助劑對NiMgAl催化劑的結構和甲烷二氧化碳重整反應性能的影響
郭章龍 黃麗瓊,2 儲 偉,* 羅仕忠
(1四川大學化學工程學院,成都610065;2云南開放大學化學工程學院,昆明650223)
1 Introduction
Recently,reducing greenhouse effects and hindering the global warming have attracted many interests,1-3such as the carbon dioxide capture,utilization and storage(CCS,CCUS)or catalytic utilizations.1,4,5The carbon dioxide reforming of methane can convert two greenhouse gases into hydrogen or the syngas.6Furthermore,the produced syngas can be used as feedstocks for the methanol synthesis7or Fischer-Tropsch synthesis.8,9
Nickel catalysts have exhibited good activities in the carbon dioxide reforming of methane reaction.However,they are reported to be sensitive to coking,which blocks active sites and results in deactivation of the catalysts.10,11Comparing to thenonpromoted metal catalysts promoted catalysts have shown higher catalytic activity and better resistance to coking.10Adding a small amount of metal promoter to nickel catalysts can improve the dispersion of the nickel nanoparticles and produce synergetic effect between promoter and Ni particles.8,12-14Promoted catalysts are of great interest for improving the catalytic properties such as activity and stability.12,15,16Pawelecet al.17studied the effect of noble metal Pt on Ni/ZSM-5 catalyst.Adding 0.5%(w)Pt to the catalyst was beneficial for the formation of easily reduced NiO nanoparticles.Garcia-Dieguezet al.18modified the Ni/nanofibrous Al2O3with Pt,the results showed that the CO2conversion and CH4conversion had been improved.The Pt promoter contributed to the reduction of NiO particles for better catalyst performance.Huanget al.19studied the influences of Mo promoter on the Ni/SBA-15 catalyst.The interaction between nickel particle and support and the nickel particle size can be adjusted by changing the atomic ratio of Mo/Ni.The promoted catalyst with atomic ratio of 0.5 showed the higher activity and better stability in the reaction of carbon dioxide dry reforming of methane.Xueet al.20prepared the Au-Cu/Co3O4promoted catalyst by coprecipitation method for the ethylene oxidation model reaction.Compared to Au/Co3O4,the Au-Cu/Co3O4promoted catalyst showed higher catalytic activity,the conversion of ethylene achieved 100%at 120°C,which was due to the higher reduction ability and the higher amount of surface oxygen active species.Yaoet al.21investigated the effects of Mn promoter on the Ni/SiO2catalyst.Mn additive can promote the dispersion of Ni species and enhance the interaction between Ni and modified support.The sample with Mn promoter showed higher activity and less sensitive to coking in the reaction of carbon dioxide reforming of methane.
In this work,the promoted catalysts were prepared using the Co or Ir or Pt additive.H2temperature-programmed reduction(H2-TPR),CO2temperature-programmed desorption(CO2-TPD),CO2/CH4temperature-programmed surface reaction(CO2/CH4-TPSR)techniques were used to investigate the effects of promoter on the catalyst structure,and performance measurements were carried out for the reaction of methane reforming with carbon dioxide.
2 Experimental
2.1 Catalysts preparation
Theγ-Al2O3support was activated at 500 °C for 3 h,and then impregnated with an aqueous solution of commercial Mg(NO3)2salt(Tianjin Bodi Chemical Co.,AR)with a controlled Mg loading amount and stirred for 45 min.Then it was dried at 110 °C overnight and calcined at 550 °C for 5 h.The new prepared support was designated as MgAl.The composite support was impregnated with an aqueous solution of commercial Ni(NO3)2·6H2O salt(The Fourth Plant of Shanghai Reagent,AR)and stirred for 45 min.The Ni loading amount was fixed at 10%(w).The precursors were dried at 80°C in air,and then calcined at 550°C for 5 h.The prepared catalyst was named as NiMgAl.The cobalt promoted nickel catalyst was prepared by adding an aqueous solution of commercial Co(NO3)2·6H2O salt(Shandong Yutai Fine Chemical Factory,AR)and stirring for 45 min.The cobalt promoter amount was 1%(w).The mixture was dried at 80°C in air,and then calcined at 550°C for 5 h.The prepared catalyst was named as NiCoMgAl.So did the samples with Ir promotion or Pt promotion.H2IrCl6·6H2O(Xi'an Kaili Chemical Co.,AR)or H2PtCl6·6H2O(idem,AR)was used as the promoter.The obtained promoted catalysts were coded as NiIrMgAl and NiPtMgAl,respectively.
