黃曲霉毒素B1對雛雞免疫器官影響的病理學觀察
于正強,陳 瑾,彭 西,方 靜,陳科杰,何 楊
(1.四川農業大學動物醫學院,成都 611130;2.西昌市農牧局,西昌 615000)
黃曲霉毒素B1對雛雞免疫器官影響的病理學觀察
于正強1#,陳 瑾2#,彭 西1*,方 靜1,陳科杰1,何 楊1
(1.四川農業大學動物醫學院,成都 611130;2.西昌市農牧局,西昌 615000)
為探明黃曲霉毒素B1對雛雞免疫器官組織學及超微結構的影響,將100只1日齡艾維茵健康公雛隨機分為4組,分別喂以對照日糧和AFB1日糧(AFB1Ⅰ、Ⅱ、Ⅲ組日糧中AFB1添加量分別為0.15、0.3和0.6 mg·kg-1),試驗期21 d。結果顯示,AFB1Ⅱ組和Ⅲ組雛雞的免疫器官臟器指數顯著下降(P<0.05)。AFB1組雛雞的免疫器官組織學損傷表現:胸腺皮質區網狀細胞周圍見較多細胞核碎片;法氏囊淋巴濾泡內細胞核碎片增多,濾泡髓質區淋巴細胞減少;脾白髓區細胞核碎片增多。超微病理學觀察,胸腺、法氏囊和脾內淋巴細胞線粒體腫脹,以染色質邊移為特征的凋亡細胞數目增多。結果表明,攝食含0.15~0.6 mg·kg-1AFB1的日糧,可不同程度地抑制雛雞免疫器官的發育,致免疫器官中的淋巴細胞數量減少、細胞核碎片增多。
黃曲霉毒素B1;胸腺;法氏囊;脾;凋亡;肉雞
黃曲霉毒素B1(Aflatoxins B1,AFB1),是由黃曲霉菌及寄生曲霉菌產生的次生代謝產物[1],是當前毒性和致癌性最強的物質之一[2]。除引起動物急性肝損傷和肝癌[3],AFB1還可導致多臟器損傷,生產性能下降和抗病能力降低[4-5]。近年來,AFB1對免疫系統的影響受到普遍關注,有關其導致免疫抑制的報道較多,AFB1主要引起畜禽巨噬細胞和淋巴細胞功能受損、淋巴細胞亞群百分率及抗體滴度下降[6-7]。家禽對AFB1較為敏感,即使攝食較低水平AFB1,也會抑制雞淋巴細胞增殖與活性,導致免疫能力低下[8]。因尚未見有飼料中梯度水平AFB1對雞免疫器官組織病理學影響的系統研究資料,本試驗以1日齡艾維茵肉雞為研究對象,闡明AFB1日糧致雛雞免疫器官組織和超微病理學損傷的特征,以期為進一步認識免疫抑制與免疫器官組織學變化之間的關系提供參考依據。
1 材料與方法
1.1 實驗動物與日糧
試驗選用1日齡艾維茵健康公雛100只購于溫江正大畜禽有限公司。
基礎日糧以玉米-豆粕為主配制而成,其中蛋白質含量、能量以及維生素和微量元素添加量均參照肉雞NRC(2004)的營養標準。AFB1日糧的配制方法:分別將1.5、3和6 mg AFB1固體粉末溶于30 mL甲醇中,再將30 mL溶液逐級混進10 kg基礎日糧中,然后將混有甲醇的日糧置于37 ℃烘箱中烘干,待甲醇揮發后取出。對照組日糧配制:取30 mL甲醇混入10 kg基礎日糧后,以同樣方法烘干。由此方法配制而成的對照組、AFB1Ⅰ組、AFB1Ⅱ組和AFB1Ⅲ組日糧中,AFB1的濃度分別為0、0.15、0.3和0.6 mg·kg-1。
1.2 試驗的總體設計和動物處理
100只1日齡艾維茵健康公雛,按初始體重無差異原則隨機分為4組,每組25只,其中對照組雛雞采食基礎日糧,AFB1Ⅰ、Ⅱ、Ⅲ組雛雞采食日糧中AFB1的濃度分別為0.15、0.3和0.6 mg·kg-1。試驗在四川農業大學獸醫院基礎實驗樓試驗場進行。所有試驗肉雞均采用網上平養。試驗開始前對雞舍進行清理并先后采用甲醛熏蒸法、高錳酸鉀和石灰消毒法進行全面消毒。采用紅外燈加溫,第一周室溫保持在33 ℃左右,以后每周降2 ℃,相對濕度控制在65%~67%。采用連續光照,自然通風,自由采食和飲水。定期打掃圈舍衛生,試驗期為21 d。
1.3 臨床觀察
試驗期間,每天觀察雞的采食、飲水及精神狀況,并記錄臨床癥狀。試驗第7、14和21天,對各組雞只稱重,統計各組間的體重和料肉比差異,并對雞只的精神狀態及生長發育狀態對比照相記錄。
