噬藻體PaV-LD主要衣殼蛋白、穿孔素和內(nèi)肽酶基因的克隆及表達(dá)分析
李三華高惡斌歐 銅張奇亞
(1.中國(guó)科學(xué)院水生生物研究所, 淡水生態(tài)與生物技術(shù)國(guó)家重點(diǎn)實(shí)驗(yàn)室, 武漢 430072; 2.中國(guó)科學(xué)院大學(xué), 北京 100049)
噬藻體PaV-LD主要衣殼蛋白、穿孔素和內(nèi)肽酶基因的克隆及表達(dá)分析
李三華1,2高惡斌1歐 銅1,2張奇亞1,2
(1.中國(guó)科學(xué)院水生生物研究所, 淡水生態(tài)與生物技術(shù)國(guó)家重點(diǎn)實(shí)驗(yàn)室, 武漢 430072; 2.中國(guó)科學(xué)院大學(xué), 北京 100049)
最近闡明了水華藍(lán)藻噬藻體PaV-LD (Planktothrix agardhiiVirus isolated from Lake Donghu)的全基因組序列, 這是一個(gè)含有142個(gè)ORF的雙鏈DNA噬藻體。在此, 我們對(duì)其主要衣殼蛋白基因073R, 內(nèi)肽酶和穿孔素基因123L-124L(PaV-LD基因組中兩個(gè)相鄰的ORF)進(jìn)行了基因克隆與表達(dá)分析。將073R克隆后構(gòu)建原核表達(dá)質(zhì)粒pET-32a-073R, 并用IPTG進(jìn)行誘導(dǎo)表達(dá), 073R融合蛋白經(jīng)純化后, 進(jìn)行免疫小鼠制備抗體;通過Western blot檢測(cè)經(jīng)噬藻體感染宿主細(xì)胞后073R的表達(dá)時(shí)序, 結(jié)果顯示在宿主細(xì)胞裂解之初, 即PaV-LD感染48h以后073R開始表達(dá), 表明073R是一個(gè)晚期基因; 同時(shí)073R推導(dǎo)的氨基酸序列與34株噬藻(菌)體及2株藻病毒(感染真核藻的病毒)的主要衣殼蛋白的氨基酸序列進(jìn)行序列比對(duì), 顯示073R與無尾的藻病毒衣殼蛋白親緣關(guān)系更近。PCR擴(kuò)增內(nèi)肽酶和穿孔素基因123L-124L, 并構(gòu)建質(zhì)粒pOP123L-124L, 將其轉(zhuǎn)入模式藻集胞藻PCC6803細(xì)胞中, 質(zhì)粒pOP123L-124L與藻集胞藻PCC6803基因組發(fā)生重組, 形成重組藻; 測(cè)定了重組藻與野生藻的生長(zhǎng)速率, 并繪制生長(zhǎng)曲線; 制備超薄切片, 進(jìn)一步比較和觀察重組藻與野生藻的超微結(jié)構(gòu)的變化。結(jié)果顯示重組藻與野生藻存在生長(zhǎng)速率與超微形態(tài)的顯著差異。
水華藍(lán)藻噬藻體PaV-LD; 主要衣殼蛋白基因(073R); 內(nèi)肽酶基因(123L); 穿孔素基因(124L)
據(jù)報(bào)道, 浮游病毒是水生態(tài)環(huán)境中豐度最高的微生物, 它們有豐富的遺傳多樣性和廣泛的地域分布[1—9]。噬藻體是感染藍(lán)藻(藍(lán)細(xì)菌)的病毒, 作為浮游病毒的一個(gè)類群, 國(guó)內(nèi)外相關(guān)領(lǐng)域的學(xué)者已對(duì)其開展了廣泛研究[10—14]。
尤其是近些年, 關(guān)于我國(guó)最大的城中淺水型湖泊—武漢東湖中的浮游病毒形態(tài)多樣性、季節(jié)分布及宏基因組分析等已有系列報(bào)道[15—17]。經(jīng)多次分離和較長(zhǎng)時(shí)間培養(yǎng), 我們從東湖水樣中分離獲得了一株能夠感染水華藍(lán)藻的無尾噬藻體PaV-LD, 與已知有尾噬藻體目中的長(zhǎng)尾科(Siphoviridae)、肌尾科(Myoviridae)和短尾科(Podoviridae) 成員不相同, PaV-LD缺少尾部結(jié)構(gòu), 呈多面體形態(tài), 這也是無尾噬藻體的第一次報(bào)道[18]。從噬藻體PaV-LD全基因組序列中, 可知該噬藻體有142個(gè)潛在基因, 其中包括編碼主要衣殼蛋白基因073R、內(nèi)肽酶基因123L和穿孔素基因124L[19]。
通常病毒的主要衣殼蛋白基因是保守的結(jié)構(gòu)蛋白基因, 并且其決定了病毒的形態(tài), 在病毒顆粒的組裝及病毒侵染等過程中起著重要的作用[20—22]。已知內(nèi)肽酶是一種水解細(xì)菌(包括藍(lán)藻)細(xì)胞壁肽聚糖中間部分肽鏈的酶[23,24]; 而穿孔素是一種溶細(xì)胞素,能使細(xì)胞膜形成小孔, 從而引起細(xì)胞的裂解[25]。現(xiàn)已知在PaV-LD基因組中, 內(nèi)肽酶基因與穿孔素基因是連接在一起的, 形成 123L-124L。為深入認(rèn)識(shí)PaV-LD的結(jié)構(gòu)特征及基因功能, 我們對(duì)該噬藻體的073R、123L-124L分別進(jìn)行了克隆和表達(dá); 通過073R的原核表達(dá)及其抗體制備, 測(cè)定了噬藻體PaV-LD感染宿主細(xì)胞后主要衣殼蛋白基因的表達(dá)時(shí)序; 并進(jìn)一步分析了123L-124L表達(dá)產(chǎn)物對(duì)宿主細(xì)胞的生長(zhǎng)速率和超微形態(tài)的影響。
1 材料與方法
1.1 材料
噬藻體、菌株及載體質(zhì)粒 噬藻體PaV-LD是由本實(shí)驗(yàn)室從武漢東湖水樣中分離得到并保存[18]。噬藻體PaV-LD的擴(kuò)增、提純及基因組核酸的制備、以及感受態(tài)大腸桿菌(Escherichia coli) DH5α、BL21(DE3)的制備, 均采用常規(guī)方法[18,19]。pMD18-T克隆載體為TAKARA公司產(chǎn)品; 原核表達(dá)載體pET-32a(+)為Novagen公司產(chǎn)品。
酶和生化試劑 KpnⅠ和Hind III 限制性內(nèi)切酶、T4 DNA 連接酶、T4 DNA聚合酶購(gòu)自TAKARA公司; DNA Marker、Taq DNA 聚合酶購(gòu)自北京全式金生物技術(shù)有限公司; 蛋白分子量Marker購(gòu)自Fermentas公司; 醋酸纖維膜購(gòu)自MILIPORE公司; 堿性磷酸酶標(biāo)記的抗鼠IgG 二抗購(gòu)自VECTOR公司。PCR 凝膠回收試劑盒、質(zhì)粒提取試劑盒購(gòu)自TIAN GEN公司; 其余試劑均為國(guó)產(chǎn)分析純。
