基于1H-苯并咪唑-2-羧酸的三個(gè)鑭系超分子化合物的結(jié)構(gòu)、熱分解動(dòng)力學(xué)和熒光性質(zhì)
段林強(qiáng) 喬成芳, 魏 青,* 夏正強(qiáng) 陳三平,*張國春 周春生 高勝利,
(1西北大學(xué)化學(xué)與材料科學(xué)學(xué)院,合成與天然功能分子化學(xué)教育部重點(diǎn)實(shí)驗(yàn)室,西安710069; 2商洛學(xué)院化學(xué)與化學(xué)工程系,陜西省尾礦資源綜合利用重點(diǎn)實(shí)驗(yàn)室,陜西商洛726000)
1 Introduction
In recent years,the self-assembly of metal-organic frameworks(MOFs)has been attracting more and more attention for their fascinating architectures and potential applications as new materials in ion exchange,magnetism,gas absorption,catalysis,and luminescence.1-6Particularly for the optical applications,extensive efforts have been devoted to the study of lanthanide-based MOFs(LnOFs),because the narrow f→f transitions derived from 4f electrons of lanthanide ions could lead to interesting and excellent luminescence emissions in visible or near-infrared regions.7-10Up to now,numerous one-dimensional(1D),two-dimensional(2D),and three-dimensional(3D)lanthanide compounds have been synthesized with appropriate bridging organic ligands,and many of them exhibited extraordinary luminescence and intriguing network topologies due to the high coordination number,flexible coordination environments,and large radii of lanthanide ions.11-18It can be concluded from the fruitful reports of LnOFs that the polytopic organic ligands containing O-or N-donors are the preferred choice for preparation of LnOFs.
Based on the consideration above,we focused on a flexible heterocyclic ligand, 1H-benzimidazole-2-carboxylic acid (H2BIC),which consists of benzimidazole ring bearing carboxylate group(Scheme 1).Related reports indicated that H2BIC was not only a reaction medium in synthesis but also a potential multifunctional organic linker for construction of MOFs.19-22Actually,H2BIC has many remarkable features.It contains two carboxyl O atoms and two imidazole N atoms, which can provide many coordination fashions,from terminal monodentate coordination or N,O-bidentate-chelating to chelating-bridging or bi(chelating-bridging)modes,giving rise to various LnOFs with charming architectures and functions.In addition,the rigid benzimidazole ring is beneficial for the stability of the framework,and the carboxylate group could further strengthen the conjugated systems when coordinating with lanthanide ions,resulting in better sensitization for lanthanide luminescence.
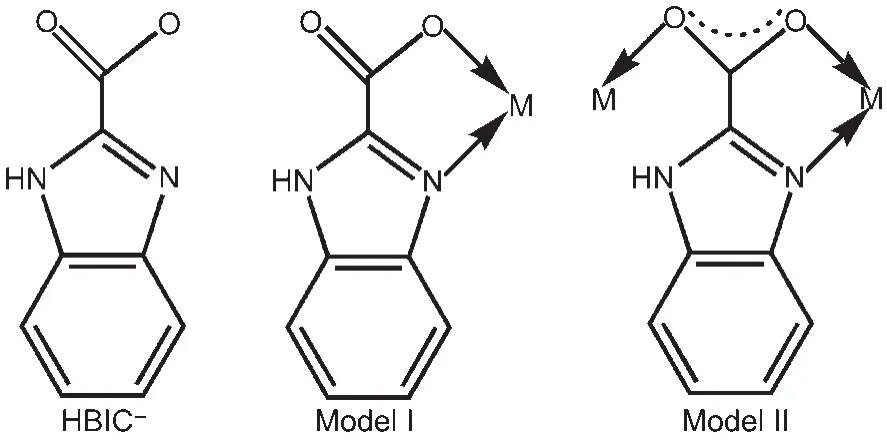
Scheme 1 Constitutional formula and coordination modes of H2BIC ligand
Hence,in this contribution,the multidentate H2BIC ligand was used to construct new LnOFs[Ln=Sm(1),Ho(2),Yb(3)] under hydrothermal conditions.In order to evaluate the thermal effect on the target structures,thermogravimetry(TG)analyses and thermonalysis kinetics for compound 1 were performed.Additionally,the luminescent properties of compounds 1 and 3 were investigated.