1.4 病理學觀察
1.4.1 免疫器官臟器指數 試驗的第7、14和21天每組隨機抽取5只剖殺,立即取胸腺、法氏囊和脾,去除其周圍脂肪和結締組織后,用電子天平稱其凈重,根據如下公式計算臟器指數。
臟器指數=(臟器凈重/空腹體重)×100%
1.4.2 組織學觀察 剖殺尸檢后,取雞的胸腺、法氏囊和脾,固定于4%的多聚甲醛溶液中,脫水包埋,石蠟切片,HE染色后于Olympus顯微鏡下觀察,并用Nikon數碼顯微照相機記錄組織病理變化。
1.4.3 超微結構觀察 試驗第21天,每組各剖殺3只雛雞,取胸腺、法氏囊和脾,用雙面刀片修成1 mm×1 mm×3 mm細條后,固定于2.5%的戊二醛中,丙酮脫水,環氧樹脂包埋,切片染色后透射電鏡下觀察記錄超微結構的病理變化。
1.5 數據處理
2 結 果
2.1 臨床觀察
試驗期間AFB1Ⅰ組雞均未出現明顯臨床癥狀,AFB1Ⅱ、Ⅲ組雞飲欲增加,食欲下降,雞精神沉郁,嗜眠,羽毛松亂而無光澤,同時較對照組生長發育遲緩;體重檢測結果顯示,7日齡,各組間差異不顯著(P>0.05);14日齡,AFB1Ⅱ組雞平均體重顯著低于對照組(P<0.05);21日齡,AFB1Ⅲ組雞體重顯著低于對照組(P<0.05),見表1。料肉比統計結果顯示,7日齡和14日齡,各組間差異不顯著(P>0.05);21日齡,AFB1Ⅱ、Ⅲ組料肉比顯著高于對照組(P<0.05);AFB1Ⅱ、Ⅲ組全期累積料肉比顯著高于對照組(P<0.05)。見表2。
2.2 剖解變化及免疫器官臟器指數
7日齡,與對照組比較,AFB1各組雞無明顯眼觀變化。14日齡開始,與對照組比較,AFB1Ⅱ、Ⅲ組雞免疫器官出現肉眼變化,表現為胸腺與脾體積減小,顏色加深;法氏囊體積減小,顏色加深。到21日齡時,AFB1Ⅱ、Ⅲ組雛雞免疫器官體積減小的病變更明顯。
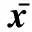
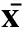
Table 1 Changes of body weight in chickens (±s,n=5)g
“*”表示與對照組相比差異顯著(P<0.05),“**” 表示與對照組相比差異極顯著(P<0.01)。表2、3與本表相同
“*” represent difference (P<0.05) between the group and control group,“**” represent significantly difference (P<0.01) between the group and control group.The same as the table 2 and table 3
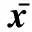
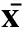
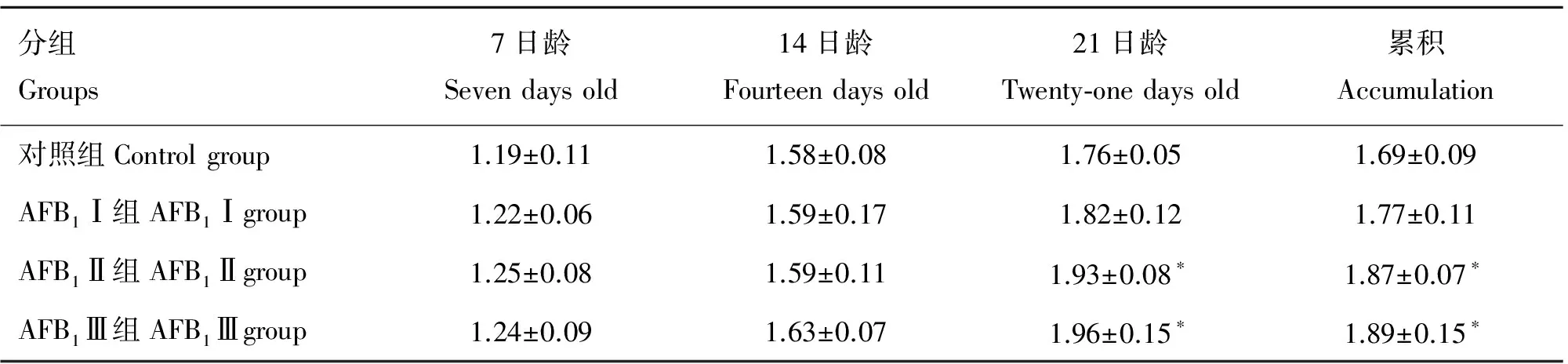
分組Groups7日齡Sevendaysold14日齡Fourteendaysold21日齡Twenty?onedaysold累積Accumulation對照組Controlgroup1.19±0.111.58±0.081.76±0.051.69±0.09AFB1Ⅰ組AFB1Ⅰgroup1.22±0.061.59±0.171.82±0.121.77±0.11AFB1Ⅱ組AFB1Ⅱgroup1.25±0.081.59±0.111.93±0.08?1.87±0.07?AFB1Ⅲ組AFB1Ⅲgroup1.24±0.091.63±0.071.96±0.15?1.89±0.15?