2.2 Catalytic performance measurement
The catalytic activity measurements were performed under atmospheric pressure using a continuous fixed-bed flow reactor at a temperature of 650-800°C.Typically,100 mg of the catalyst was loaded in the quartz-reactor.The molar ratio of CH4to CO2was 1:1,and gas hourly space velocity(GHSV)was 36000 mL·h-1·g-1.The catalyst was reduced in the reactor with H2at 700°C for 1 h before the test.The effluent gases from the reactor were analyzed on-line using a SC-200 model gas chromatograph(the ninth factory of Sichuan Instrument)with a TDX-01 column and a thermal conductivity detector(TCD).
The CH4conversion(XCH4)and CO2conversion(XCO2)were calculated as follows:
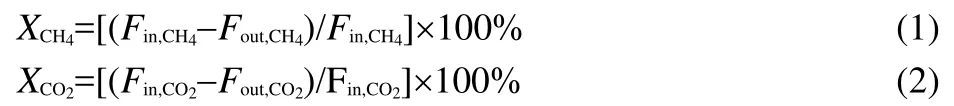
whereFin,iis the flow rate of each component in the inlet feed,Fout,iis the flow rate of each component in the outlet feed.
2.3 Sample characterizations
Temperature-programmed reduction experiments were carried out in a fixed-bed reactor.50 mg sample was loaded,and the 5%H2/N2gas mixture at a flow rate of 30 mL·min-1was introduced.The reactor temperature was raised linearly from 100 to 900 °C at a heating rate of 10 °C·min-1by a temperature controller.The hydrogen consumption was analyzed on-line using a SC-200 gas chromatograph(the ninth factory of Sichuan Instrument)with a thermal conductivity detector.
The surface species in the reforming process were investigatedviatemperature-programmed surface reaction using a fixed-bed reactor.Before TPSR experiment,the catalyst was reduced under hydrogen flow at 700°C for 1 h.Upon cooling down to room temperature under flowing Ar,the CH4with a flow rate of 30 mL·min-1was switched in.The temperature of the reactor was raised linearly to 800 °C at a heating rate of 10 °C·min-1by a temperature controller for CH4-TPSR experiment.The products were analyzed on-line using a Hiden QIC-20 mass spectrometer(the Hiden Company of British).After coking reaction with CH4for 30 min at 700°C,CO2-TPSR was performed.The system was cooled down to 50°C in Ar atmosphere.Then the CO2gas at a flow reaction rate of 30 mL·min-1was introduced.The temperature of the reactor was raised linearly to 800 °C at a heating rate of 10 °C·min-1by a temperature controller for CO2-TPSR experiment.The products were analyzed on-line by a QIC-20 mass spectrometer.
CO2-TPD was used to determine the basicity of samples.The catalyst was treated at 300°C for 1 h in Ar,and then CO2was introduced for 1 h at room temperature.After purging with Ar for 1 h to remove CO2in the gas phase,the sample was heated from room temperature to 800 °C with a heating rate of 10 °C·min-1and the desorbed CO2was detected on-line by a Hiden QIC-20 mass spectrometer(the mass signal atm/z=44 was used to determine the amount of desorbed CO2).
