雜色曲霉轉(zhuǎn)化孕酮的研究
楊海英,李忠孝,沈廣劍,張振宇,李淑娟,杜 剛
(云南民族大學(xué) 民族藥資源化學(xué)國(guó)家民族事務(wù)委員會(huì)-教育部重點(diǎn)實(shí)驗(yàn)室,云南 昆明 650500)
雜色曲霉轉(zhuǎn)化孕酮的研究
楊海英,李忠孝,沈廣劍,張振宇,李淑娟,杜 剛
(云南民族大學(xué) 民族藥資源化學(xué)國(guó)家民族事務(wù)委員會(huì)-教育部重點(diǎn)實(shí)驗(yàn)室,云南 昆明 650500)
利用雜色曲霉對(duì)孕酮進(jìn)行生物轉(zhuǎn)化,通過(guò)HPLC對(duì)轉(zhuǎn)化產(chǎn)物進(jìn)行檢測(cè),硅膠柱層析分離純化轉(zhuǎn)化產(chǎn)物,得到3個(gè)轉(zhuǎn)化產(chǎn)物.運(yùn)用NMR、MS、IR等波譜數(shù)據(jù)對(duì)轉(zhuǎn)化產(chǎn)物進(jìn)行結(jié)構(gòu)鑒定,確定轉(zhuǎn)化產(chǎn)物結(jié)構(gòu)為:雄烯二酮(AD);睪酮;雄二烯二酮(ADD).研究結(jié)果表明,雜色曲霉菌株能夠裂解孕酮C-17位的側(cè)鏈,生成19個(gè)碳的轉(zhuǎn)化產(chǎn)物,并對(duì)裂解產(chǎn)物進(jìn)行結(jié)構(gòu)修飾.
生物轉(zhuǎn)化;雜色曲霉;孕酮;結(jié)構(gòu)鑒定
篩選優(yōu)良菌株、發(fā)掘新的生物催化劑是發(fā)展甾體藥物工業(yè)的重要課題.1952年美國(guó)普強(qiáng)公司的Murray和Peterson利用黑根霉轉(zhuǎn)化孕酮11位羥基化,并實(shí)現(xiàn)了工業(yè)化生產(chǎn),這一工作使可的松的合成步驟減少到十一步,大大降低了生產(chǎn)成本,到1967年可的松的生產(chǎn)成本已降到1美元/g[1-3].這是第一個(gè)成功的生物轉(zhuǎn)化工業(yè)化項(xiàng)目,證明了微生物技術(shù)可以應(yīng)用于大規(guī)模的甾體藥物生產(chǎn).自此以后,微生物轉(zhuǎn)化甾體化合物的研究被廣泛開(kāi)展,微生物轉(zhuǎn)化甾體類藥物成為生物轉(zhuǎn)化在大規(guī)模工業(yè)應(yīng)用中最成功的范例之一,在甾體藥物生產(chǎn)中具有高選擇性、高轉(zhuǎn)化率、條件溫和及低化學(xué)污染等優(yōu)點(diǎn)[4].
孕酮是一種孕激素,是合成皮質(zhì)激素的重要中間體,也是最早進(jìn)行微生物轉(zhuǎn)化研究的甾體化合物,雄烯二酮(AD)和雄二烯二酮(ADD)為最具市場(chǎng)價(jià)值的甾體藥物中間體,市場(chǎng)容量達(dá)每年10億美元[5].本研究以實(shí)驗(yàn)室保藏雜色曲霉菌株對(duì)孕酮進(jìn)行生物轉(zhuǎn)化初步研究,為該菌株在甾體藥物研發(fā)中的應(yīng)用提供依據(jù).
1 儀器試劑
1.1 材料
孕酮購(gòu)自江蘇省鹽城信誼醫(yī)藥化工有限公司,經(jīng)NMR及HPLC檢測(cè)純度>98%.
1.2 微生物菌株
雜色曲霉分離自滇重樓根狀莖,為本實(shí)驗(yàn)室保藏菌株,實(shí)驗(yàn)室編號(hào)YNCA0098.