對雛雞胸腺、法氏囊及脾進行臟器指數統計的結果顯示,7日齡和14日齡時,AFB1各組與對照組相比差異均不顯著(P>0.05);21日齡時,AFB1Ⅱ組雛雞的胸腺及脾的臟器指數顯著低于對照組(P<0.05),AFB1Ⅲ組雛雞的胸腺、法氏囊及脾的臟器指數均顯著低于對照組(P<0.05),見表3。
2.3 病理組織學變化
組織學觀察結果顯示,與對照組比較,3個AFB1組雛雞的胸腺、法氏囊和脾出現程度不同的病理變化,并表現為劑量效應關系。
正常雛雞的胸腺皮質區的網狀細胞形態清晰,周圍偶見有少量細胞核碎片,皮質和髓質區的毛細血管數量少(圖1a)。AFB1組胸腺淤血表現為髓質區毛細血管擴張充血及數量相對增多(圖1b);增多的細胞核碎片主要位于胸腺皮質區的網狀細胞核周圍,且導致網狀細胞核形態不清(圖1c、d)。
雛雞法氏囊的正常形態學結構表現為淋巴濾泡皮髓質分界清晰,細胞均勻排列,其中散見有少量細胞核碎片(圖1e)。AFB1組雛雞法氏囊的病變特征是,淋巴濾泡髓質區的淋巴細胞明顯減少、排列較稀疏,皮質及髓質均見有大量小空洞,細胞核碎片顯著增多,且主要位于空洞內(圖1f、g)。21日齡時,AFB1Ⅲ組一只雛雞的法氏囊淋巴濾泡數量減少、體積縮小,濾泡之間大量纖維結締組織增生(圖1h)。
正常雛雞的脾白髓區淋巴細胞排列均一。AFB1組雛雞的脾紅髓區淤血,脾小結及動脈周圍淋巴鞘見有一定量空洞,細胞核碎片增多,且主要位于空洞內(圖1i、j、k 和l)。
對胸腺、法氏囊和脾的主要病變進行統計(表4),結果顯示,7日齡時,AFB1Ⅰ組未見有明顯的病變,AFB1Ⅱ、Ⅲ組1/5或2/5出現病變,隨試驗期延長,3個AFB1組的病變率逐漸升高,至21日齡時,AFB1Ⅱ組的病變率為2/5~4/5,AFB1Ⅲ組的病變率為4/5。該結果顯示,飼糧中AFB1會導致雛雞免疫器官的病理損傷,胸腺和脾的病變特征:淤血及細胞核碎片增多;法氏囊的病變特征:細胞核碎片增多及淋巴細胞數量減少。
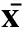
Table 3 Changes of organ index of immune organ (±s)g·kg-1
表4 胸腺、法氏囊和脾主要病變的發生率(n=5)
Table 4 Incidence of major lesions in thymus,bursa of Fabricius and spleen (n=5)
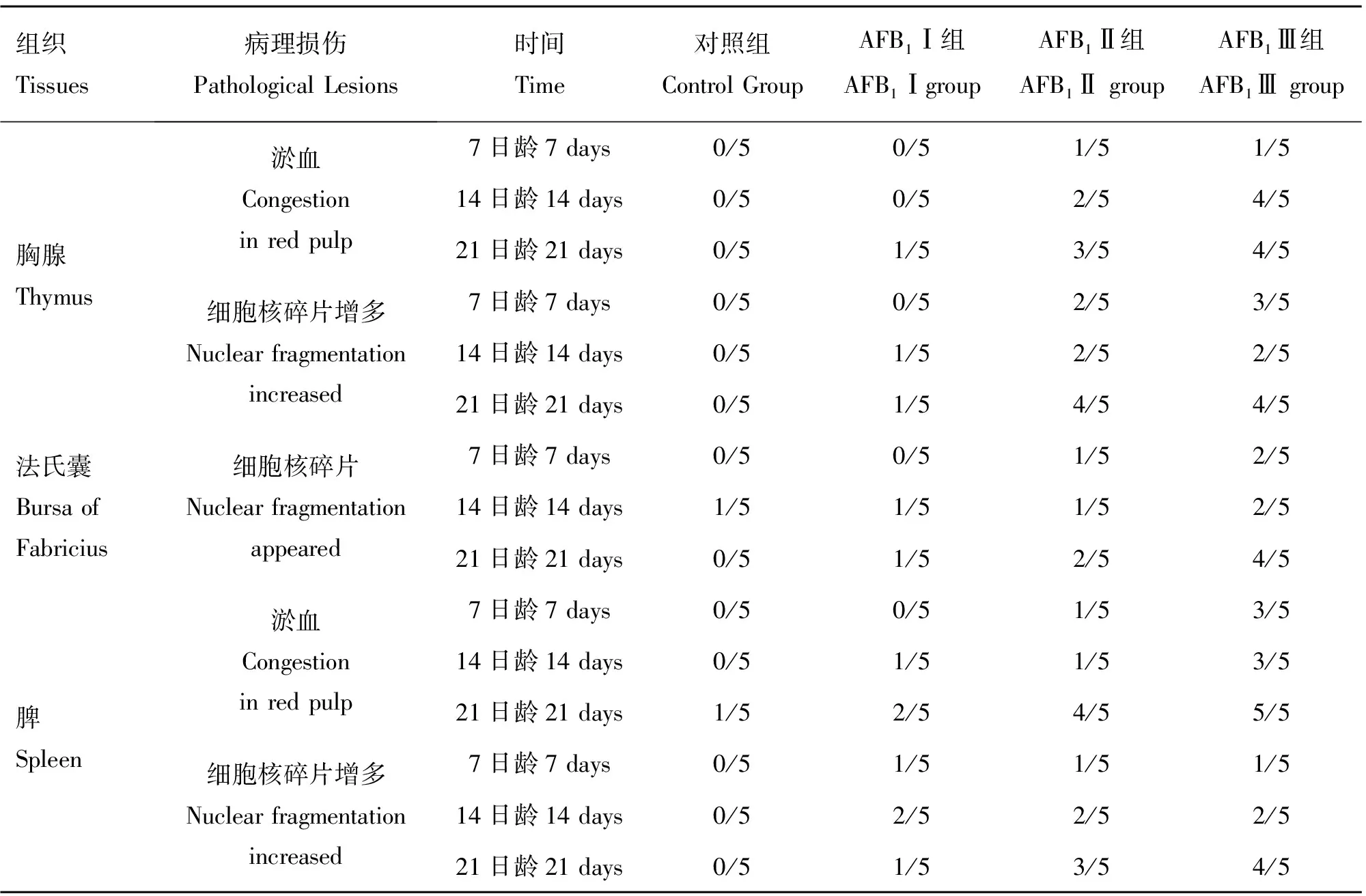
組織Tissues病理損傷PathologicalLesions時間Time對照組ControlGroupAFB1Ⅰ組AFB1ⅠgroupAFB1Ⅱ組AFB1ⅡgroupAFB1Ⅲ組AFB1Ⅲgroup胸腺Thymus淤血Congestioninredpulp細胞核碎片增多Nuclearfragmentationincreased7日齡7days0/50/51/51/514日齡14days0/50/52/54/521日齡21days0/51/53/54/57日齡7days0/50/52/53/514日齡14days0/51/52/52/521日齡21days0/51/54/54/5法氏囊BursaofFabricius細胞核碎片Nuclearfragmentationappeared7日齡7days0/50/51/52/514日齡14days1/51/51/52/521日齡21days0/51/52/54/5脾Spleen淤血Congestioninredpulp細胞核碎片增多Nuclearfragmentationincreased7日齡7days0/50/51/53/514日齡14days0/51/51/53/521日齡21days1/52/54/55/57日齡7days0/51/51/51/514日齡14days0/52/52/52/521日齡21days0/51/53/54/5
