Experimental Investigation to Evaluate LiFePO4Batteries Anode and Cathode Elastic Properties under Cyclic Temperature Loading Conditions
Sergey Verlinski,Nimitt Patel,Tyler Arsenault,Philip Yuya,Pier Marzocca
1.Faculty of Mechanics and Mechanical Engineering,State Engineering University of Armenia,Yerevan,0009,Republic of Armenia;2.Mechanical and Aeronautical Engineering Department,Clarkson University,New York,13699,USA
1 Introduction
The need to develop and deploy large-scale,cost-effective,renewable energy is becoming increasingly important.Lithium-ion batteries are one of the most popular types of rechargeable battery for portable electronics.Developing technologies to produce flexible batteries with good performance in combination with high specific strength is strongly desired[1].Various approaches to fabricate structural electrodes to enhance the mechanical properties have been reported in Ref.[1].When blends of traditional electrode with other materials are used,as is commonly the case,device performance directly depends on the nanoscale morphology and phase separation of the blend components.Both anode and cathode materials are layered structures,which allow lithium ions to stay in or pass through them.On the cathode side,intercalated lithium compound such as Lithium iron phosphate (LiFePO4),lithium manganese oxide(LiMn2O4),and lithium cobalt oxide(LiCoO2)are commonly used.LiFePO4is a promising candidate for high-energy-density low-cost batteries.This material has numerous advantages,among which,it is environmentally friendly and has minimal hazard with significantly higher safety[1-5].Synthesis and electrochemical properties of LiFePO4and LiFePO4/C composite powders were investigated.The study involved the evaluation of temperature effect on specific capacity of battery and dependence on firing temperature at three temperatures associated with voltages varying between 2.5Vand 4.3V.However,the cyclic charge/discharge process of a battery occurs between different temperatures,therefore the mechanical properties are also expected to change significantly within the operational range of temperatures,from room temperature to 600°C.LixFePO4(0<x<1)is phase separating at room temperature and undergoes a phase transformation between heterosite FePO4and triphylite LiFePO4during the charge and discharge processes.
Nanoindentation method is extensively used to characterize the mechanical behavior of small volumes of material with spatial resolutions in the range from nanometer to micrometer.The technique relies on the local deformation induced on a material′s surface with an indenter of known properties under the application of a given load.The load applied on the indenter and the corresponding displacement of penetrated indenter into a sample is continuously monitored during the loading and unloading processes.In Lithiumion batteries,the technique has been used to study the changes in mechanical properties due to phase transformation of anode coating[6-9].In order to better control the thermodynamical properties determining its electrochemical performance,a better understanding of its elastic properties is needed.At present,experimental data on mechanical properties such as elastic constants or bulk moduli are not available since LixFePO4is usually synthesized as sintered powder and the growth of larger crystals is known to be very difficult.To fulfill the gap in knowledge currently existing in lithium-ion battery material properties,the objective of this work is to apply characterization technique based on nanoindentation(NI)and scanning electron microscopy(SEM)methods to understand their nonlinear mechanical characteristics behavior and their primary dependence on temperature,which changes during charge/discharge process.While such materials could be exposed to high temperature during their lifecycle,the tests performed at ambient temperature would no longer predict the reliability with greater accuracy.
2 Experimental Methods
2.1 SEM measurements
A new off-the-shelf LiFePO4battery is used for this experiment.Anode and cathode constituents are carefully extracted from the new battery and tests are conducted on the individual constituents before and after the battery is subjected to cyclic loading by charging/discharging operations.The cathode and anode material character-istics are evaluated using SEM.Micrographs of the material morphology and structure are taken by SEM (JEOL JSM-7400F).Images are taken with a 15kV accelerating voltage and working distances of 6—15mm.It can provide constituent material characteristics along with structural composition and percentage of chemical elements.An example of LiFePO4cathode SEM picture is provided in Fig.1.The olivine crystal structure is evident.

Fig.1 SEM micrograph of new cathode
As indicated earlier,the LiFePo4battery was subjected to cyclic loading to follow a charge/discharge process 2 000times.The battery was cycled between 2.2Vand 3.2V,with an average of 2.7V.Charge continued until the voltage reached 3.2V,and then a discharge was initiated.The process was stopped after 2 000consecutive cycles.SEM is used before and after this cyclic process to evaluate any change in the crystal structure and measure the quantities of chemical elements and the structure of the cathode constituent.In Fig.2,an SEM image of the cycled cathode is provided.The thermo-electrical cyclic process has significantly changed the structure of the battery component.
Similarly,the new anode material(before cycling)is analyzed using SEM (Fig.3)to evaluate both structural composition and percentage of chemical elements.After a similar charge/discharge process conducted for the cathode,the anode is also analyzed under SEM,and its structure is reported in Fig.4.