3 Results and discussion
3.1 Catalytic performances
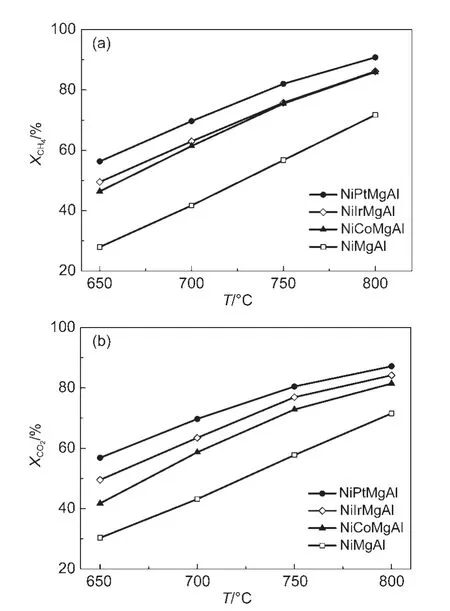
Fig.1 Catalytic performances of the four catalysts in carbon dioxide dry reforming of methane
The catalytic performances of the four catalysts are shown in Fig.1.The carbon dioxide conversion of NiMgAl unpromoted catalyst was 43%at 700°C,while it was 59%for the cobalt promoted catalyst,63%for the Ir promoted sample,and 70%for the Pt promoted catalyst.Comparing to the primitive nickel catalyst,the promoted catalyst shows higher activity,while the Pt promoted catalyst gives the better one.The higher activity of the promoted catalyst is attributed to promoting effects,which could increase the reducibility of Ni nanoparticles.22Meanwhile,there is also a similar tendency for the methane conversion.The catalytic activity was increasing with the temperature rising from 650 to 800°C,the catalyst with Pt promoter has the higher activity among the four catalysts.
According to Arrhenius formula,the relationship between logarithm of CH4conversion and reciprocal of temperature is shown in Fig.2.Reaction activation energies of these catalysts were calculated from the resulting trend line slop value.The activation energies of four catalysts are shown in Table 1.The results show that the activation energy was decreased by adding the promoter.Compared to that of the NiMgAl catalyst,the activation energy of NiPtMgAl catalyst was decreased from 51.79 to 26.43 kJ·mol-1.
3.2 H2temperature-programmed reduction
The H2-TPR profiles are shown in Fig.3.The various promoters have different effects on the Ni/MgO-Al2O3based catalysts.The TPR results are indicated in Table 2.There are three reduction peaks for the unpromoted nickel catalyst(α, β, γ there peaks).The α peak was corresponded to the reduction of free nickel oxide species for which there was weak interaction with the support.The β peak was corresponded to more dis-persed Ni species on the surface,while the γ peak was attributed to the spinel-like species which were more difficult to be reduced.For the promoted catalysts,the temperatures of reduction peaks were shifted to lower temperatures for each of the α,β,γ there peaks.In addition,there was a fourth reduction peak at a lower temperature(δ peak).It is about 320,200,and 200 °C for the promoted samples with promoters Co,Ir,and Pt,respectively.This is attributed to the reduction of Co(Ir or Pt).22-24From Table 2,the Pt promoted catalyst possessed the larger percentage of reduced species.It is reported that noble metal Pt can activate and divide hydrogen molecule to hydrogen atoms,and then favor the reduction of nickel oxide species.26Comparing to the unpromoted NiMgAl catalyst,there were less γ-peak for the promoted catalyst with cobalt or iridium additive(Table 2).