2 Experimental
2.1 Materials and methods
All of the reagents were purchased and used without further purification.1H-benzimidazole-2-carboxylic acid(97%)was purchased from Xi?an Chemblossom Pharmaceutical Technology Co.,Ltd.,SmCl3·6H2O(99.9%),HoCl3·6H2O(99.9%), YbCl3·6H2O(99.9%),and NaOH(97%)were purchased from Sigma-Aldrich.Elemental analyses(C,H,N)were performed on a Perkin-Elmer III elemental analyzer(USA).Infrared(IR) spectra were recorded with a Tensor 27 spectrometer(Bruker Optics,Ettlingen,Germany)in the range of 4000-400 cm-1using powdered samples on a KBr pellets.Powder X-ray diffraction(PXRD)patterns were recorded on a Bruker D8 Advance diffractometer(Germany).Thermogravimetric measurements were performed with a Netzsch STA 449C apparatus(Germany)under air atmosphere from 20 to 1000°C.Differential scanning calorimetry(DSC)experiments were performed using a CDR-4P thermal analyzer of Shanghai Balance Instrument factory under a flow of air atmosphere from 20 to 1000°C.Luminescence spectra were measured at room temperature with an Edinburgh FL-FS90 TCSPC system(UK).
2.2 Syntheses
2.2.1 Synthesis of compound[Sm(HBIC)3]n(1)
A mixture containing SmCl3·6H2O(0.0365 g,0.1 mmol), H2BIC(0.0162 g,0.1 mmol),NaOH(0.0040 g,0.1 mmol),and H2O(6 g,333.6 mmol)was kept heating in a 10 mL stainless steel reactor with a Teflon liner at 110°C for 72 h.The reaction system was then slowly cooled to room temperature at a rate of 5°C·h-1,and the colorless platy crystals were obtained, washed with water,and dried in air.Yield:42%based on Sm(III).Anal.calcd.forC24H15N6SmO6(Mr=633.77):C 45.48%,H 2.39%,N 13.26%;Found:C 45.43%,H 2.45%,N 13.35%.IR(KBr)ν/cm-1:3122(m),3009(m),2919(w),2637 (w),1642(s),1614(vs),1522(s),1460(s),1396(vs),1332 (s),748(s),642(m).
2.2.2 Synthesis of compound[Ho(HBIC)3]n(2)
Being followed the same procedure as for the preparation of 1 except that SmCl3·6H2O was replaced with HoCl3·6H2O (0.0379 g,0.1 mmol).The colorless platy crystals were obtained,washed with water and dried in air.Yield:40%based on Ho(III).Anal.calcd.for C24H15N6HoO6(Mr=648.35):C 44.46%,H 2.33%,N 12.96%;Found:C 45.37%,H 2.41%,N 12.92%.IR(KBr)ν/cm-1:3119(m),3003(m),2922(w),2640 (w),1647(s),1611(vs),1519(s),1456(s),1399(vs),1329 (s),744(s),647(m).
2.2.3 Synthesis of compound[Yb(HBIC)3]n(3)
Following the same procedure as for the preparation of compound 1 but YbCl3·6H2O(0.0384 g,0.1 mmol)was used instead of SmCl3·6H2O.The white powders were obtained. Yield:35%based on Yb(III).Anal.calcd.for C24H15N6YbO6(Mr=656.46):C 43.91%,H 2.30%,N 14.62%;Found:C 43.80%,H 2.42%,N 14.55%.IR(KBr)ν/cm-1:3115(m),3012 (m),2925(w),2645(w),1644(s),1616(vs),1515(s),1465 (s),1401(vs),1335(s),781(s),650(m).
2.3 X-ray crystallography
Diffraction data for compounds 1 and 2 were collected on a BrukerSmartApex IICCD diffractometer(Germany) equipped with graphite monochromated Mo Kαradiation(λ= 0.071073 nm)using ω and φ scan mode at 296(2)K.Absorption correction were applied using the SADABS program. Their structures were solved by direct methods and refined with full-matrix least-squares refinements based on F2using SHELXS-97 and SHELXL-97.23,24All non-hydrogen atoms were refined with anisotropic displacement parameters.Hydrogen atoms were placed in geometrically calculated positions. Crystal data and structure refinement details for compounds 1 and 2 are summarized in Table 1,selected bond lengths and angles are shown in Table S1(see Supporting Information),and hydrogen-bonding interactions are listed in Table S2(see Supporting Information).