1.2 方法
073R的克隆及原核表達(dá)載體的構(gòu)建 利用引物073R-F/R(引物序列見表 1), 以噬藻體PaV-LD基因組作為模板進(jìn)行PCR擴(kuò)增073R, PCR 反應(yīng)體系: 滅菌雙蒸水17 μL, 10×Taq Buffer (不含 Mg2+) 2.5 μL, 25mmol/L MgCl22.5 μL, dNTP混合物0.5 μL,上下游引物各0.5 μL, Taq 聚合酶 0.5 μL, PaV-LD基因組核酸1 μL。PCR 反應(yīng)程序: 94℃預(yù)變性4min, (94℃ 30s, 53℃ 30s, 72℃ 90s, 30個(gè)循環(huán)), 最后72℃延伸10min。
擴(kuò)增產(chǎn)物經(jīng)1%瓊脂糖凝膠進(jìn)行電泳, 將回收后的目的基因片段與克隆載體pMD18-T連接, 構(gòu)成重組質(zhì)粒pMD18-T-073R, 再對(duì)大腸桿菌DH5α感受態(tài)細(xì)胞進(jìn)行轉(zhuǎn)化后, 用質(zhì)粒提取試劑盒提取重組質(zhì)粒, 并對(duì)其進(jìn)行PCR鑒定和基因全長(zhǎng)測(cè)序。重組質(zhì)粒pMD18-T-073R中主要衣殼蛋白基因片段經(jīng)KpnⅠ和Hind III 雙酶切, 回收后定向插入經(jīng)同樣雙酶切過的原核表達(dá)載體pET-32a(+)中, 構(gòu)建原核表達(dá)質(zhì)粒pET-32a-073R, 再轉(zhuǎn)化大腸桿菌BL21 (DE3)感受態(tài)細(xì)胞。用質(zhì)粒提取試劑盒提取質(zhì)粒, 經(jīng)雙酶切后, 電泳驗(yàn)證。
073R原核誘導(dǎo)表達(dá)、蛋白純化及抗血清制備將驗(yàn)證后正確的陽性克隆接種至200 mL Amp+LB培養(yǎng)基中37℃(225rpm/min)培養(yǎng)至A600約為0.6時(shí),加入IPTG(使終濃度為1 mmol/L)誘導(dǎo), 5h后, 經(jīng)超聲波破碎, 然后利用 Ni-NTA樹脂親和層析純化融合蛋白[26]。
將純化的融合蛋白免疫BALB/C小鼠制備抗體,先取純化蛋白400 μL(約240 μg)與400 μL完全佐劑充分混勻乳化, 腹腔注射; 7d后加強(qiáng)免疫, 共4次,每次間隔7d, 均取純化蛋白400 μL與等體積的不完全佐劑混合乳化, 腹腔注射; 第5次免疫后一周取血, 將血于室溫放置30min或4℃靜置6h, 2000 r/min離心2min, 吸取上層, 分裝后于?80℃保存?zhèn)溆谩?/p>
073R表達(dá)時(shí)序的Western blotting分析 先取對(duì)數(shù)期藻置于細(xì)胞六板孔內(nèi), 然后接種PaV-LD病毒懸液(按體積比1∶5), 同時(shí)以等體積的BG11培養(yǎng)基取代病毒懸液設(shè)為對(duì)照, 于25℃光照培養(yǎng)[27]。分別在0h、12h、24h、36h、48h、60h、72h、84h、96h收集感染PaV-LD的藻液, 經(jīng)28000 r/min 離心1h,沉淀用50 μL TE(pH 8.0)重懸, 加50 μL 蛋白上樣緩沖液, 沸水煮5min, 用5%的濃縮膠, 12%的分離膠進(jìn)行SDS-PAGE電泳, 轉(zhuǎn)膜后用含5%脫脂奶粉的TBS (0.02 mol/L Tris-HCl pH 7.4; 154 mmol/L NaCl)孵育1h, 然后用含有0.1%吐溫?20的TBS-T洗3次后, 分別用制備的小鼠血清(PBS 1∶500稀釋)、堿性磷酸酶標(biāo)記的羊抗兔IgG(PBS 1∶1000稀釋)孵育1h, 用TBS-T洗三次后, 采用NBT/BCIP顯色試劑盒進(jìn)行顯色, 隨時(shí)觀察顯色情況, 最后用雙蒸水終止反應(yīng)[28]。
073R的進(jìn)化分析 采用軟件DNA Star Lasergene 7.1, 對(duì)073R推導(dǎo)的氨基酸序列與來自GeneBank數(shù)據(jù)庫(kù)的34株噬藻(菌)體及2株藻病毒的主要衣殼蛋白基因進(jìn)行序列比對(duì)分析, 利用MEGA4軟件作進(jìn)化分析和構(gòu)建進(jìn)化樹。
124L-123L的PCR擴(kuò)增、重組與轉(zhuǎn)染質(zhì)粒的構(gòu)建及模式藻轉(zhuǎn)化 利用引物124L-F和123L-R(引物序列見表1)以PaV-LD核酸為模板, 擴(kuò)增124L-123L, PCR反應(yīng)體系及反應(yīng)程序參見前面所述(稍有變動(dòng): 54℃退火30s, 72℃延伸1min);電泳回收后將上述兩個(gè)基因插入到pMD18-T載體中, 構(gòu)成質(zhì)粒pT-124L-123L。然后將含有壯觀霉素抗性基因(Omega)和啟動(dòng)子(PpetE)的核酸片段Omega-PpetE組裝到質(zhì)粒pT-124L-123L中, 位于124L上游, 構(gòu)成重組質(zhì)粒pT-Omega-PpetE-124L-123L。然后利用EcoRⅠ和Hind Ⅲ從重組質(zhì)粒中切下Omega-PpetE- 124L-123L片段, 補(bǔ)平后定點(diǎn)插入到載體質(zhì)粒pKW1188所攜帶的基因slr0168中間, 構(gòu)成轉(zhuǎn)化質(zhì)粒pOP124L-123L。
集胞藻PCC6803的轉(zhuǎn)化: 收集A730為0.8左右的藻細(xì)胞, 重懸后與轉(zhuǎn)染質(zhì)粒pOP124L-123L混合,于光照條件下25℃孵育4h, 然后均勻涂布在覆有硝酸纖維素濾膜的BG11平板上; 光照培養(yǎng)24h后, 將濾膜轉(zhuǎn)移至含有10 μg/mL 壯觀霉素和50 mmol/L葡萄糖的BG11平板上再培養(yǎng)[29]。轉(zhuǎn)染質(zhì)粒進(jìn)入藻后, 與之基因組發(fā)生重組形成重組藻, 從長(zhǎng)出的重組藻中, 篩選克隆, 繼續(xù)培養(yǎng)并進(jìn)一步分析。