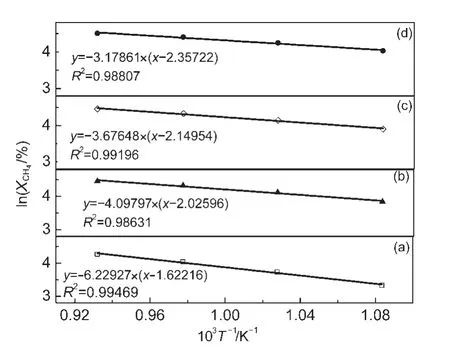
Fig.2 Logarithm of methane conversion versus the reciprocal of temperature
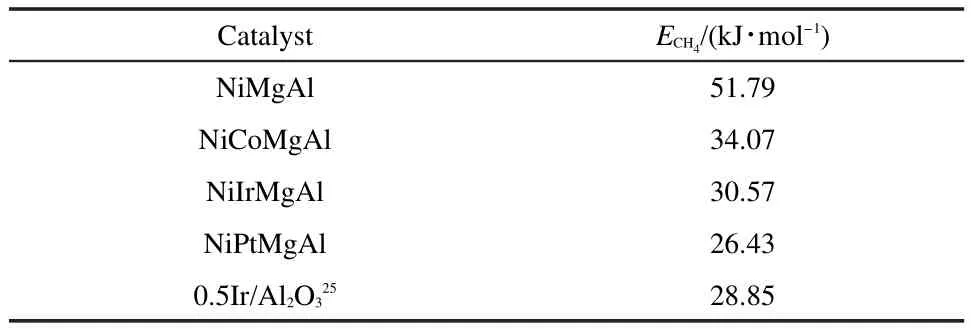
Table 1 Activation energy data obtained fromArrhenius plots

Fig.3 H2-TPR profiles of four catalysts
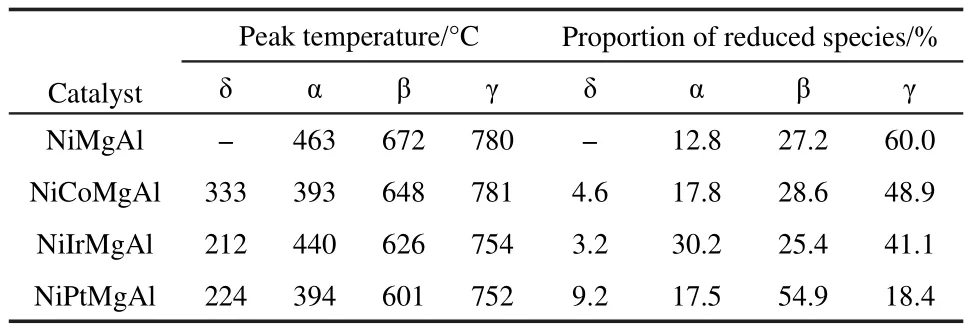
Table 2 H2-TPR results of four catalysts
3.3 CH4-TPSR and CO2-TPSR

Fig.4 CH4-TPSR profiles of catalysts
The results of CH4-TPSR experiments are shown in Fig.4.The methane starts to be activated at about 560°C for the unpromoted NiMgAl catalyst,reaching a peak temperature at 800°C.While the Pt-promoted sample(NiPtMgAl),the starting temperature of methane activating was shifted to 500°C,and the peak temperature was shifted to 700°C.The peak areawas also increased.Thus,the Pt promoted catalysts NiPtMgAl gave better activity,there is a good correlation between this result and that of H2-TPR experiments.
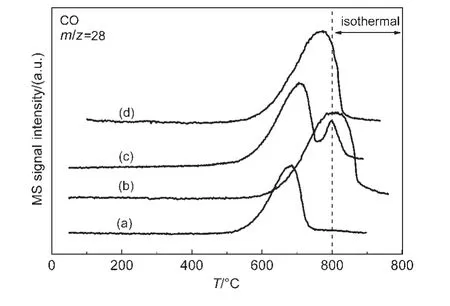
Fig.5 TPSR profiles of CO2over different catalysts
The results of CO2-TPSR are shown in Fig.5.The results show that the CO2begins to be consumedat about 500°C in the cases of NiMgAl and NiPtMgAl samples,while it shifted to a higher temperature at about 600°C in the case of NiCoMgAl or NiIrMgAl.The NiCoMgAl and NiIrMgAl catalyst have larger CO formation peak area and higher peak temperature(800°C for Co-promoted,760 °C for Ir-promoted),while it was 670 °C for NiMgAl catalyst.Therefore,there were more carbon species on the surface of NiCoMgAl or NiIrMgAl catalyst,comparing to that of unpromoted sample.
3.4 CO2-TPD
The CO2-TPD results are shown in Fig.6.The unpromoted NiMgAl sample shows a main desorption peak at about 200°C,corresponding to the desorption of weakly adsorbed CO2.While there is a tiny desorption at about 800°C.The NiCoMgAl,NiIrMgAl,and NiPtMgAl catalysts not only show a peak near 200°C,but also another desorption peak in the range of 650-800°C.The addition of metal promoter can increase the adsorbed CO2amount,while the interaction between Ni and Co,Ir,or Pt changed the surface structure of the catalysts,which can enhance the activation of CO2.27,28As seen from Fig.6,the CO2desorption peak area has been improved significantly at higher temperature,29indicating that the amount of adsorbed/desorbed CO2has been increased at the temperature,which is in the range of the reaction temperature,which is favorable for improving the reactivity.
