兩個由兩性二酸配體構筑的Cd(Ⅱ)低維配合物的合成、結構及熒光性質
丁芳芳 張 娜 張建勇*, 王 敏 高恩慶
(1上海應用技術學院材料科學與工程學院,上海201418)
(2電磁散射重點實驗室,上海200438)
(3華東師范大學,上海市綠色化學與化工過程綠色化重點實驗室,上海200062)
兩個由兩性二酸配體構筑的Cd(Ⅱ)低維配合物的合成、結構及熒光性質
丁芳芳1張 娜1張建勇*,1王 敏2高恩慶3
(1上海應用技術學院材料科學與工程學院,上海201418)
(2電磁散射重點實驗室,上海200438)
(3華東師范大學,上海市綠色化學與化工過程綠色化重點實驗室,上海200062)
利用合成的兩性離子二酸配體1-(4-carboxylatobenzyl)pyridinium-4-carboxylate(HL),通過調變合成條件,制備了2個低維的配合物{Cd(L)2·4H2O}n(1),和{[Cd(L)(N3)]·3H2O}n(2),并對其進行了元素分析(EA)、紅外光譜(IR)、熱重(TG)、熒光光譜及X-射線單晶衍射測定。分析結果顯示化合物1中八配位的Cd(Ⅱ)離子被4個L配體連接形成一維鏈狀結構。而化合物2中六配位的Cd(Ⅱ)離子被雙羧基-疊氮三重橋聯形成二維層狀結構。固體熒光測試表明配體的配位方式明顯影響其熒光發射。
兩性離子配體;晶體結構;配位聚合物;熒光;Cd(Ⅱ)配合物
0 Introduction
Overthepastdecade,theconstructionof coordination polymers(CPs)that are composed of infinite of metal ions connected by functionalized organic linkers,have been carried out by many researchers,because of not only their fascinating structures,but also their potential applications[1-3],for example in the areas of gas adsorption[4],catalysis[5],ion recognition[6]and luminescent properties[7].The multidentate ligands with conjugated groups are selected as organic linkers in the design of variable coordination frameworks because the flexibility of organic backbone, conformational preference and symmetry of organic ligands can lead to a remarkable series of materials with variousstructuresandproperties.Amongthem, aromatic multicarboxylate ligands,due to strong and versatile coordination abilities towards metal ions have been widely used for the construction of CPs in recent years.Compared with the usual carboxylate carboxylate ligands,thechemistryofzwitterioniccarboxylate ligands have been studied limitedly so far[8].As illustratedinScheme1,comparedwithnormal dicarboxylateligands,suchdicarboxylateligand bearing separated positive(pyridinium)and negative (carboxylate)charges can overcome the unbalanced competition of two anionic bridges in binding metal ions and compensating for metal charge.Our interest lies in the construction of coordination polymers based on various multicarboxylate ligands comprises zwitterionic carboxylate moieties.Recently,we have successfully constructed some newly CPs with dimensionalities from 1D to 3D using a series of zwitterionic carboxylate ligands and systematically studied the influences of metalions,shapeofthedifferentzwitterionic carboxylate ligands on the structures and properties of final compounds formed[9].As an extension of this research,we present here two new Cd(Ⅱ)complex with a flexiblezwitterionicdicarboxylate1-(4-carboxylatobenzyl)pyridinium-4-carboxylate(HL)ligand,{Cd(L)2· 4H2O}n(1),and{[Cd(L)(N3)]·3H2O}n(2).Their structures havebeencharacterizedbyX-raysingle-crystal diffraction analyses.Interestingly,the fluorescence measurements show that the emission bands can be correlated to the coordination modes of the L ligand.