重組藻的檢測(cè)與分析 (1)PCR擴(kuò)增。先制備作為模板的野生藻核酸、重組藻核酸及重組質(zhì)粒pOP124L-123L[18,19], 用啟動(dòng)子PpetE上游引物PpetE-F與124L下游引物124L-R、123L基因下游引物 123L-R和slr0168基因上游引物slr0168-F分別組成的三對(duì)檢測(cè)引物(引物序列見表1), 通過PCR擴(kuò)增, PCR產(chǎn)物經(jīng)1%瓊脂糖電泳分析。(2)生長(zhǎng)速率測(cè)定。配制缺銅的BG11液體培養(yǎng)基, 分別用該培養(yǎng)基將野生藻及重組藻初始濃度調(diào)至A730為0.009; 然后在25℃、光照[~35μE/(m2·s)]條件下添加400 nmol/L Cu2+誘導(dǎo), 另外, 在重組藻培養(yǎng)基中加入10 μg/mL 壯觀霉素, 以抑制野生藻的生長(zhǎng)。隨后每天取樣(共培養(yǎng)測(cè)定22d), 并通過分光光度計(jì)測(cè)定其A730值, 指示藻細(xì)胞的濃度; 以時(shí)間為橫坐標(biāo), 以A730值為縱坐標(biāo)繪制生長(zhǎng)曲線。(3)透射電鏡觀察。分別收集培養(yǎng)20d的野生及重組藻細(xì)胞, 用2.5%戊二醛固定后按照實(shí)驗(yàn)室常規(guī)方法制備超薄切片[18];利用JEOL 1230型透射電鏡于80kV條件下進(jìn)行超微觀察。

表1 本研究所用引物及序列(5′—3′)Tab.1 Sequences of the primers used in this study (5′—3′)
2 結(jié)果
2.1 獲得073R 的克隆與原核表達(dá)質(zhì)粒pET-32a-073R
以PaV-LD基因組為模板, 使用引物073R-F/R通過PCR的方法擴(kuò)增到特異的073R, 其分子量大小約1 kb。將這條核酸帶切膠回收后, 連接到克隆載體pMD18-T上, 經(jīng)PCR和雙酶切鑒定, 得到陽性克隆。序列測(cè)定表明pMD18-T-073R中插入基因的序列與PaV-LD 073R 序列一致。
原核表達(dá)質(zhì)粒pET-32a-073R經(jīng)KpnⅠ和Hind III雙酶切驗(yàn)證。同時(shí)對(duì)PCR檢測(cè)的陽性克隆作進(jìn)一步測(cè)序分析, 結(jié)果質(zhì)粒pET-32a-073R中插入的基因序列與073R的序列完全一致, 顯示已成功構(gòu)建PaV-LD 073R的原核表達(dá)質(zhì)粒。
2.2 073R原核表達(dá)產(chǎn)物
將誘導(dǎo)pET-32a-073R表達(dá)的樣品進(jìn)行SDSPAGE電泳分析。出現(xiàn)一條較高濃度的誘導(dǎo)表達(dá)融合蛋白帶(53 kD), 其中包括載體表達(dá)蛋白約17 kD及073R約36 kD, 但在未誘導(dǎo)的樣品中沒有相應(yīng)的蛋白帶, 經(jīng)離子交換樹脂親和層析柱后,得到純化的53 kD融合蛋白帶(圖1)。提純的融合蛋白用于免疫小鼠,制備鼠抗 PaV-LD 073R血清。
2.3 073R表達(dá)時(shí)序
利用上述制備的抗血清, 對(duì)感染PaV-LD后不同時(shí)間制備的藻細(xì)胞蛋白進(jìn)行Western blotting 分析, 檢測(cè)感染過程中 PaV-LD 073R 在宿主細(xì)胞內(nèi)的表達(dá)時(shí)序。結(jié)果顯示: 在PaV-LD感染48h后, 36 kD的特異條帶開始有微弱表達(dá); 60h至84h, 其表達(dá)量顯著增強(qiáng); 而在96h有所減弱(圖2A)。
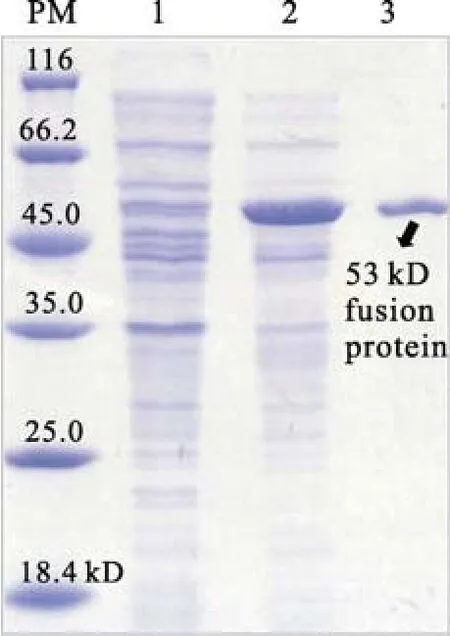
圖 1 PaV-LD 073R 原核表達(dá)產(chǎn)物SDS-PAGE分析Fig.1 Analysis the prokaryotic expression of PaV-LD 073R gene by SDS-PAGE
另外, 利用顯微鏡觀察感染噬藻體后宿主藻的形態(tài)變化, 可見在PaV-LD感染48h后, 宿主藻的絲狀體斷裂增多, 并且隨著感染時(shí)間的延長(zhǎng), 裂解量和裂解程度不斷增加, 至96h絲狀體完全斷裂消失(圖2B)。可見073R是在宿主藻細(xì)胞發(fā)生裂解時(shí)才表達(dá), 這顯示073R是在PaV-LD的感染晚期表達(dá)。
2.4 073R序列進(jìn)化地位
073R全長(zhǎng)954 bp,共編碼317個(gè)氨基酸, 與其他的34株噬藻(菌)體及2株藻病毒(感染真核藻的病毒)的主要衣殼蛋白氨基酸進(jìn)行序列比對(duì), 并借助軟件MEGA4構(gòu)建Neighbor-Joining系統(tǒng)進(jìn)化樹。結(jié)果顯示噬藻體PaV-LD 073R與有尾噬藻(菌)體 Siphoviridae, Myoviridae, Podoviridae成員的同源性不高,而與無尾藻病毒PBCV-1, EhV86的親緣關(guān)系相距較近(圖 3)。
2.5 重組質(zhì)粒pOP124L-123L與重組藻的鑒定
對(duì)重組藻與野生藻進(jìn)行PCR檢測(cè), PCR產(chǎn)物電泳結(jié)果如圖4A所示, 條帶大小與預(yù)期一致, 除在轉(zhuǎn)化質(zhì)粒pOP124L-123L中檢測(cè)到啟動(dòng)子PpetE、123L、124L、基因slr0168的N端外, 在重組藻也能檢測(cè)到上述基因片段的存在, 而在野生藻中則沒有檢測(cè)到,說明片段Omega-PpetE-124L-123L成功經(jīng)同源重組定向插入到集胞藻PCC6803基因組slr0168基因中;另外, 引物PpetE-F、124L-R、123L-R和slr0168-F的位置如圖4B所示, PCR擴(kuò)增結(jié)果也說明片段插入方向正確, 即PpetE啟動(dòng)子和Omega在基因124L的上游。圖4B顯示了將轉(zhuǎn)化質(zhì)粒pOP124L-123L轉(zhuǎn)入野生藻發(fā)生同源重組后的重組藻染色體結(jié)構(gòu)示意圖。
2.6 重組藻的生長(zhǎng)速率與超微形態(tài)發(fā)生變化