a.對照組胸腺;b.AFB1 Ⅲ組胸腺,髓質區淤血;c.AFB1Ⅱ組,胸腺皮質區出現少量空洞及細胞核碎片(→); d.AFB1 Ⅲ組,胸腺皮質區出現大量空洞及細胞核碎片(→);e.對照組雛雞的法氏囊;f.AFB1 Ⅲ,法氏囊淋巴濾泡髓質區細胞排列稀疏;g.AFB1 Ⅲ組,法氏囊大量空洞及細胞核碎片(→);h.AFB1 Ⅲ組,法氏囊間質結締組織增生; i.對照組脾;j.AFB1 Ⅲ組,脾紅髓區淤血;k.AFB1 Ⅲ組,脾動脈周圍淋巴鞘淋巴細胞減少;l.AFB1 Ⅲ組,脾動脈周圍淋巴鞘形成空洞a.Thymus of the chicken in control group;b.Thymus of the chicken in AFB1 group Ⅲ.Congestion in medulla;c.Thymus of the chicken in AFB1 group Ⅱ.A few vacuoles and nuclear debris were found in cortex (→);d.Thymus of the chicken in AFB1 group Ⅲ.More vacuoles and nuclear debris in cortex (→);e.Bursa of Fabricius of the chicken in control group;f.Bursa of Fabricius of the chicken in AFB1 group Ⅲ.Sparse medulla of follicles;g.Bursa of Fabricius of the chicken in AFB1 group Ⅲ.Vacuoles and nuclear debris (→);h.Bursa of Fabricius of the chicken in AFB1 group Ⅲ.Proliferated connective tissue in the mesenchymal of bursa;i.Spleen of the chicken in control group;j.Spleen of the chicken in AFB1 group Ⅲ.Congestion in red pulp;k.Spleen of the chicken in AFB1 group Ⅲ.Lymphocytes were decreased in periarterial lymphatic sheath;l.Spleen of the chicken in AFB1 group Ⅲ.Vacuoles in the periarterial lymphatic sheath圖1 21日齡雛雞的胸腺、法氏囊和脾病理組織學變化(HE染色400×)Fig.1 Thymus,bursa of Fabricius and spleen were in the chicken from the four groups at 21 days old (HE,400×)
2.4 超微結構變化