3 Results and discussion
3.1 Structure description
Powder X-ray diffraction indicates that compound 3 is isomorphous to compound 2 in Fig.S1(see Supporting Information).The structures of compounds 1 and 2 determined using single-crystal X-ray diffraction,are isostructural,crystallizing in monoclinic space group P21/n.Here,we take compound 1 for representative example to describe the structure of the coordination compounds in detail.As shown in Fig.1,each Sm(III)ion center is eight-coordinated by three nitrogen atoms and five carboxyl oxygen atoms from five HBIC-ligands,resulting in a slightly distorted two-capped triangle prism geometry.The Sm―O bond distances are in the range of 0.2351(4)-0.2497(4) nm,and Sm―N bond lengths vary from 0.2535(5)to 0.2577 (5)nm,which are slightly longer than the lengths of Ho―O [0.2302(3)-0.2461(3)nm]and Ho―N[0.2461(4)-0.2526(4) nm]in compound 2.However,the bond angles of O―Sm―O change from 70.79(14)°to 156.96(15)°,and the bond angles of N―Sm―N range from 70.44(17)°to 151.91(17)°,slightly smaller than those of O―Ho―O[70.26(11)°-157.13(12)°] and N―Ho―N[71.16(13)°-152.70(13)°]in compound 2(Table S1).The tiny differences may be ascribed to the effect of lanthanide contraction and soft and hard acid-alkali.
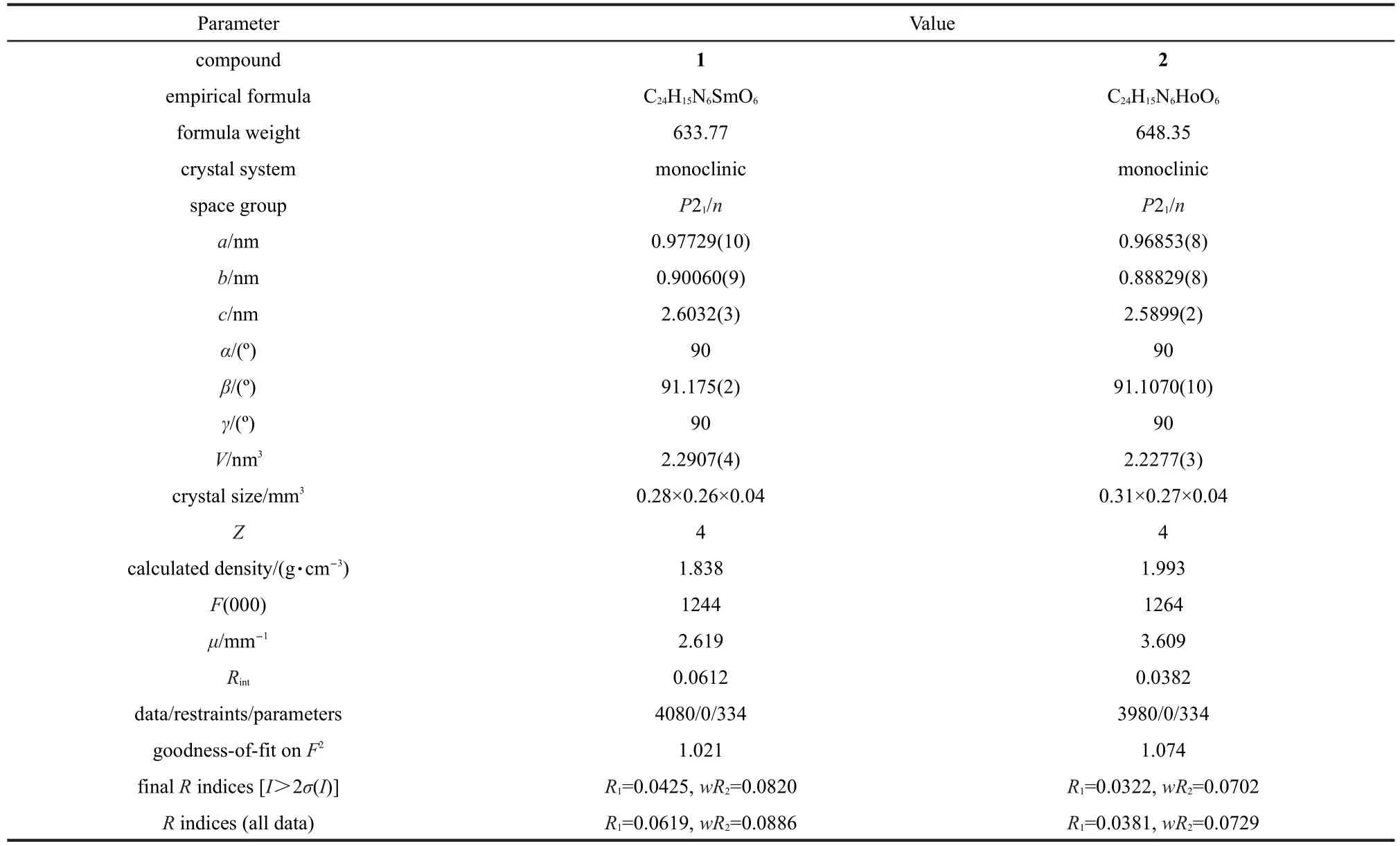
Table 1 Crystal data and structure refinement summary for compounds 1 and 2
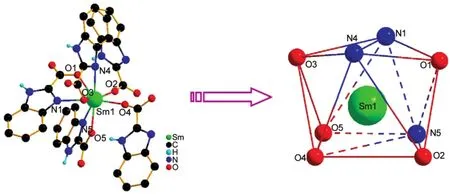
Fig.1 Coordination environment and coordination polyhedron geometry of Sm(III)in compound 1