通過測(cè)定, 野生藻從培養(yǎng)第6天開始生長(zhǎng)迅速加快, 在第6至第18天期間A730近乎呈直線上升,從0.204上升到1.181, 直至第19天達(dá)到最大值1.225, 其后生長(zhǎng)速度減緩, 趨于平穩(wěn)。而同時(shí)重組藻從第6天開始雖然生長(zhǎng)速度也明顯加快, 但在第6至第18天期間A730僅從0.167升到0.698, 直至第21天才達(dá)到其最大值0.737, 顯然重組藻較之野生藻生長(zhǎng)速度明顯變慢(圖5)。
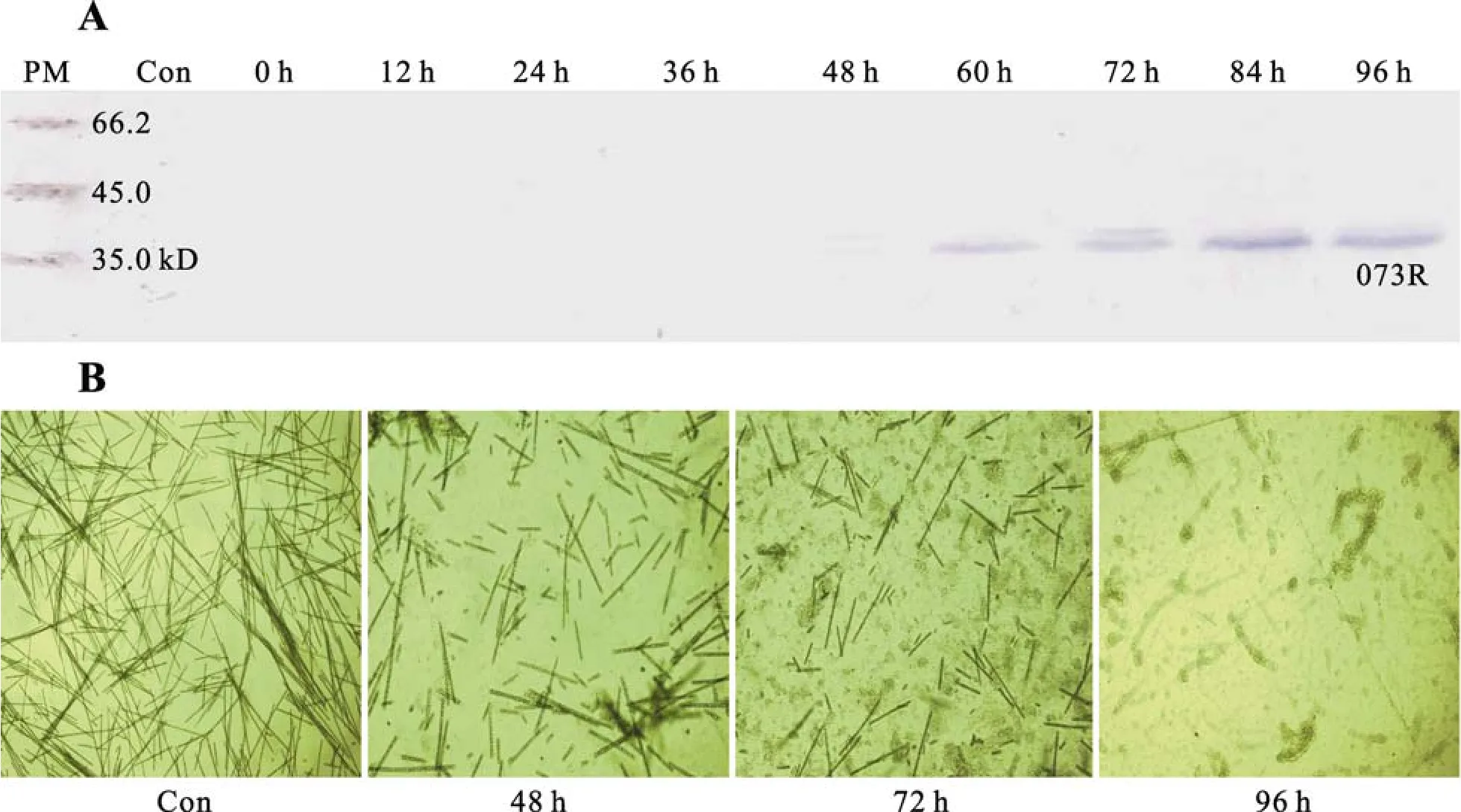
圖2 Western blot 分析 073R 的表達(dá)時(shí)序及宿主藻感染PaV-LD后不同時(shí)間顯微圖Fig.2 Analysis of the temporal expression of the 073R gene by Western blot and optical microphotographs of host algae cultures after infected by PaV-LD

圖3 073R編碼蛋白 (星號(hào), PaV-LD) 與有尾噬藻(菌)體及無尾藻病毒主要衣殼蛋白(MCP)的系統(tǒng)進(jìn)化分析Fig.3 Phylogenetic analysis of 073R encoding proteins (star, PaV-LD), MCP of tailed cyanophages (or phages) and tailless phycovirus
超微觀察顯示, 野生藻細(xì)胞外周呈現(xiàn)電子密度較低的細(xì)胞壁是清晰完整的(圖6 Wt); 而重組藻部分細(xì)胞壁則很模糊甚至消失, 且細(xì)胞原生質(zhì)體局部腫脹, 細(xì)胞內(nèi)容物有溢出趨勢(shì)(圖6 Re中箭頭所示)。表明重組藻這部分細(xì)胞壁已受損或溶解。
3 討論

圖4 PCR鑒定重組藻的電泳圖和重組藻染色體結(jié)構(gòu)示意圖Fig.4 Confirmation of recombinant Synechocystis sp.PCC6803 by PCR and chromosome structure of the recombinant
衣殼是病毒的蛋白質(zhì)外殼, 由于主要衣殼蛋白基因具有結(jié)構(gòu)的相對(duì)穩(wěn)定性和序列的保守性, 使人們一直將其作為病毒分類的重要參考[30]。近年來,由于主要衣殼蛋白基因在噬藻體進(jìn)化關(guān)系和多樣性分析方面的靈敏和可靠性, 常被作為標(biāo)志基因[31,32]。本研究對(duì)PaV-LD 073R的進(jìn)化分析顯示, PaV-LD與已知有尾噬藻(菌)體親緣關(guān)系較遠(yuǎn), 而與無尾藻病毒的親緣關(guān)系相對(duì)較近, 這與電鏡觀察到的無尾形態(tài)學(xué)特征相吻合。這表明PaV-LD的進(jìn)化地位居于原核噬藻體與真核藻病毒之間, 且推測(cè)衣殼蛋白基因的進(jìn)化可能與噬藻體有無尾巴關(guān)系密切。
病毒感染宿主后, 要通過基因組的表達(dá)完成自身所需生物大分子的合成, 且基因表達(dá)按一定時(shí)間程序展現(xiàn), 因此了解噬藻體基因的表達(dá)時(shí)序有助于揭示這類病毒生命周期及與宿主的相互作用[33,34]。在原核表達(dá)073R并制備抗體的基礎(chǔ)上, 我們采用Western blotting與顯微觀察相結(jié)合的方法, 明確了073R起始表達(dá)的時(shí)間與宿主藻絲的裂解時(shí)間同步,即073R在其宿主藻絲狀體斷裂后才能檢測(cè)到有所表達(dá), 顯示073R是PaV-LD的晚期表達(dá)基因。
一般認(rèn)為, 噬菌體要感染細(xì)胞就要穿過細(xì)胞壁將其核酸注入宿主細(xì)胞內(nèi), 這個(gè)過程需要具有肽聚糖水解活性的酶及穿孔素等發(fā)揮作用[23], 而大多數(shù)噬菌體至少需要細(xì)胞內(nèi)溶素和穿孔素兩種蛋白才能裂解宿主細(xì)胞[35], 使子代噬菌體能順利釋放。有證據(jù)表明, 來源于噬菌體P22的細(xì)胞溶解素和穿孔素能成功裂解集胞藻PCC6803[36]。由于PaVLD的自然宿主藻尚無現(xiàn)成的遺傳操作系統(tǒng),在此借用模式藍(lán)藻集胞藻PCC6803表達(dá)PaV-LD 的基因。在獲得含123L-124L重組藻的基礎(chǔ)上, 分析結(jié)果顯示, 與野生藻相比,重組藻的生長(zhǎng)速率及細(xì)胞壁的超微形態(tài)都發(fā)生顯著變化。已有報(bào)道介紹, 在實(shí)驗(yàn)室條件下, 當(dāng)外源基因插入slr0168基因中, 檢測(cè)不到其對(duì)集胞藻PCC6803有影響[29]。這就表明重組藻細(xì)胞壁受損是由于轉(zhuǎn)入了噬藻體PaV-LD 的123L-124L所致, 且這兩個(gè)基因確實(shí)發(fā)揮了內(nèi)肽酶與穿孔素的作用, 從而導(dǎo)致重組藻細(xì)胞壁損傷或溶解, 并使重組藻細(xì)胞生長(zhǎng)速率減緩。
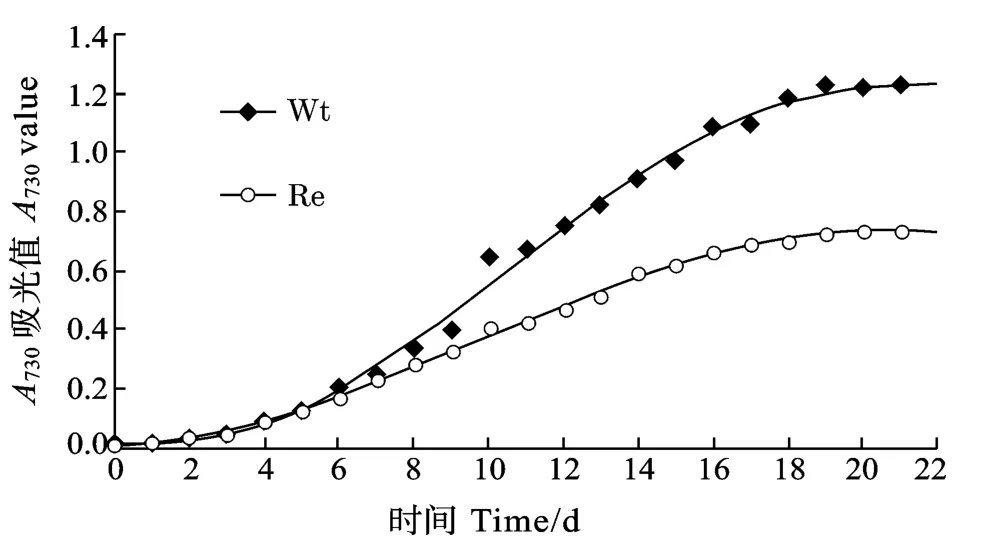
圖5 野生藻和重組藻的生長(zhǎng)速率Fig.5 Growth curves of wild type and the Recombinant of Synechocystis sp.PCC6803

圖 6 野生藻和重組藻的生長(zhǎng)速率及超微形態(tài)Fig.6 Growth curves and ultramicroscopic morphology of Wild type and the Recombinant of Synechocystis sp.PCC6803
水生病毒學(xué)研究的對(duì)象包括水生動(dòng)物病毒和藍(lán)藻病毒 (噬藻體) 等。近期, 有專家指出, 我國(guó)水產(chǎn)動(dòng)物病毒病原基因組及其功能基因的研究快速發(fā)展,因此為獲得水產(chǎn)動(dòng)物抗病性狀和候選抗病相關(guān)基因奠定了基礎(chǔ)[37]。盡管與水產(chǎn)動(dòng)物病毒學(xué)研究相比,我國(guó)噬藻體基因組及功能基因的相關(guān)研究尚在起步階段, 但本研究提示: 通過基因組及功能基因研究,不僅有益于人們了解噬藻體的侵染機(jī)制, 也將有助于噬藻體重要功能基因的發(fā)現(xiàn), 并就此可進(jìn)一步研發(fā)噬藻體控藻技術(shù)。