2.4.1 胸腺 與對照組比較,AFB1Ⅲ組雛雞的胸腺中的淋巴細胞核周隙擴張,線粒體腫脹,嵴斷裂溶解,甚至消失呈空泡狀(圖2a、b);凋亡細胞數量增多,凋亡細胞的染色質或濃縮邊移形成月牙形或花環狀貼于核膜下,或聚集成不規則團塊狀。凋亡細胞常位于網狀細胞附近或被吞噬于網狀細胞的細胞質內(圖2c)。
2.4.2 法氏囊 與對照組比較,凋亡細胞數量增多。凋亡細胞核染色質凝聚邊移形成馬蹄形、月牙形等,部分網狀細胞內見有被吞噬的凋亡細胞(圖2d、e、f)。
2.4.3 脾 與對照組(圖2g)比較,AFB1Ⅲ組雛雞的脾中淋巴細胞的核周隙擴張和線粒體腫脹(圖2h)。脾內凋亡的淋巴細胞和漿細胞染色質凝聚邊移,呈花環形或月牙形(圖2i)。
3 討 論
3.1 雛雞生長狀況及臨床癥狀觀察
AFB1為一類致癌物質,高劑量導致急性死亡,低劑量長期暴露導致慢性中毒,畜禽表現生長不良,免疫能力低下,飼料轉化率降低,死亡率增加,蛋雞產蛋率下降等[9]。試驗期間,AFB1Ⅱ、Ⅲ組(0.3、0.6 mg·kg-1)雞出現食欲下降、飲欲增加的臨床癥狀;體重和料肉比的統計結果表明,AFB1Ⅱ、Ⅲ組雞只飼料轉化率降低,生長發育受到抑制。A.Marchioro等[10]用AFB1處理科寶肉雞結果顯示生長抑制、體重下降,本試驗結果與其一致。雛雞采食量下降可能與飼料品質惡化,適口性下降有關[11]。引起雛雞體重減輕、生長抑制的可能原因:攝入的黃曲霉毒素一方面可破壞腸道上皮細胞的完整性和通透性,影響消化酶的分泌,進而影響營養物質的消化吸收[12-13];另一方面可導致腎上腺皮質激素、生長激素等合成紊亂,引起營養成分體內代謝障礙[14]。
a.對照組胸腺; b.AFB1Ⅲ組,胸腺核周隙明顯擴張;c.AFB1Ⅲ組,胸腺兩個凋亡的淋巴細胞;d.對照組法氏囊;e.AFB1Ⅲ組,法氏囊細胞凋亡;f.AFB1Ⅲ組,法氏囊網狀細胞內吞噬有凋亡細胞;g.對照組脾;h.AFB1Ⅲ組,脾淋巴細胞核周隙擴張,線粒體呈空泡狀(→);i.AFB1Ⅲ組,脾漿細胞凋亡a.Thymus of the chicken in control group;b.Thymus of the chicken in AFB1 group Ⅲ.Dilated perinuclear cisternae;c.Thymus of the chicken in AFB1 group Ⅲ.Two apoptotic thymocytes;d.Bursa of Fabricius of the chicken in control group;e.Bursa of Fabricius of the chicken in AFB1 group Ⅲ.Apoptotic cell in bursa of Fabricius;f.Bursa of Fabricius of the chicken in AFB1 group Ⅲ.Apoptotic cells swallowed by reticular cell;g.Spleen of the chicken in control group;h.Spleen of the chicken in AFB1 group Ⅲ.Dilated perinuclear cisternae and vacuolated mitochondrial in lymphocytes (→);i.Spleen of the chicken in AFB1 group Ⅲ.Apoptotic plasmocyte in spleen圖2 21日齡雛雞的胸腺、法氏囊和脾的超微結構Fig.2 The thymus,bursa of Fabricius and spleen in the chicken from the control and AFB1 groups at 21 days of age