Scheme 1Synthetic route to the zwitterionic dicarboxylate ligand 1-(4-carboxylatobenzyl)pyridinium-4-carboxylate ligand(HL)
1 Experimental
1.1 General
All the metal salts and 4-(bromomethyl)benzoic acid ethyl ester,ethyl isonicotinate were purchased and used without further purification.
1H NMR spectra were recorded on a Bruker AVANCEIII NMR spectrometer at 400 MHz,using DMSO-d6as a locking solvent.Elemental analyses (EA)were determined on an Elementar Vario ELIII analyzer.FTIR spectra were recorded in the range of 500~4 000 cm-1using KBr pellets on a Nicolet NEXUS670spectrophotometer.Thermogravimetric analyses(TGA)were carried out on a Mettler Toledo TGA/SDTA851 instrument under flowing air at a heating rate of 10℃·min-1.Powder X-ray diffraction data were collected on a Bruker D8 ADVANCE diffractometer equipped with Cu Kα(λ=0.154 18 nm) at a scan speed of 5°·min-1.Excitation and emission spectra were recorded on an F-4500 luminescence spectrophotometer.
1.2 Synthesis
1.2.1 Synthesis of the ligand HL
The ligand HL was prepared according to theliterature following a method(Scheme 1)as described below[10].4-(bromomethyl)benzoic acid ethyl ester(15.0 mmol,3.46 g)was dissolved in 20 mL of absolute ethanol followed by the dropwise addition of ethyl isonicotinate(10.0 mmol,1.52 g)and the reaction mixture was refluxed under vigorously stirred for 72 h. Theexcessethanolwasthenevaporatedunder reduced pressure,yielding crude products,which was then recrystallized from hot H2O to give the ester of HL.The resultant was dissolved in 10wt%HCl(100 mL)and was subsequently refluxed for 24h giving colorless powder after removal of the solvent.Removal of bromide and chloride ions with moist silver(I) oxide afforded the colorless HL.Diamond shaped crystals suitable for single-crystal X-ray diffraction analyses were grown by crystallization from H2O (Yield:86%).Elemental analysis Calcd.for C14H13NO5(M=275.26)(%):C,61.09;H,4.76;N,5.09.Found(%): C,61.16;H,4.57;N,5.36.IR(KBr disc,cm-1)3 448 (s),3 050(m),1 675(s),1 634(s),1 563(m),1 376 (m),759(m),542(m).1H NMR(400 MHz,DMSO-d6, TMS,25℃):δ 12.96(m,1H,-COOH),9.22(d,1H,Py-H),8.41(d,1H,Py-H),7.98(d,1H,Ph-H),7.64(d,1H, Ph-H);6.05(m,1H,-CH2),
1.2.2 Synthesis of{Cd(L)2·4H2O}n(1)
Compound 1 was prepared by the following method.A solution of HL(0.10 mmol,0.027 g)in DMSO(3 mL)was added dropwise to a stirred aqueous solution(2 mL)of CdCl2·4H2O(0.10 mmol, 0.03 g);then NaN3(0.2 mmol,0.013 g)dissolved in H2O(2 mL)was added slowly.The resulting mixture was stirred for 1 h at room temperature and then filtered to remove any possible precipitate.The clear colorless solution was left to evaporate undisturbed at roomtemperature.Colorlessblockcrystalsof1 appeared after two days(Yield:44%).Elemental analysis Calcd.for C28H28N2O12Cd(M=696.92)(%):C, 48.21;H,4.05;N,4.02.Found(%):C,48.07;H,4.23; N,3.85.IR(KBr,cm-1):3 433(br),3 118(w),3061(m), 3 020(w),2 694(m),2 584(w),2 551(w),2 445(w), 2 403(w),1 795(w),1 707(vs),1 641(m),1 614(m), 1 576(m),1 510(w),1 462(m),1 394(vs),1 323(w), 1 240(vs),1 173(s),1 117(s),1 045(w),1 017(w),928 (m),906(w),870(m),835(m),746(s),681(m).
1.2.3 Synthesis of{[Cd(L)(N3)]·3H2O}n(2)
Colorless crystals of 2 were obtained by slow diffusion in an H-shaped tube.An aqueous solution(3 mL)containing HL(0.10 mmol,0.027 g)and NaN3(0.50 mmol,0.033 g)and the solution of CdCl2·4H2O (0.10mmol,0.03 g)in the same solvent(3 mL)were added into,respectively,the two arms of a H-shaped tube,and then about 15 mL of ethanol was carefully added till the bridge of the tube was filled.Slow diffusion between the two solutions afforded rodshaped crystals of 2 within one week.Yield:59%. Elemental analysis Calcd.for C14H16N4O7Cd(M= 464.71)(%):C,36.18;H,3.47;N,12.06.Found(%): C,40.29;H,3.56;N,11.97.IR(KBr,cm-1):3 444 (br),3 113(m),3 049(m),2 964(w),2 071(vs),1 624 (vs),1 562(vs),1 456(m),1 392(vs),1 192(w),1 186 (w),1 134(w),1 045(w),864(w),806(m),768(s),690 (m).
1.3 Crystallographic Studies
Single crystal diffraction data of HL,1 and 2 were respectively collected on a Bruker APEXⅡdiffractometer equipped with a CCD area detector and graphite-monochromated Mo Kα radiation(λ=0.071 073 nm)atroomtemperature.Empiricalabsorption corrections were applied using the SADABS program[11]. The structure was solved by the direct method and refined by the full-matrix least-squares method on F2, with all non-hydrogen atoms refined with anisotropic thermal parameters[12].Hydrogen atoms were placed in calculated positions and refined isotropically using the riding model.For compounds 1 and 2,the final structures have a large volume fraction of solvent accessible voids(22.5 and 33.7%for 1 and 2, respectively)containing a number of residual electron density peaks,which may be attributed to solvent H2O molecules but could not be satisfactorily modeled, perhaps due to the heavy disorder and the limited quality of the dataset.To improve the refinement,the SQUEEZEroutinewithinthePLATONsoftware package[13]was applied to subtract the scattering contributions of the solvent molecules to the intensity data.In compound 2,the orthorhombic Imma groupimposes C2vpoint symmetry on the HL ligand,so the pyridyl nitrogen atom(N4)and the benzene carbon atoms(C5)wererefinedtooccupythesame crystallographic position with the same displacement parameters and bisected occupancy factors.Pertinent crystallographicdataandstructurerefinement parameters are summarized in Table 1.
CCDC:1031439,HL;1031440,1;1031441,2.