致謝:
質(zhì)粒T-Omega-PpetE 和pKW1188由中國(guó)科學(xué)院水生生物研究所徐旭東研究員實(shí)驗(yàn)室惠贈(zèng), 謹(jǐn)致謝忱。
[1] Zhang Q Y.Virioplankton [J].Acta Hydrobiologica Sinica, 2002, 26(6): 691—696 [張奇亞.浮游病毒.水生生物學(xué)報(bào), 2002, 26(6): 691—696]
[2] Sullivan M B, Waterbury J B, Chisholm S W.Cyanophages infecting the oceanic cyanobacterium Prochlorococcus [J].Nature, 2003, 424(6952): 1047—1051
[3] Hambly E, Suttle C A.The viriosphere, diversity, and genetic exchange within phage communities [J].Current Opinion in Microbiology, 2005, 8(4): 444—450
[4] Suttle C A.Marine viruses—major players in the global ecosystem [J].Nature Reviews Microbiology, 2007, 5(10): 801—812
[5] Zhang Q Y, Gui J F.A kind of strategic bio-resource not to be neglected—freshwater and marine viruses and their roles in the global ecosystem [J].Bulletin of the Chinese Academy of Sciences, 2009, 24(4): 414—420 [張奇亞, 桂建芳.一類不可忽視的戰(zhàn)略生物資源——淡水與海水中的病毒及其在生態(tài)系統(tǒng)中的作用.中國(guó)科學(xué)院院刊, 2009, 24(4): 414—420]
[6] Liu X, Zhang Q, Murata K, et al.Structural changes in a marine podovirus associated with viral genome release into Prochlorococcus [J].Nature Structural & Molecular Biology, 2010, 17(7): 830—836
[7] Fischer M G, Suttle C A.A virophage at the origin of large DNA transposons [J].Science, 2011, 332(6026): 231—234
[8] Pope W H, Jacobs-Sera D, Russell D A, et al.Expanding the Diversity of Mycobacteriophages: Insights into Genome Architecture and Evolution [J].PloS One, 2011, 6(1): e16329
[9] Thompson L R, Zeng Q, Kelly L, et al.Phage auxiliary metabolic genes and the redirection of cyanobacterial host carbon metabolism [J].Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(39): E757—E764
[10] Zhao Y J, Cheng K, Shi Z L, et al.Isolation and identification of the first cyanophage in China [J].Progress in Nature Science, 2002, 12(9): 923—927 [趙以軍, 程凱, 石正麗, 等.我國(guó)首株噬藻體(藍(lán)藻病毒)的分離與鑒定.自然科學(xué)進(jìn)展, 2002, 12(9): 923—927]
[11] Yoshida M, Yoshida T, Kashima A, et al.Ecological dynamics of the toxic bloom-forming cyanobacterium Microcystis aeruginosa and its cyanophages in freshwater [J].Applied and Environmental Microbiology, 2008, 74(10): 3269—3273
[12] Gao E B, Li S H, Lü B, et al.Analysis of the cyanophage (PaV-LD) infection in host cyanobacteria under different culture conditions [J].Acta Hydrobiologica Sinica, 2012, 36(3): 420—425 [高惡斌, 李三華, 呂波, 等.水華藍(lán)藻噬藻體對(duì)不同條件培養(yǎng)的宿主細(xì)胞感染性分析.水生生物學(xué)報(bào), 2012, 36(3): 420—425]
[13] Sabehi G, Shaulov L, Silver D H, et al.A novel lineage of myoviruses infecting cyanobacteria is widespread in the oceans [J].Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(6): 2037-2042
[14] Liu L, Guo Z L, Huang P, et al.Isolation and identification of a cyanophage in Lake Taihu [J].Acta Hydrobiologica Sinica, 2012, 36(2): 339—343 [劉露, 郭宗樓, 黃樸, 等.一株太湖水域藍(lán)藻噬藻體的分離與鑒定.水生生物學(xué)報(bào), 2012, 36(2): 339—343]