3.2 AFB1對雛雞免疫器官的形態學損傷
試驗結束時,AFB1Ⅱ、Ⅲ組(0.3、0.6 mg·kg-1)雛雞胸腺、法氏囊和脾的臟器指數下降。M.Manafi等[15]研究發現,肉雞日糧中AFB1含量為0.5 mg·kg-1時,胸腺和法氏囊的臟器指數顯著低于對照組。F.C.Quist等[16]研究發現,火雞日糧中AFB1含量超過0.1 mg·kg-1時,脾的臟器指數顯著低于對照組。本試驗結果與上述研究結果一致,表明AFB1對雛雞免疫器官的生長發育有一定的抑制作用。
組織學觀察結果顯示,雛雞采食含AFB10.3和0.6 mg·kg-1的日糧后,3個免疫器官均出現細胞核碎片增加以及淋巴細胞減少的病理變化。N.A.Omar[17]對小鼠脾的研究結果顯示,AFB1可導致脾中淋巴細胞減少以及形成空洞,本試驗中脾的病變與之相符。細胞核碎片的增多表明采食AFB1會導致免疫器官中的壞死淋巴細胞數量增多,超微病理學觀察結果證實淋巴細胞主要以凋亡的方式死亡,本研究采用流式細胞術亦檢測到AFB1組脾細胞的凋亡率升高[18]。壞死細胞增多可能引起淋巴細胞減少和實質萎縮[19-20],并導致免疫器官的臟器指數下降。在胸腺和脾中還觀察到淤血的病變,提示AFB1會導致胸腺和脾的血液循環障礙,進而導致細胞病變。AFB1Ⅲ組一只雛雞的法氏囊還出現間質結締組織增生的現象,這可能是較多量細胞壞死后機體的修復性反應,該結果還提示同種屬動物的不同個體對飼料中AFB1的敏感性存在差異。結構是功能的基礎,免疫器官出現病理損傷,雛雞的細胞和體液免疫功能必然下降。
超微結構的觀察顯示,AFB1可引起免疫器官核膜擴張;線粒體腫脹,嵴斷裂消失或形成空泡;淋巴細胞發生凋亡的頻率增高,凋亡細胞表現為染色質凝聚邊移形成月牙形或花環狀貼于核膜下,凋亡細胞多位于網狀細胞周圍或被網狀細胞吞噬。L.Rainbow等[21]對小鼠脾的研究也表明,AFB1引起脾淋巴細胞核膜擴張、線粒體嵴斷裂消失的病變。這些超微變化表明AFB1主要引起膜系統的損傷。大量的研究表明,AFB1能引起過多的脂質過氧化反應[22-24],脂質過氧化產生的自由基攻擊細胞膜系統,引起細胞膜、線粒體或內質網損傷;線粒體損傷后可觸發依賴于線粒體調控的Caspase途徑而引起細胞凋亡[25]。凋亡細胞主要位于網狀細胞周圍且凋亡細胞能夠被網狀細胞吞噬,這與組織學觀察到細胞核碎片主要位于網狀細胞周圍的現象相吻合,表明壞死或凋亡的淋巴細胞可能被網狀內皮細胞吞噬清除。
4 結 論
雛雞飼料中AFB1含量達0.15~0.6 mg·kg-1時,可不同程度地抑制雛雞免疫器官的發育,導致胸腺、法氏囊和脾組織中淋巴細胞數量減少,細胞核碎片與凋亡細胞數目增多。
[1] CONY P J,BHATNAGAR D.Variability among atoxigenic aspergillus flavus strains in ability to prevent aflatoxin contamination and production of aflatoxin biosynthetic pathway enzymes[J].ApplEnvironMicrobiol,1994,60(7):2248-2251.