Fig.2 SEM micrograph of cathode subjected to cyclic process(2 000times)
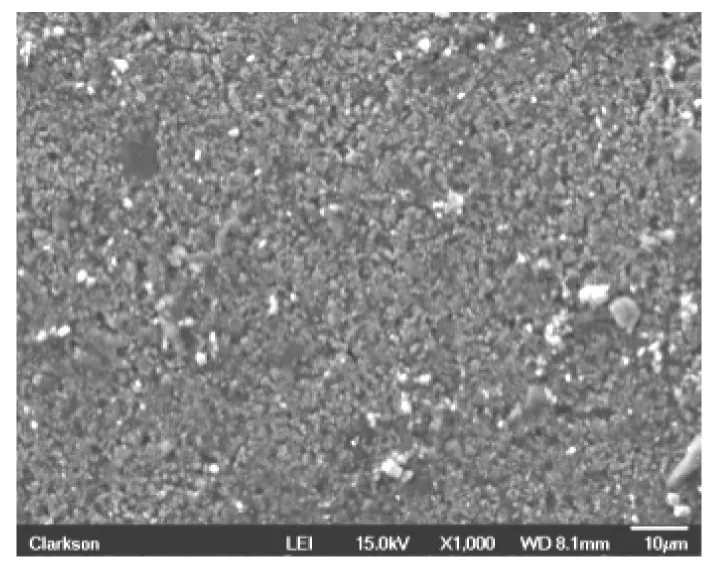
Fig.3 SEM micrograph of new anode

Fig.4 SEM micrograph of anode subjected to cyclic process(2 000times)
The thicknesses of the cathode and anode layers are also measured.In Fig.5the thickness of cathode is displayed.The two LiFePO4layers and copper substrate are about 93μm.
Fig.6presents the thickness of the anode.Two LiFePO4layers and aluminum substrate are about 172μm.The thickness information is used to plan the successive test to characterize the local mechanical properties.

Fig.5 Micrograph of cathode thickness

Fig.6 Micrograph of anode thickness
2.2 Nanoindentation measurements
To characterize the local mechanical properties at different temperatures,high temperature quasi-static nanoindentation tests are performed using a TI-950Triboindenter (Hysitron Inc.,MN).It is also important to mention that the TI-950Hysitron Tribolndentor can only measure up to 10μm from the surface.In order to confirm the reliability of results,the machine is well calibrated before performing tests.A Berkovich tip(three-sided diamond pyramid)is used to indent the materials at different locations using a trapezoidal load function(Fig.7),resulting in an average maximum indentation depth of 100—200nm which is lower than 10%of the material thickness.

Fig.7 Trapezoidal load function used for the tests
In quasi-static nanoindentation experiments,the Oliver-Pharr method[10]is used to extract the indentation modulus and hardness from the forcedisplacement curves.The reduced modulus(Er)is calculated from the unloading portion of the forcedisplacement curve according to the relation
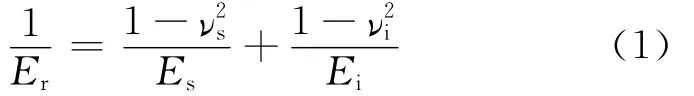
where Eandνare the Young′s modulus and Poisson′s ratio,respectively,and the subscripts correspond to the sample(s)and diamond indenter tip(i)elastic properties.Parameters for the indenter are E=1 140GPa andν=0.07.Hardness(H)is the ratio of the maximum force to the contact area,namely