1.3 轉(zhuǎn)化過(guò)程及粗提物制備
發(fā)酵培養(yǎng)基含3 g/L NaNO3,1 g/L K2PO4,0.5 g/L MgSO4·7H2O,0.5 g/L KCl,0.01 g/L FeSO4·7H2O,30 g/L蔗糖,pH 6.5,孕酮1.0 g/L,孕酮加少量乙酸乙酯溶解,再加4 g/L吐溫80混勻,加水形成乳濁液加入1 L培養(yǎng)基,進(jìn)行放大培養(yǎng)發(fā)酵.250 mL錐形瓶裝入100 mL發(fā)酵液,共發(fā)酵8 L.置于28 ℃,220 r/min振蕩培養(yǎng)7 d.發(fā)酵液用乙酸乙酯萃取,菌絲體用甲醇浸泡超聲提取,真空濃縮合并得到粗提物浸膏.
1.4 HPLC分析
色譜柱:Agilent ZORAX SB-C18柱/4.6×150 mm,5 μm;檢測(cè)器:二級(jí)管陳列檢測(cè)器(DAD);流動(dòng)相(體積比):甲醇/水=60/40;流速:1.0 mL/min;檢測(cè)波長(zhǎng):245 nm;柱溫:40 ℃;進(jìn)樣量:5 μL.取0.05 g粗提物,加入1 mL甲醇溶解,用0.45 μm過(guò)濾膜過(guò)濾,進(jìn)行HPLC分析.
1.5 孕酮傳化產(chǎn)物的分離純化
正相硅膠(200~300 目):樣品=10∶1(V/V)裝柱上樣,以V(石油醚)∶V(乙酸乙酯)為10∶1~1∶5逐級(jí)梯度洗脫,初步分離劃段.通過(guò)TLC檢測(cè),含轉(zhuǎn)化產(chǎn)物組分再次用正相硅膠柱進(jìn)行分離,以V(氯仿)∶V(甲醇)為100∶1~60∶1梯度洗脫.
2 實(shí)驗(yàn)結(jié)果
2.1 轉(zhuǎn)化結(jié)果HPLC分析
雜色曲霉YNCA0098轉(zhuǎn)化底物孕酮后發(fā)酵液的HPLC圖見(jiàn)圖1.
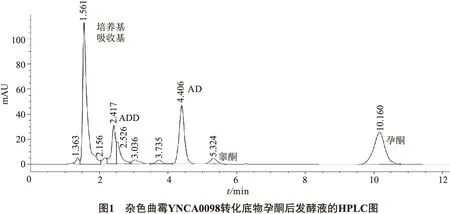
由HPLC圖可以看出,雜色曲霉YNCA0098轉(zhuǎn)化孕酮不完全,轉(zhuǎn)化產(chǎn)物極性變大.添加的底物孕酮出現(xiàn)在10.160 min,轉(zhuǎn)化反應(yīng)新出現(xiàn)3個(gè)峰,分別為5.324 min,4.406 min和2.417 min,經(jīng)后期分離及結(jié)構(gòu)鑒定,確定5.324 min峰為睪酮峰,4.406 min峰為雄烯二酮峰,2.417 min峰為雄二烯二酮峰.
2.2 孕酮轉(zhuǎn)化產(chǎn)物結(jié)構(gòu)鑒定
雜色曲霉轉(zhuǎn)化孕酮(1)得到的3個(gè)轉(zhuǎn)化產(chǎn)物,經(jīng)核磁共振、紅外光譜、質(zhì)譜、熔點(diǎn)檢測(cè),結(jié)構(gòu)鑒定分別為:雄烯二酮(AD)(2);睪酮(3);雄二烯二酮(ADD)(4).