[2] 侯然然,張敏紅.霉菌毒素對畜禽的危害及其防控方法的研究進展[J].中國畜牧獸醫,2007,34(1):13-16. HOU R R,ZHANG M H.Damage of mycotoxins on animals and advances in study on methods of revention[J].ChinaAnimalHusbandry&VeterinaryMedicine,2007,34(1):13-16.(in Chinese)
[3] AGUILAR F,HUSSAIN S P,CERUTTI P.Aflatoxin B1induces the transversion of G-T in codon 249 of the p53 tumor suppressor gene in human hepatocytes[J].ProcNatlAcadSciUSA,1993,90(18):8586-8590.
[4] 史瑩華,許梓榮,王成章.黃曲霉毒素對豬生長性能及免疫和抗氧化指標的影響[J].中國獸醫學報,2007,27(5):733-736. SHI Y H,XU Z R,WANG C Z.Effects of aflatoxin on growth performance and immunology and antioxidant indexes in pigs[J].ChineseJournalofVeterinaryScience,2007,27(5):733-736.(in Chinese)
[5] JIANG Y,JOLLY P E,ELLIS W O,et al.Aflatoxin B1albumin adduct levels and cellular immune status in Ghanaians[J].IntImmunol,2005,17(6):807-814.
[6] GHOSH R C,CHAUHAN H V,JHA G J.Suppression of cell-mediated immunity by purified aflatoxin B1in broiler chicks[J].VetImmunolImmunopathol,1991,28(2):165-172.
[7] CORRIER D E.Mycotoxicosis:mechanisms of immunosuppression[J].VetImmunolImmunopathol,1991,30(1):73-87.
[8] THAXTON J P,TUNG H T,HAMILTON P B.Immunosuppression in chickens by aflatoxin[J].PoultSci,1974,53(2):721-725.
[9] 石達友,李鵬飛,郭銘生,等.不同劑量黃曲霉毒素B1對雛鴨生長性能的影響[J].中國獸醫雜志,2010,46(4):22-23. SHI D Y,LI P F,GUO M S,et al.Effects of different doses of aflatoxin B1on growth performance in ducklings[J].ChineseJournalofVeterinaryMedicine,2010,46(4):22-23.(in Chinese)
[10] MARCHIORO A,MALLMANN A,DIEL A,et al.Effects of aflatoxins on performance and exocrine pancreas of broiler chickens[J].AvianDis,2013,57(2):280-284.
[11] KRYUKOV V,KRIVSTOV V,KRUPIN V,et al.Effect of aflatoxin on protein utilization by broilers[J].Pticeprvodsvo,1992,3:13-15.
[12] 劉艷麗,汪銘書,程安春,等.人工感染黃曲霉毒素雛鴨的病理學動態變化[J].中國獸醫科學,2006,36(5):396-400. LIU Y L,WANG M S,CHENG A C,et al.Pathological development of ducklings infected experimentally with aflatoxin[J].VeterinaryScienceinChina,2006,36(5):396-400.(in Chinese)
[13] ZHANG S,PENG X,FANG J,et al.Effects of aflatoxin B1exposure and sodium selenite supplementation on the histology,cell proliferation,and cell cycle of jejunum in broilers[J].BiolTraceElemRes,2014,160(1):32-40.
[14] HSIEH D P H.Mode of action of mycotoxins[M].Mycotoxins in food.Cambridge:Academic Press,1987:149-176.
[15] MANAFI M,UMAKANTHA B,ALI M N,et al.Study of the combination effects of aflatoxin and T-2 toxin on performance parameters and internal organs of commercial broilers[J].GlobalVet,2012,8(4):393-396.