On the specially designed heating stage,the samples are secured with compression clips in order to avoid slippage and obtain efficient thermal conductivity.Temperature is varied from 30°C to 400°C with the steps of 30,50,100,200,300 and 400°C.Between each temperature increase,at least 30min is allowed to elapse for the sample to equilibrate.Before initiating the indentations,the tip is brought in contact with the sample for 5min in order to equilibrate the temperature at the specimen-tip contact.
3 Results and Discussions
Quantitative analysis of measurements for the cathode from SEM images is provided in Table 1.The presence of copper is explained by the fact that the cathode substrate material is copper.Quantitative measurements for the 2 000 times cycled material are presented in Table 2.
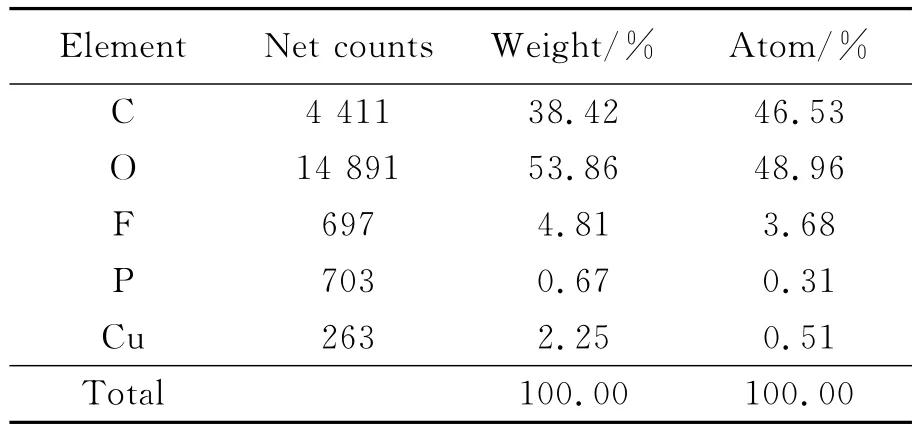
Table 1 Quantitative analysis of new cathode chemical elements

Table 2 Quantitative analysis of cycled cathode chemical elements
After 2 000cycles of discharge-recharge the oxygen is fully fired,a large percentage of carbon remains.For the new anode the percentages of chemical elements are presented in Table 3.The presence of Aluminum and Magnesium is explained by the fact that the anode substrate material is Aluminum rich Magnesium.Alloys of Al-Mg system are characterized by a combination of satisfactory strength,good ductility,weld ability and very good corrosion resistance.Cycled material quantitative measurements of chemical elements are presented in Table 4.The evaluated Young′s moduli for new and cycled LiFePO4cathode and anode materials are provided in Figs.8,9,respectively.It was observed that,the materialsincrease in reduced modulus with the rise in temperature up to 200°C.However,for temperatures beyond 200°C,the reduced modulus values start decreasing with increase in temperature.The material is composed of several base materials.Such variations in the reduced modulus could be from the response of different materials at elevated temperatures.The adhesion of the materials with the copper substrate at the higher temperatures could also considerably affect the reduced modulus values.The experimental results use Eq.(1)to compute the Young′s modulus Eusing values of generalized gradient approximation(GGA)of Poisson′s ration supplied to Eq.(1).

Table 3 Quantitative analysis of new anode chemical elements

Table 4 Quantitative analysis of cycled anode chemical elements

Fig.8 Young′s modulus for new and cycled 2 000 times LiFePO4cathode
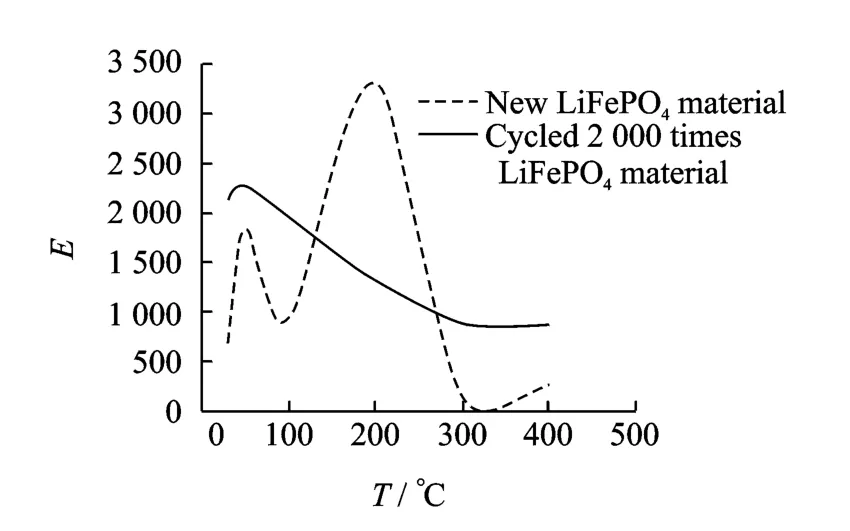
Fig.9 Young′s modulus for new and cycled 2 000 times LiFePO4anode
The results show that the cycled LiFePO4cathode material has modulus of elasticity around 2.5times lower than a new material.This is explained by the fact that oxygen is fired during the cycles and softer carbon is mostly left on the samples.Interestingly,in both new and cycled cathode materials,the maximum value of the modulus of elasticity is found in the proximity of 200°C,while after 250°C the modulus of elasticity starts decreasing.The modulus of elasticity of new material is 5.87GPa,while the cycled material has an elastic modulus of 2.18GPa.On the other hand,the maximum value of the modulus of elasticity for the new anode,as it is for the cathode,is near 200°C,while it is near 50°C for the cycled material.The maximum value of the modulus of elasticity of new material is about 3.3GPa while it is about 2.62GPa for the cycled material.
The maximal hardness for the new and cycled cathode as well as for the new and cycled anode materials at room temperature,50,100,200,300and 400°C are reported in Fig.10.