化合物的理化鑒定結(jié)果如下:
雄烯二酮(AD)(2),白色粉末,m.p.171~172 ℃;IR(KBr)νmax:3 449,2 952,2 919,2 849,1 735,1 663,1 128 cm-1;1H NMR(CDCl3,400 MHz)δ:5.69(1H,s,H-4),0.86(3H,s,H-18),1.16(3H,s,H-19);13C NMR(CDCl3,100 MHz)δ:35.6(t,C-1),34.9(t,C-2),199.3(C=O,C-3), 124.1(d,C-4),170.4(s,C-5),32.5(t,C-6),30.7(t,C-7),35.1(d,C-8),54.7(d,C-9),38.6(s,C-10),20.3(t,C-11),35.7(t,C-12),47.5(s,C-13),50.8(d,C-14),21.7(t,C-15),31.2(t,C-16),220(C=O,C-17),14.7(q,C-18),17.3(q,C-19).ESI-MS(m/z):285.2[M-H]-;309.2[M+Na]+.分子式:C19H26O2.與文獻(xiàn)[6-8]對(duì)照一致.
睪酮(3),白色針狀結(jié)晶,m.p.142~144 ℃;IR(KBr)νmax:3 398,2 929,1 654,1 131,1 066 cm-1.1H NMR(CDCl3,400 MHz)δ:5.70(1H,s,H-4),0.76(3H,s,H-18),1.16(3H,s,H-19);13C NMR(CDCl3,100 MHz,δ):36.8(t,C-1),37.4(t,C-2),202.0(C=O,C-3), 124.1(d,C-4),175.1(s,C-5),34.9(t,C-6),32.9(t,C-7),36.8(d,C-8),55.5(d,C-9),40.0(s,C-10),21.7(t,C-11),37.7(t,C-12),44.9(s,C-13),51.8(d,C-14),24.2(t,C-15),30.6(t,C-16),220(C=O,C-17),11.6(q,C-18),17.7(q,C-19);ESI-MS(m/z)311.2[M+Na]+,327.2 [M+K]+.分子式:C19H28O2.與文獻(xiàn)[6]對(duì)照一致.
雄二烯二酮(ADD)(4),白色粉末.m.p.139~141 ℃,IR(KBr)νmax:3 455,2 936,2 845,1 739,1 657,1 129,1 064 cm-1;1H NMR(CDCl3,400 MHz)δ:7.01(1H,m,H-2),6.21(1H,m,H-2),6.03(1H,m,H-4),0.92(3H,s,H-18),1.22(3H,s,H-19);13C NMR(CDCl3,100 MHz)δ:155.4(d,C-1),127.6(d,C-2),186.1(C=O,C-3), 124.0(d,C-4),168.4(s,C-5),32.3(t,C-6),31.1(t,C-7),35.0(d,C-8),52.2(d,C-9),44.4(s,C-10),22.0(t,C-11),32.5(t,C-12),47.6(s,C-13),50.3(d,C-14),21.9(t,C-15),35.6(t,C-16),219.6(C=O,C-17),14.8(q,C-18),18.7(q,C-19);ESI-MS(m/z):284.2[M-H]-,307.2[M+Na]+.分子式:C19H24O2.與文獻(xiàn)[7,9]對(duì)照一致.

2.3 轉(zhuǎn)化途徑推測(cè)
根據(jù)化合物在轉(zhuǎn)化過(guò)程中出現(xiàn)順序及結(jié)構(gòu)特點(diǎn),推測(cè)孕酮首先裂解為AD,17位羰基被還原為羥基,得到睪酮,C-1和C-2之間脫氫得到ADD.孕酮生物轉(zhuǎn)化路線推測(cè)如圖2.