Further analysis reveals that the partly deprotonated HBIC-exhibits two coordination modes,as shown in Scheme 1.Mode I adopts a simple N,O-bidentate-chelating fashion,and Mode II features aμ2-bridge fashion connecting two Sm(III)ions,in which one Sm(III)ion is coordinated one imidazole N atom and one carboxyl O atom in N,O-bidentate motif,and the other Sm(III)ion is monodentatly bonded by the other carboxyl O atom.As shown in Fig.2(a),the adjacent Sm(III)centers are bridged by HBIC-ligands with Mode II to generate a 2D structure,where some HBIC-ligands with Mode I just chelate the Sm(III)ions to stable the configuration of the metal centers and the whole network.The topological analysis method was used to describe intuitively and understand the structural characteristics of compound 1,as illustrated in Fig.2(b),the 2D framework can be simplified to be a(4,4)net through defining the Sm(III)centers as knots and HBIC-ligands as spacers.In the net,the size of each grid is 0.67171(6)nm×0.69699(7)nm, which is larger than that in compound 2[0.66425(5)nm× 0.68974(5)nm].
Notably,another obvious structural feature of compound 1 is the existence of the strong hydrogen bonds between the carboxylate groups and imidazole rings[N(6)―H(6N)···O(6)#1, 0.2818(7)nm,N(3)―H(3)···O(5)#2,0.2788(7)nm,and N(2)―H(2)···O(1)#3,0.2723(6)nm(#1:-x+1,-y+1,-z;#2:-x+ 1/2,y+1/2,-z+1/2;#3:-x+3/2,y+1/2,-z+1/2)](Table S2), which are crucial for stabilizing the whole structure of compound 1.These abundant hydrogen bonds eventually link the 2D layers into a 3D supramolecular framework(Fig.3).
3.2 IR spectrum
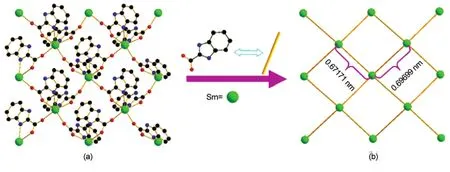
Fig.2 (a)2D network of compound 1 and(b)simplified 2D structure with(4,4)topologyAll H atoms and partial ligands in(a)are omitted for clarity.