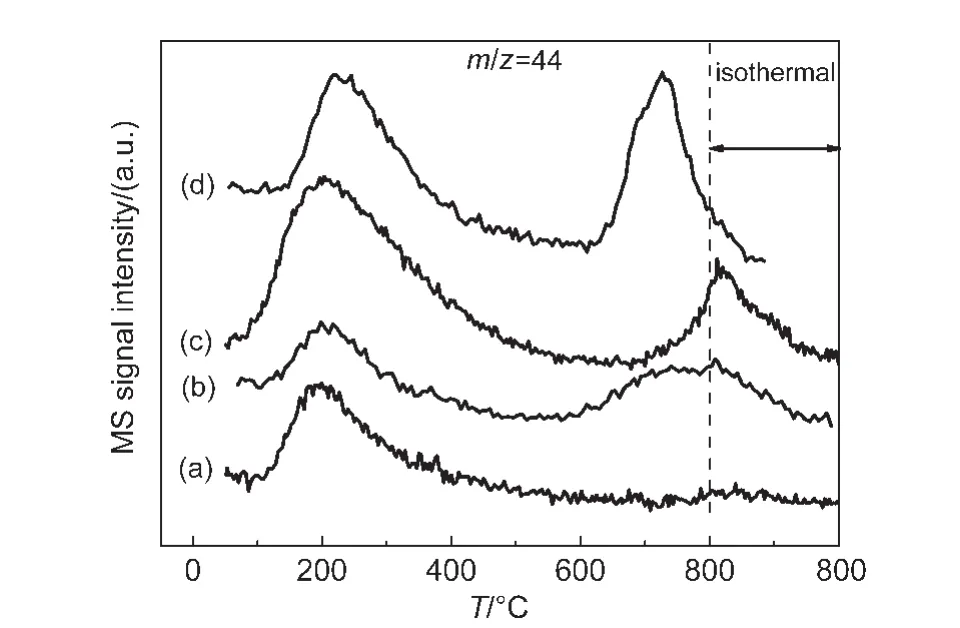
Fig.6 CO2-TPD results of different catalysts
4 Conclusions
(1)The enhanced new catalyst with promoter Co,Ir,or Pt can decrease the activation energy and improve the catalyst activity of the unpromoted catalyst,for the methane dry reforming reaction with carbon dioxide.Furthermore,the catalyst with Pt promoter has the higher catalytic activity.
(2)Adding promoter can enhance the reduction of nickle active ingredient,and it is favorable for the methane reforming reaction.
(3)TPSR results demonstrate that the catalyst with Pt promoter can improve the activation of CH4at lower temperature;CO2-TPD results show that adding promoter can improve adsorbed CO2amount than that of the unpromoted catalyst in the reaction temperature range.
(1) Wang,Q.;Luo,J.Z.;Zhong,Z.Y.;Borgna,A.Energ.Environ.Sci.2011,4,42.doi:10.1039/c0ee00064g
(2)Chu,W.;Ran,M.F.;Zhang,X.;Wang,N.;Wang,Y.F.;Xie,H.P.;Zhao,X.S.J.Energy Chem.2013,22,136.doi:10.1016/S2095-4956(13)60018-2
(3) Hansen,J.;Sato,M.Proc.Natl.Acad.Sci.U.S.A.2004,101,16109.doi:10.1073/pnas.0406982101
(4) von derAssen,N.;Jung,J.;Bardow,A.Energ.Environ.Sci.2013,6,2721.doi:10.1039/c3ee41151f
(5) Serrano-Ruiz,J.C.;Dumesic,J.A.Energ.Environ.Sci.2011,4,83.doi:10.1039/c0ee00436g
(6)Ashcroft,A.T.;Cheetham,A.K.;Green,M.L.H.;Vernon,P.D.F.Nature1991,352,225.doi:10.1038/352225a0
(7) Ramos,L.;Zeppieri,S.Fuel2013,110,141.doi:10.1016/j.fuel.2012.12.045
(8)Chu,W.;Wang,L.N.;Chernavsk,P.A.;Khodakov,A.Y.Angew.Chew.Int.Edit.2008,47,5052.doi:10.1002/anie.v47:27
(9) Ha,K.S.;Bae,J.W.;Woo,K.J.;Jun,K.W.Environ.Sci.Technol.2010,44,1412.doi:10.1021/es902784x
(10)Wang,N.;Chu,W.;Zhang,T.;Zhao,X.S.Chem.Eng.J.2011,170,457.doi:10.1016/j.cej.2010.12.042
(11)Aldashukurova,G.B.;Mironenko,A.V.;Mansurov,Z.A.;Shikina,N.V.;Yashnik,S.A.;Kuznetsov,V.V.;Ismagilov,Z.R.J.Energy Chem.2013,22,811.doi:10.1016/S2095-4956(13)60108-4
(12)Zhang,J.G.;Wang,H.;Dalai,A.K.J.Catal.2007,249,300 300.doi:10.1016/j.jcat.2007.05.004