3 結(jié)語(yǔ)
雜色曲霉YNCA0098轉(zhuǎn)化孕酮得到3個(gè)轉(zhuǎn)化產(chǎn)物,通過(guò)NMR、MS、IR等波譜數(shù)據(jù)對(duì)轉(zhuǎn)化產(chǎn)物進(jìn)行結(jié)構(gòu)鑒定,確定為AD、ADD和睪酮,推測(cè)轉(zhuǎn)化過(guò)程為孕酮首先裂解為AD,17位羰基被還原為羥基,得到睪酮,C-1和C-2之間脫氫得到ADD.由YNCA0098轉(zhuǎn)化孕酮發(fā)酵液的HPLC分析圖譜上可以看出,底物孕酮轉(zhuǎn)化不完全,經(jīng)分離得到三個(gè)轉(zhuǎn)化產(chǎn)物.轉(zhuǎn)化過(guò)程為AD、ADD和睪酮的生產(chǎn)提供了新的可能途徑,在下一步工作中還需優(yōu)化轉(zhuǎn)化條件,提高孕酮的轉(zhuǎn)化率,同時(shí)提高轉(zhuǎn)化的專一性.
[1] DONOVA M,EGOROVA O.Microbial steroid transformations:current state and prospects[J].Appl Microbiol Biotechnol,2012,94(6):1423-1447.
[2] 諸有義.生物合成藥物學(xué)[M].北京:化學(xué)工業(yè)出版社,2000:649.
[3] 諸秉根.甾體激素和其作用機(jī)制發(fā)現(xiàn)的歷史回顧[J].醫(yī)學(xué)與哲學(xué),1997,18(4):193-195.
[4] CARBALLEIRA J D,QUEZADA M A,HOYOS P,et al.Microbial cells as catalysts for stereoselective red-ox reactions[J].Biotechnol Adv,2009,27(6):686-714.
[5] CARBALLEIRA J D,QUEZADA M A,HOYOS P,et al.Microbial cells as catalysts for stereoselective red-ox reactions[J]. Biotechnol Adv,2009,27(6):686-714.
[6] BLUNT J W,STOTHERS J B.13C n.m.r.spectra of steroids —a survey and commentary[J].Organic Magnetic Resonance,1977,9(8):439-464.
[7] KIRK D N,MILLER B W,LATIF S A,et al.18-substituted steroids .16.synthesis of 6-beta-hydroxy-aldosterone and 6-alpha-hydroxy-aldosterone and their 17-alpha-isomers[J].J Chem Res-S,1989,6 :164-165.
[8] FARAMARZI M A,YAZDI M T,AMINI M.Microbial production of testosterone and testololactone in the culture of Aspergillus terreus[J].World J Microb Biot,2004,20:657-660.
[9] KIRK D N,TOMS H C,DOUGLAS C,et al.A survey of the high-field 1H NMR spectra of the steroid-hormones,their hydroxylated derivatives,and related-compounds[J].J Chem Soc,Perkin Trans 2,1990,9:1567-1594.
(責(zé)任編輯 王 琳)
Biotransformation of progesterone byAspergillusversicolor
YANG Hai-ying,LI Zhong-xiao,SHEN Guang-jian,ZHANG Zhen-yu,LI Shu-juan,DU Gang
(Key Laboratory of Chemistry in Ethnic Medicinal Resources,State Ethnic Affairs Commission and Ministry of Education of China,School of Chemistry and Biotechnology,Yunnan Minzu University,Kunming 650500,China)
Progesterone was transformed byAspergillusversicolorand three transformation products were obtained. These products were elucidated by13C NMR,1H NMR,FTIR and MS. The structures of these compounds were androstenedione,testosterone and androstanedienedione. The results showed that the progesterone could be split at C-17 byAspergillusversicolor.
biotransformation;Aspergillusversicolor; progesterone; structural identification
2014-11-23.
云南省教育廳科學(xué)研究基金重點(diǎn)項(xiàng)目(2013Z038);云南民族大學(xué)化學(xué)與生物技術(shù)學(xué)院SRT項(xiàng)目(2013HXSRT05).
楊海英(1975-),女,碩士,副教授,碩士生導(dǎo)師.主要研究方向:天然產(chǎn)物及生物催化.
杜剛(1973-),男,博士研究生,副教授,碩士生導(dǎo)師.主要研究方向:生物催化.
O629.2
A
1672-8513(2015)02-0126-03