In the IR spectra of compounds 1-3,the powerful peaks at approximately 1640,1610,1520,1460,and 750 cm-1can be ascribed to the asymmetric and symmetric stretching vibrations of carboxyl and phenyl groups from the H2BIC ligands.The respective values of the vas(COO-)(182 cm-1)and vs(COO-)(198 cm-1)clearly indicate that the carboxylate groups are coordinated with the metal ions in both monodentate and bidentate-chelating coordination modes.25In addition,the characteristic absorption bands at approximately 3120 and 1610 cm-1represent the N―H and C=N stretching vibrations from imidazole rings,respectively.
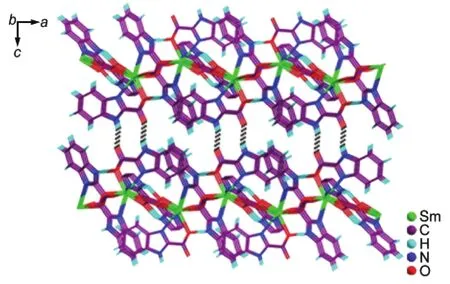
Fig.3 3D network in compound 1 constructed by N―H···O hydrogen-bonding interactions
3.3 Thermogravimetric analysis
In order to evaluate the stability of the coordination compounds,the thermalgravimetric(TG)analyses were carried out between 20 and 1000°C at a heating rate of 10°C·min-1under air atmosphere.TG data of compounds 1-3 plotted in Fig.4 possess similar thermal decomposition process,which further demonstrates that the frameworks 1-3 are isostructural.Compound 1 is taken for a representative example to study the thermostability of the coordination compounds.TG curve of compound 1 indicates that compound 1 could be stable up to 360°C, showing high thermal stability.Then one continuous mass loss of 74.25%occurred in the range 360-700°C is attributed to the decomposition of the framework.The observed residual is 25.75%,which is nearly in agreement with the calculated value of 27.50%.The final solid powder is identified as Sm2O3.
3.4 Non-isothermal thermoanalysis kinetics
As observed from TG curve,compound 1 is transformed into Sm2O3in one process.From equations(1)and(2),thermodecomposition kinetics parameters of the apparent activation energy(E)and the pre-exponential factor(A)of the process were determined by two most common Kissinger and Ozawa-Doyle methods.26,27
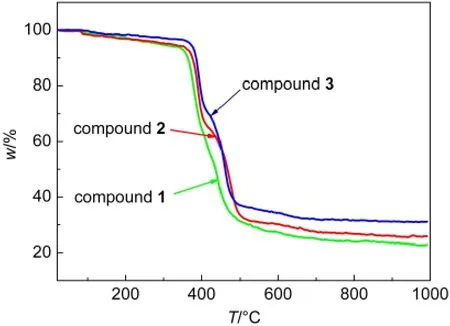
Fig.4 TG curves of compounds 1-3
The Kissinger and Ozawa-Doyle equations are as follows, respectively:

where,Tpis the peak temperature,R is the gas constant,β is the linear heating rate,and C is constant.
Herein,the non-isothermal kinetics analysis of compound 1 is discussed based on DSC curves at different heating rates.In viewed of the exothermic peak temperatures measured at four different heating rates of 5,10,15,and 20°C·min-1(Fig.5), Kissinger and Ozawa-Doyle methods are applied to explore the kinetics parameters of the compound[Sm(HBIC)3]n.The relevant parameter values are calculated and listed in Table 2, it is obvious to notice that EKand EOare 199.3 and 205.2 kJ· mol-1,respectively,which is of seemly significance to evaluate the process by thermoanalysis kinetics method when compound 1 could be employed as precursor for preparation of Sm2O3with different morphologies and sizes.