[15] Liu Y M, Zhang Q Y, Yuan X P.Studies on abundance and morphological diversity of virioplankton in the Donghu Lake, Wuhan [J].Acta Hydrobiologica Sinica, 2005, 29(1): 1—6 [劉艷鳴, 張奇亞, 袁秀平.武漢東湖浮游病毒的豐度及多樣性.水生生物學(xué)報(bào), 2005, 29(1): 1—6]
[16] Liu Y M, Zhang Q Y, Yuan X P, et al.Seasonal variation of virioplankton in a eutrophic shallow lake [J].Hydrobiologia, 2006, 560(1): 323—334
[17] Liu Y M, Yuan X P, Zhang Q Y.Spatial distribution and morphologic diversity of virioplankton in Lake Donghu, China [J].Acta Oecologica-International Journal of Ecology, 2006, 29: 328—334
[18] Gao E B, Yuan X P, Li R H, et al.Isolation of a novel cyanophage infectious to the filamentous cyanobacterium Planktothrix agardhii (Cyanophyceae) from Lake Donghu, China [J].Aquatic Microbial Ecology, 2009, 54(2): 163—170
[19] Gao E B, Gui J F, Zhang Q Y.A Novel Cyanophage with a Cyanobacterial Nonbleaching Protein A Gene in the Genome [J].Journal of Virology, 2012, 86(1): 236—245
[20] Bowman B R, Baker M L, Rixon F J, et al.Structure of the herpesvirus major capsid protein [J].The EMBO Journal, 2003, 22(2): 757—765
[21] Chen R, Neill J D, Noel J S, et al.Inter-and intragenus structural variations in calicivimses and their functional implications [J].Journal of Virology, 2004, 78(12): 6469—6479
[22] Pope W H, Weigele P R, Chang J, et al.Genome Sequence, Structural Proteins, and Capsid Organization of the Cyanophage Syn5: A “Horned” Bacteriophage of Marine Synechococcus [J].Journal of Molecular Biology, 2007, 368(4): 966—981
[23] Moak M, Molineux I J.Peptidoglycan hydrolytic activities associated with bacteriophage virions [J].Molecular Microbiology, 2004, 51(4): 1169—1183
[24] Liu X, Kong S, Shi M, et al.Genomic analysis of freshwater cyanophage Pf-WMP3 infecting cyanobacterium Phormidium foveolarum: the conserved elements for a phage [J].Microbial Ecology, 2008, 56(4): 671—680
[25] Gründling A, Manson M D, Young R.Holins kill without warning [J].Proceedings of the National Academy of Sciences of the United States of America, 2001, 98(16): 9348— 9352
[26] He L B, Ke F, Zhang Q Y.Cloning, expression and localization analysis of a sequence conserved gene (RGV-12L) from Rana grylio virus [J].Acta Hydrobiologica Sinica, 2010, 34(6): 1166—1171 [何利波, 柯飛, 張奇亞.蛙虹彩病毒一個(gè)序列保守基因(RGV-12L)的克隆表達(dá)及定位分析.水生生物學(xué)報(bào), 2010, 34(6): 1166—1171]
[27] Gao E B, Li S H, Lü B, et al.The analysis of the cyanophage (PaV-LD) infection in host cyanobacteria under different culture conditions [J].Acta Hydrobiologica Sinica, 2012, 36(3): 420—425 [高惡斌, 李三華, 呂波, 等.水華藍(lán)藻噬藻體對(duì)不同條件培養(yǎng)的宿主細(xì)胞感染性分析.水生生物學(xué)報(bào), 2012, 36(3): 420—425]
[28] Chen Z Y, Liu H, Li Z Q, et al.Development and characterization of monoclonal antibodies to spring viraemia of carp virus [J].Veterinary Immunology and Immunopathology, 2008, 123 (3-4): 266—276
