metastatic/advanced breast cancer pretreated with anthracyclines.Br J Cancer 2002;86:1367-72.
11.Fabi A, Vidiri A, Ferretti G, et al.Dramatic regression of multiple brain metastases from breast cancer with Capecitabine: another arrow at the bow? Cancer Invest 2006;24:466-8.
12.Gajria D, Chandarlapaty S.HER2-amplified breast cancer:mechanisms of trastuzumab resistance and novel targeted therapies.Expert Rev Anticancer Ther 2011;11:263-75.
13.Scaltriti M, Eichhorn PJ, Cortés J, et al.Cyclin E amplification/overexpression is a mechanism of trastuzumab resistance in HER2+ breast cancer patients.Proc Natl Acad Sci U S A 2011;108:3761-6.
14.Junttila TT, Parsons K, Olsson C, et al.Superior in vivo efficacy of afucosylated trastuzumab in the treatment of HER2-amplified breast cancer.Cancer Res 2010;70:4481-9.
15.Spector NL, Blackwell KL.Understanding the mechanisms behind trastuzumab therapy for human epidermal growth factor receptor 2-positive breast cancer.J Clin Oncol 2009;27:5838-47.
16.Cristofanilli M, Hayes DF, Budd GT, et al.Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer.J Clin Oncol 2005;23:1420-30.
 Chinese Journal of Cancer Research2012年4期
Chinese Journal of Cancer Research2012年4期
- Chinese Journal of Cancer Research的其它文章
- Combination chemotherapy with paclitaxel, cisplatin and fluorouracil for patients with advanced and metastatic gastric or esophagogastric junction adenocarcinoma: a multicenter prospective study
- NRS2002 assesses nutritional status of leukemia patients undergoing hematopoietic stem cell transplantation
- Activation of sonic hedgehog signaling pathway is an independent potential prognosis predictor in human hepatocellular carcinoma patients
- High-risk endometrial cancer may be benefit from adjuvant radiotherapy plus chemotherapy
- Circulating tumor cells (CTCs) in breast cancer: a diagnostic tool for prognosis and molecular analysis
- Perivascular epithelioid cell tumor of male pelvic cavity: a case report and literature review