3.5 Photoluminescent property
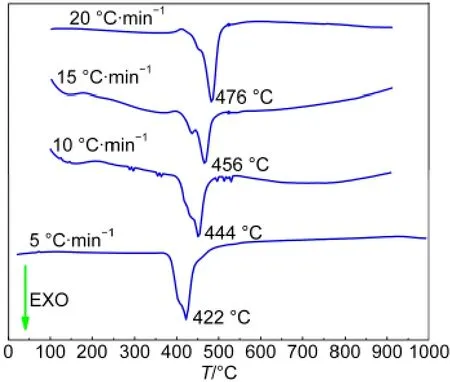
Fig.5 DSC curves of[Sm(HBIC)3]nat different heating rates

Table 2 Peak temperatures of the exothermic stage at different heating rates and the thermoanalysis kinetic parameters
The luminescent properties of compounds 1 and 3 were investigated in the solid state at room temperature.As shown in Fig.6(b),the emission spectrum of compound 1 exhibits a series of sharp lines characteristic of the Sm(III)energy-level structure upon excitation at 348 nm(Fig.6(a)).The characteristic emission bands of Sm(III)ion centered at 565,595,643, 702 nm are attributed to the transitions of4G5/2→6HJ/2(J=5,7,9, 11,respectively).29,30The4G5/2→6H5/2(565 nm)transition consists of the relatively weak band observed in the spectrum,4G5/2→6H7/2(595 nm)starts as a shoulder then splits into two poor-defined peaks but is the most intense observed in the spectrum,and the4G5/2→6H9/2transition is also a poorly resolved peak at 643 nm.31Yb(III)is a special example among the near-infrared-emitting lanthanide ions,because Yb(III)ion has only one excited 4f level,namely,the2F5/2level.Hence,under excitation of 752 nm(Fig.6(c)),the broad emission spectrum of compound 3(Fig.6(d))is observed in the range 900-1050 nm at λmax=972 nm,which is assigned to the characteristic transition of2F5/2→2F7/2.32Both the characteristic lanthanide emission bands observed in compounds 1 and 3 reveal that the HBIC-ligand shows good sensitization for lanthanide luminescence,suggesting compounds 1 and 3 could be anticipated as optical materials.
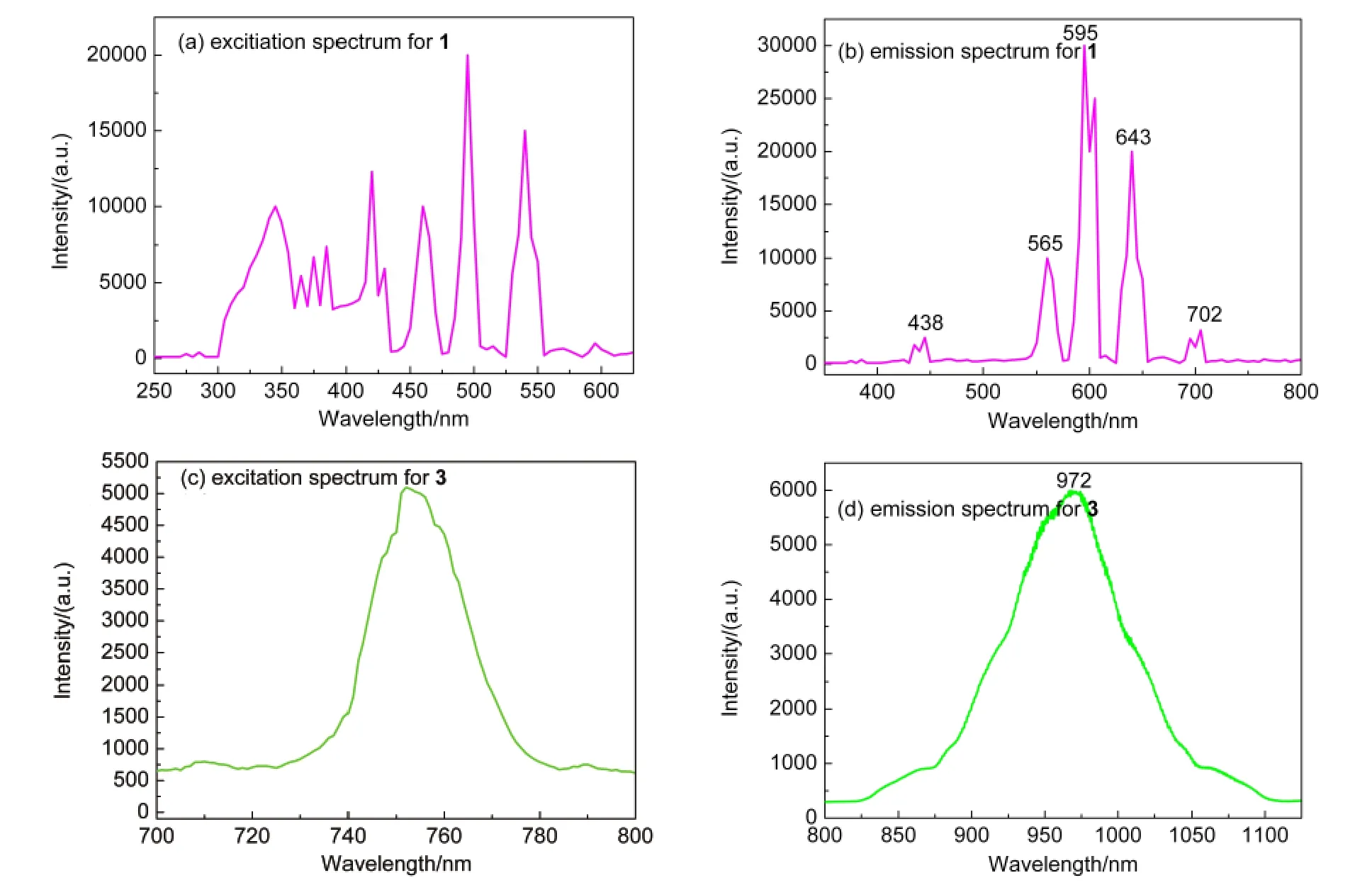
Fig.6 Luminescence excitation and emission spectra of compounds 1 and 3 in the solid state at room temperature
4 Conclusions