Fig.10 Max hardness for new and cycled LiFePO4 cathode and anode
Fig.10shows that a large variability in hardness is present at low temperature 25—100°C,and at larger temperature above 300°C.A minimum of hardness is reached around 200°C for the new and cycled cathode and for the new anode,while the cycled anode hardness remains unchanged.These results show certain consistency.
4 Conclusions
This paper discusses the influence of temperature on the elastic properties of the cathode and anode of LiFePO4battery.Quantitative measurements including the chemical composition and structure by SEM,and elastic modulus and hardness by nano-indenter for new or cycled cathode and anode materials,are conducted.A peculiar behavior is noticed,namely the elasticity modulus has its maximum value for both cathode and an-ode materials near 200°C,with the exception of the cycled anode material which has its maximum around 50°C.
Acknowledgements
The authors would like to thank the National Science Foundation and Advanced Technologies(NFSAT),the grant No.TFP-12-06.This work also was supported by Clarkson University Mechanical and Aeronautical Engineering Department,and Clarkson University Center for Advanced Material Processing.
[1] Array Power & Cleaning Energytech,Inc.The introduction of lithium iron phosphate battery[EB/OL].http://www.appowertech.com/case-2show.asp?id=16,2011-05-19.
[2] Kwon S J,Kim C W,Jeong W T,et al.Synthesis and electrochemical properties of olivine LiFePO4as a cathode material prepared by mechanical alloying[J].Journal of Power Sources,2004,137:93-99.
[3] Takashi M,Tobishima S,Takei K,et al.Characterization of LiFePO4as the cathode material for rechargeable lithium batteries[J].Journal of Power Sources,2001,511:97-98,
[4] Cai L,White R E.Mathematical modeling of a lithium ion battery with thermal effects in COMSOL Inc.Multiphysics(MP)software[J].Journal of Power Sources,2011,196:5985-5989.
[5] Jeon D H,Baek S M.Thermal modeling of cylindrical lithium ion battery during discharge cycle[J].Energy Conversion and Management,2011,52:2973-2981.
[6] Chen J,Bull S J,Roya S,et al.Nanoindentation and nanowear study of Sn and Ni-Sn coatings[J].Tribology International,2009,42(6):779-791.
[7] Zhu J,Zeng K,Lu L.Cycling effects on surface morphology,nanomechanical and interfacial reliability of LiMn2O4cathode in thin film lithium ion batteries[J].Electrochimica Acta,2012,68:52-59.
[8] Caceres D,Vergara I,Gonzales R,et al.Nanoindentation on MgO crystals implanted with Lithium ion[J].Nuclear Instruments and Methods in Physics Research B,2002,191:154-157.
[9] Li X,Diao D,Bhusan B.Fracture mechanism of thin amorphous carbon films in nanoindentation[J].Acta Materialia,1997,45(11):4453-4461.
[10]Oliver W C,Pharr G M.An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments[J].Journal of Materials Research,1992,7(6):1564-1583.
 Transactions of Nanjing University of Aeronautics and Astronautics2014年2期
Transactions of Nanjing University of Aeronautics and Astronautics2014年2期
- Transactions of Nanjing University of Aeronautics and Astronautics的其它文章
- Temperature-Dependence of Microstructure Evolution in a Ferroelectric Single Crystal with Conducting Crack
- Optimization of Injection Molding Process of Bearing Stand Based on BP Network Method
- Influence of Patch Side of Heat-Ray Absorbing Film on One-Dimensional Unsteady Thermal Stresses in Window Glass
- Problem of Circular Hole in Thermopiezoelectric Media with Semi-permeable Thermal Boundary Condition
- One-Dimensional-Unsteady Thermal Stress in Heat-Ray Absorbing Sheet Glass:Influence of a Sudden Weather Change
- Thin Film Lithium-Ionbatteries Crack Initiation Due to Thermal and Electric Effects