[16] QUIST C F,BOUNOUS D I,KILBURN J V,et al.The effect of dietary aflatoxin on wild turkey poults[J].JWildlDis,2000,36(3):436-444.
[17] OMAR N A.Effect of some aflatoxins on a lymphatic organ (spleen) of male albino rats (histopathological study)[J].EgyptHospMed,2012,48(7):357-367.
[18] CHEN J,CHEN K,YUAN S,et al.Effects of aflatoxin B1on oxidative stress markers and apoptosis of spleens in broilers[J].ToxicolIndHealth,2013,0748233713500819.
[19] HASANZADEH S,HOSSEINI E,REZAZADEH L.Effects of aflatoxin B1on profiles of gonadotropic (FSH and LH),steroid (testosterone and 17β-estradiol) and prolactin hormones in adult male rat[J].IranJVetRes,2011,12(4):332-336.
[20] ORTATATLI M,OGUZ H,HATIPOGLU F,et al.Evaluation of pathological changes in broilers during chronic aflatoxin (50 and 100 ppb) and clinoptilolite exposure[J].ResVetSci,2005,78(1):61-68.
[21] RAINBOW L,MAXWELL S M,HENDRICKSE R G.Ultrastructural changes in murine lymphocytes induced by aflatoxin B1[J].Mycopathologia,1994,125(1):33-39.
[22] YANG X J,LU H Y,LI Z Y,et al.Cytochrome P450 2A13 mediates aflatoxin B1-induced cytotoxicity and apoptosis in human bronchial epithelial cells[J].Toxicology,2012,300(3):138-148.
[23] MEKI A R,ABDEL-GHAFFAR S K,EL-GIBALY I.Aflatoxin B1induces apoptosis in rat liver:protective effect of melatonin[J].NeuroEndocrinolLett,2001,22(6):417-426.
[24] BERNABUCCI U,COLAVECCHIA L,DANIELI P P,et al.Aflatoxin B1and fumonisin B1affect the oxidative status of bovine peripheral blood mononuclear cells[J].ToxicolInVitro,2011,25(3):684-691.
[25] ABRAHAM M C,SHAHAM S.Death without caspases,caspases without death[J].TrendsCellBiol,2004,14(4):184-193.
(編輯 白永平)
Effect of Aflatoxin B1on Pathological Changes of Immune Organs in Broilers
YU Zheng-qiang1#,CHEN Jin2#,PENG Xi1*,FANG Jing1,CHEN Ke-jie1,HE Yang1
(1.CollegeofVeterinaryMedicine,SichuanAgricuturalUniversity,Chengdu611130,China; 2.AnimalHusbandryBureauofXichang,Xichang615000,China)
The aim of the current study was to investigate the histopathological and ultrastructural changes caused by dietary AFB1in broilers.One hundred one-day-old avian male broilers were randomly divided into four equal groups and were fed for 21 days as follows:a control diet and three AFB1addition diets containing 0.15,0.3 and 0.6 mg·kg-1AFB1,respectively.The results showed that the relative weight of the three organs were lower than those of the control group (P<0.05).Histopathologically,in the AFB1groups,there were increased nuclear debris around the reticulocytes in the cortex of the thymus;the number of lymphocytes was decreased in the medulla,and increased nuclear debris can be observed in the lymphoid follicle of the Bursa of Fabricius;more nuclear debris appeared around lymphoid follicles and lymphatic sheath in the chicken spleens.The ultrastructural changes were mitochondria swelling and increased apoptotic cells characterized as chromatin margination in the lymphocytes of the three immune organs.These results indicated that when the contents of dietary AFB1were from 0.15 to 0.6 mg·kg-1,the development of immune organs could be inhibited,and the major lesions of the three immune organs were the decrease of lymphocytes and the increase of nuclear debris.
aflatoxin B1;thymus;bursa of Fabricius;spleen;apoptosis;broilers
10.11843/j.issn.0366-6964.2015.08.022
2014-11-12
“教育部長江學者和創新團隊發展計劃”創新團隊(IRT 0848);四川省科技廳資助項目(2013FZ0072)
于正強(1990-),男,重慶榮昌人,碩士,主要從事動物病理學研究,E-mail:mine_yzq@163.com;陳 瑾(1988-),女,四川冕寧人,碩士,主要從事動物病理學研究, E-mail:chenjin19880808@126.com。于正強、陳瑾為并列第一作者
*通信作者:彭 西(1973-),教授,E-mail:pengxi197313@163.com
S852.35;S856.9
A
0366-6964(2015)08-1447-08