In summary,three new compounds[Ln(HBIC)3]n[Ln=Sm (1),Ho(2),Yb(3)]were hydrothermally synthesized and characterized.In their isomorphous structures,the HBIC-ligands connect lanthanide ions with two new coordination modes to form 2D planes,which are further assembled into 3D supramolecular frameworks through strong hydrogen-bonding interactions.TG analyses indicate that compounds 1-3 exhibit good thermostability.The apparent activation energy of thermodecomposition of the compound 1,EKand EOwere respectively determined to be 199.3 and 205.2 kJ·mol-1by the Kissinger and Ozawa-Doyle methods.Luminescent property investigations indicate that compounds 1 and 3 exhibit characteristic transitions in visible and near-infrared regions,respectively, suggesting that the HBIC-ligand is a good sensitizer for lanthanide ions luminescence.
Supporting Information: available free of charge via the internet at http://www.whxb.pku.edu.cn.
(1)Lv,Y.K.;Zhan,C.H.;Feng,Y.L.CrystEngComm 2010,12, 3052.doi:10.1039/b925546j
(2) Lv,Y.K.;Zhan,C.H.;Jiang,Z.G.;Feng,Y.L.Inorg.Chem. Commun.2010,13,440.doi:10.1016/j.inoche.2010.01.007
(3) Halder,G.J.;Kepert,C.J.;Moubaraki,B.;Murray,K.S.; Cashion,J.D.Science 2002,298,1762.doi:10.1126/ science.1075948
(4)Min,K.S.;Suh,M.P.J.Am.Chem.Soc.2000,122,6834.doi: 10.1021/ja000642m
(5) Dong,Y.B.;Jiang,Y.Y.;Li,J.;Ma,J.P.;Liu,F.L.;Tang,B.; Huang,R.Q.;Batten,S.R.J.Am.Chem.Soc.2007,129,4520. doi:10.1021/ja0701917
(6)Sudik,A.C.;Millward,A.R.;Ockwig,N.W.;C?té,A.P.;Kim, J.;Yaghi,O.M.J.Am.Chem.Soc.2005,127,7110.doi: 10.1021/ja042802q
(7) Bünzli,J.C.G.;Piguet,C.Chem.Rev.2002,102,1897.doi: 10.1021/cr010299j
(8)Dias,A.D.B.;Viswanathan,S.Chem.Commun.2004,1024.
(9) Ronson,T.K.;Lazarides,T.;Adams,H.Chem.Eur.J.2006,12, 9299.doi:10.1002/chem.200600698
(10)Yu,Y.Y.;Liu,J.F.;Li,H.Q.;Zhao,G.L.Acta Phys.-Chim. Sin.2010,26,1535.[余玉葉,劉建風(fēng),李花瓊,趙國良.物理化學(xué)學(xué)報(bào),2010,26,1535.]doi:10.3866/PKU.WHXB20100622
(11)Zhu,W.H.;Wang,Z.M.;Gao,S.Inorg.Chem.2007,46,1337. doi:10.1021/ic061833e
(12)Ying,S.M.;Mao,J.G.Cryst.Growth Des.2006,6,964.doi: 10.1021/cg050554j
(13) Do,K.;Muller,F.C.;Muller,G.J.Phys.Chem.A 2008,112, 6789.doi:10.1021/jp804463e
(14) Long,D.L.;Blake,A.J.;Champness,N.R.;Wilson,C.; Schr?der,M.Angew.Chem.Int.Edit.2001,40,2443.