(13) Crisafulli,C.;Scire,S.;Minico,S.;Solarino,L.Appl.Catal.AGen.2002,225,1.doi:10.1016/S0926-860X(01)00585-3
(14) Meshkani,F.;Rezaei,M.Int.J.Hydrog.Energy2010,35,10295.doi:10.1016/j.ijhydene.2010.07.138
(15)Chen,Y.G.;Tomishige,K.;Yokoyama,K.;Fujimoto,K.Appl.Catal.A-Gen.1997,165,335.doi:10.1016/S0926-860X(97)00216-0
(16)Jose-Alonso,D.S.;Illan-Gomez,M.J.;Roman-Martinez,M.C.Int.J.Hydrog.Energy2013,38,2230.doi:10.1016/j.ijhydene.2012.11.080
(17) Pawelec,B.;Damyanova,S.;Arishtirova,K.;Fierro,J.L.G.;Petrov,L.Appl.Catal.A-Gen.2007,323,188.doi:10.1016/j.apcata.2007.02.017
(18) Garcia-Dieguez,M.;Pieta,I.S.;Herrera,M.C.;Larrubia,M.A.;Alemany,L.J.J.Catal.2010,270,136.doi:10.1016/j.jcat.2009.12.010
(19) Huang,T.;Huang,W.;Huang,J.;Ji,P.Fuel Process.Technol.2011,92,1868.doi:10.1016/j.fuproc.2011.05.002
(20) Xue,W.J.;Zhang,X.Y.;Li,P.;Liu,Z.T.;Hao,Z.P.;Ma,C.Y.Acta Phys.-Chim.Sin.2011,27,1730.[薛雯娟,張新艷,李鵬,劉昭鐵,郝鄭平,麻春艷.物理化學學報,2011,27,1730.]doi:10.3866/PKU.WHXB20110719
(21)Yao,L.;Zhu,J.Q.;Peng,X.X.;Tong,D.M.;Hu,C.W.Int.J.Hydrog.Energy2013,38,7268.doi:10.1016/j.ijhydene.2013.02.126
(22)Wang,L.;Li,D.L.;Koike,M.;Watanable,H.;Xu,Y.;Nakagawa,Y.;Tomishige,K.Fuel2013,112,654.doi:10.1016/j.fuel.2012.01.073
(23)Lin,W.W.;Cheng,H.Y.;He,L.M.;Yu,Y.C.;Zhao,F.Y.J.Catal.2013,303,110.doi:10.1016/j.jcat.2013.03.002
(24) de Miguel,S.R.;Vilella,I.M.J.;Maina,S.P.;Jose-Alonso,D.S.;Roman-Martinez,M.C.;Illan-Gomez,M.J.Appl.Catal.AGen.2012,435,10.
(25)El-Temtamy,S.A.;Ghoneim,S.A.;El-Morsy,A.K.;El-Naggar,A.Y.;El-Salamouny,R.A.Petrol Sci.Technol.2009,27,1661.doi:10.1080/10916460802455897
(26)Yu,X.P.;Wang,N.;Chu,W.;Liu,M.Chem.Eng.J.2012,209,623.doi:10.1016/j.cej.2012.08.037
(27) Li,C.L.;Fu,Y.L.;Bian,G.Z.Acta Phys.-Chim.Sin.2003,19,902.[李春林,伏義路,卞國柱.物理化學學報,2003,19,902.]doi:10.3866/PKU.WHXB20031004
(28)Qin,Z.Q.;Gao,W.G.;Wang,H.;Han,C.;Guo,W.Chemical Industry and Engineering Progress2013,32,820.[覃志強,高文桂,王 華,韓 沖,郭 偉.化工進展,2013,32,820.]
(29)Wang,N.;Shen,K.;Yu,X.P.;Qian,W.Z.;Chu,W.Catal.Sci.Technol.2013,3,2278.doi:10.1039/c3cy00299c