[29] Gao H, Tang Q, Xu X D.Construction of copper-induced gene expression platform in Synechocystis sp.6803 [J].Acta Hydrobiologica Sinica, 2007, 31(2): 240—243 [高宏, 唐蜻,徐旭東.集胞藻 PCC6803 銅離子誘導(dǎo)表達(dá)平臺(tái)的構(gòu)建.水生生物學(xué)報(bào), 2007, 31(2): 240—243]
[30] Zhan Q Y, Xiao F, Li Z Q, et al.Characterization of an iridovirus from the cultured pig frog Rana grylio with lethal syndrome [J].Diseases of Aquatic Organisms, 2001, 48(1): 27—36
[31] Larsen J B, Larsen A, Bratbak G, et al.Phylogenetic analysis of members of the phycodnaviridae virus family, using amplified fragments of the Major Capsid Protein gene [J].Applied and Environmental Microbiology, 2008, 74(10): 3048—3057
[32] Rowe J M, Fabre M F, Gobena D, et al.Application of the major capsid protein as a marker of the phylogenetic diversity of Emiliania huxleyi viruses [J].FEMS Microbiology Ecology, 2011, 76(2): 373—390
[33] Lindell D, Jaffe J D, Johnson Z I, et al.Photosynthesis genes in marine viruses yield proteins during host infection [J].Nature, 2005, 438(7064): 86—89
[34] Ferrer M D, Quiles-Puchalt N, Harwich M D, et al.RinA controls phage-mediated packaging and transfer of virulence genes in Gram-positive bacteria [J].Nucleic Acids Research, 2011, 39(14): 5866—5878
[35] Wang I N, Smith D L, Young R.Holins: The protein clocks of bacteriophage infections [J].Annual Review Microbiology, 2000, 54(1): 799—825
[36] Liu X, Curtiss R 3rd.Nickel-inducible lysis system in Synechocystis sp.PCC6803 [J].Proceedings of the National Academy of Sciences of the United States of America, 2009, 106(51): 21550—21554
[37] Gui J F, Zhu Z Y, Molecular basis and genetic improvement of economically important traits in aquaculture animals [J].Chinese Science Bulletin, 2012, 57(15): 1751—1760
CLONING AND EXPRESSION ANALYSIS OF MAJOR CAPSID PROTEIN GENE, ENDOPEPTIDASE AND HOLIN GENE OF CYANOPHAGE PAV-LD
LI San-Hua1,2, GAO E-Bin1, OU Tong1,2and ZHANG Qi-Ya1,2
(1.State Key Laboratory of Freshwater Ecology and Biotechnology, Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan 430072, China; 2.University of Chinese Academy of Sciences, Beijing 100049, China)
he 142 open reading frame (ORF) of the genome of cyanophage, PaV-LD (Planktothrix agardhiivirus isolated from the Lake Donghu), was elucidated recently.However, the characteristic of genomic, phylogenetic position, and mechanism of infection remained to be examined on PaV-LD, a novel tailless caynophage.With the relatively stable structure and evolutionarily conserved sequence, the major capsid protein (MCP) gene has been an important reference in the classification of these viruses.Endopeptidase and holin were considered to play an important role in the progress of invasion and release of viruses.The MCP gene (073R), endopeptidase gene (123L), and holin gene (124L) of cyanophage PaV-LD were cloned and the expressions were analysed.PaV-LD073Rwas amplified and cloned, and the prokaryotic expression plasmid pET-32a-073R was constructed.The prokaryotic expression products of PaV-LD073Rwas analyzed by SDS-PAGE after being induced with isopropyl beta-D-thiogalactopyranoside (IPTG).The fusion protein was purified via affinity chromatography and was then used to immunize BALB/C mice to prepare the antiserum against PaV-LD 073R.Next the antiserum was used for Western blotting analysis and the temporal expression pattern of PaV-LD073Rwas characterized during PaV-LD infection at different times.The results of western blotting indicated that the MCP was initially expressed at 48h post-infection (p.i.) and developed into particularly enhanced from 60h p.i.to 84h p.i.The data also demonstrated that073Rwas a late expression gene of the tailless cyanophage genome.To further investigate the phylogenetic position of PaV-LD, a phylogenetic tree was constructed using the Neighbor-Joining method with 34 tailed cyanophages and 2 phycoviruses (viruses that infect eukaryotic algae).The analysis of the phylogenetic tree revealed that the amino acid sequences in PaV-LD 073R grouped more closely with tailless phycoviruses instead of the tailed cyanophages, indicating that PaV-LD had the morphological characteristics of the tailless phycoviruses.PaV-LD endopeptidase gene and holin gene (two adjacent ORFs in the genome of PaV-LD,123L-124L) were amplified using polymerase chain reaction (PCR) and the plasmid contained a spectinomycin resistance gene, then the promoter genes pPetE and 123L-124L were constructed and integrated into the genome of Synechocystis sp.PCC6803 via homologous recombination.The growth curves of the recombinant and the wild type of Synechocystis sp.PCC 6803 were drawn, and the growth rate of the recombinant was found to be significantly slower than the wild type.Ultrastructural morphology observation with transmission electron microscopy showed that the cell wall of recombinant was damaged partly or fully, causing protoplast swelling and the leakage of cell contents.Results suggested that PaV-LD 123L-124L could express the functional proteins that played a role in dissolving cell wall and slowing down cell growth in the recombinant Synechocystis sp.PCC 6803.
Cyanophage infecting bloom-forming cyanobacteria PaV-LD; Major capsid protein gene (073R); Endopeptidase gene (123L); Holin gene (124L)
X179
A
1000-3207(2013)02-0252-09
10.7541/2013.12
2012-03-27;
2012-10-09
中國(guó)科學(xué)院先導(dǎo)專項(xiàng)項(xiàng)目(KSCX2-EW-Z-3); 國(guó)家自然科學(xué)基金項(xiàng)目(31072239); 淡水生態(tài)與生物技術(shù)國(guó)家重點(diǎn)實(shí)驗(yàn)室基金(2011FBZ12)資助
李三華(1987—), 男, 山西人; 碩士研究生; 從事水生病毒及分子生物學(xué)研究。E-mail: lisanhua1987@163.com
張奇亞, Tel: +86-027-68780792; E-mail: zhangqy@ihb.ac.cn