(15) Long,D.L.;Hill,R.J.;Blake,A.J.;Champness,N.R.; Hubberstey,P.;Proserpio,D.M.;Wilson,C.;Schr?der,M. Angew.Chem.Int.Edit.2004,43,1851.doi:10.1002/anie. 200352625
(16)Cui,Y.;Ngo,H.L.;White,P.S.;Lin,W.Chem.Commun.2002, 1666.
(17)Qin,C.;Wang,X.L.;Wang,E.B.;Su,Z.M.Inorg.Chem. 2005,44,7122.doi:10.1021/ic050906b
(18)Zhang,M.B.;Zhang,J.;Zheng,S.T.;Yang,G.Y.Angew. Chem.Int.Edit.2005,44,1385.doi:10.1002/anie.200461424
(19) Wagner,E.;Wittmann,H.J.;Elz,S.;Strasser,A.Bioorg.Med. Chem.Lett.2011,21,6274.doi:10.1016/j.bmcl.2011.09.001
(20)Nandhakumar,R.;Soo,A.Y.;Hong,J.;Ham,S.;Kim,K.M. Tetrahedron 2009,65,666.doi:10.1016/j.tet.2008.11.022
(21) Zheng,S.R.;Cai,S.L.;Fan,J.;Xiao,T.T.;Zhang,W.G.Inorg. Chem.Commun.2011,14,818.doi:10.1016/j.inoche. 2011.02.017
(22) Fan,J.;Cai,S.L.;Zheng,S.R.;Zhang,W.G.Acta Crystallogr. Sect.C:Cryst.Struct.Commun.2011,67,m346.
(23) Sheldrick,G.M.SHELXS-97,Program for Solution of Crystal Structures;University of Gottingen:Germany,1997.
(24) Sheldrick,G.M.SHELXL-97,Program for Refinement of Crystal Structures;University of Gottingen,Germany,1997.
(25) Nakamoto,K.Infrared Spectra and Raman Spectra of Inorganic and Coordination Compounds;Wiley VCH:New York,1986.
(26) Kissinger,H.E.Anal.Chem.1957,29,1702.doi:10.1021/ ac60131a045
(27) Ozawa,T.Bull.Chem.Soc.Jpn.1965,38,1881.doi:10.1246/ bcsj.38.1881
(28) Hu,R.Z.;Gao,S.L.;Zhao,F.Q.;Shi,Q.Z.;Zhang,T.L.; Zhang,J.J.Thermal Analysis Kinetics;Science Press:Beijing, 2007.[胡榮祖,高勝利,趙風(fēng)起,史啟禎,張同來,張建軍.熱分析動(dòng)力學(xué).北京:科學(xué)出版社,2007.]
(29) Su,S.Q.;Chen,W.;Qin,C.;Song,S.Y.;Guo,Z.Y.;Li,G.H.; Song,X.Z.;Zhu,M.;Wang,S.;Hao,Z.M.;Zhang,H.J.Cryst. Growth Des.2012,12,1808.doi:10.1021/cg201283a
(30)Liu,M.S.;Yu,Q.Y.;Cai,Y.P.;Su,C.Y.;Lin,X.M.;Zhou,X. X.;Cai,J.W.Cryst.Growth Des.2008,8,4083.doi:10.1021/ cg800526y
(31) Li,Z.F.;Yu,J.B.;Zhou,L.;Zhang,H.J.;Deng,R.P.Inorg. Chem.Commun.2008,11,1284.doi:10.1016/j.inoche. 2008.08.008
(32)Huang,W.;Wu,D.Y.;Zhou,P.;Yan,W.B.;Guo,D.;Duan,C. Y.;Meng,Q.J.Cryst.Growth Des.2009,9,1361.doi:10.1021/ cg800531y