Table 1Crystallographic data and structure refinements parameters for compounds HL,1 and 2
2 Results and discussion
Both compounds were prepared by the reaction of cadmium(Ⅱ)chloride,sodium azide and the HL ligands.However,small changes in the syntheses seem to influence on the final structure.For the synthesis of 1,although cadmium(Ⅱ)chloride and sodium azide were first well mixed in water before a DMSO solution of HL ligands was added dropwise,the azide ions did not coordinate to the Cd(Ⅱ)centers,which may be due to the precipitation of the azide ions by Cd(Ⅱ)ions. When the cadmium(Ⅱ)chloride was diffused slowly into the water solution of HL ligands with sodium azide in an H-shaped tube,compound 2 was isolated, in which the azide ion bridged the neighboring Cd(Ⅱ)centers.It should be noted that in the synthesis of compound 2,a large excess of sodium azide was necessary for the crystallization of the compounds.The azide ions not only act as ligands to bind Cd(Ⅱ)ions, but also could serve to facilitate the deprotonation of HL.
2.1 Thermal analyses and PXRD patterns
Thermogravimetric analysis(Fig.1a)indicates that compound 1 lost its guest water molecules in the range of 25~90℃.The weight loss(10.9%) correspondstothereleaseoffourguestwater molecules(Calcd.10.3%)per formula,and the dehydrated solid shows no weight loss until ca.280℃.Compound 2 loses the water molecules(Found 12.3%,Calcd.11.6%)upon heating from room temperature to 105℃,and there is no significant weight loss until 270℃,above which a rapiddecomposition occurs.The phase purity of the two productswasalsoconfirmedbycomparingthe experimental PXRD patterns with those calculated from the single-crystal structural data(Fig.1b).

Fig.1TGA and PXRD patterns for compounds 1 and 2

Thermal ellipsoids at 30%probabilityFig.2ORTEP representation of the crystal structure for HL
2.2 Description of the structure
AsshowninScheme1,thezwitterionic dicarboxylate ligand(HL)was synthesized by the reaction of 4-(bromomethyl)benzoic acid ethyl ester withethylisonicotinateinabsoluteethanoland hydrolysis.Single-crystal X-ray diffraction analyses revealed that HL crystallized in the monoclinic P21/c space group.As shown in Fig.2,the HL free ligand adopts a highly twisted conformation,in which the two aromatic rings are perpendicular to each other with a large dihedral angle of 75.97(10)°,and the∠C8-C7-N1 angle is 114.24(21)°.Adjacent HL ligands are interlinked into 1D chains through strong hydrogen bonding interactions,which involves the carboxylate oxygen atom(O3)and hydroxyl groups(O1-H)from the other carboxylate groups(H1…O3 0.127 4(51) nm,O1…O3 0.249 3(3)nm).
X-ray analyses reveal that compound 1 possesses 1D polymeric coordination chain with a space group C2/c.The molecular structure is shown in Fig.3,and selected bond distances and angles are given in Table 2.The Cd(Ⅱ)ion is coordinated to four chelating carboxylate groups from four L ligands with the Cd-O bond distances in the range of 0.237 6~0.253 9 nm. The L ligand adopts bis(chelate)mode(μ4,η2)to bridge neighboring Cd(Ⅱ)ions forming 1D coordination polymer chains[Cd(L)2]n,extending along the(101) direction,with a long Cd…Cd distance of 1.293 nm. Due to the V-shaped conformation of the L ligands, the two aromatic rings are titled to each other giving a dihedral angle of 79.31(07)°,and the∠C8-C7-N1 angle is 109.54(17)°.Compared with the free HL ligand,both the angles change larger,maybe due to the coordination to Cd(Ⅱ),changing the rigidities and conjugation.The L ligands are hump alternately up and down from the chain,resulting in 1D rhombic channel along the b direction,occupied by the guest water molecules.PLATON calculations revealed that the guest-accessible volume is 0.672 2 nm3per unit cell[13],comprising 22.5%of the crystal volume.
As shown in Fig.4,the individual ribbon chains are further packed into 3D supramolecular networkvia the hydrogen bonding interactions between the coordinated carboxylate oxygen atoms(O2,O4)and guest water molecules.

Fig.3(a)Coordination environments in 1((b)View of the 1D ribbon chain of 1 linked by the L ligands

Fig.43D supramolecular network constructed via the hydrogen bonds

Table 2Selected bond(nm)and angles(°)for compound 1
SinglecrystalX-rayanalysesrevealedthat compound 2 crystallized in the orthorhombic space group Imma and containing 2D coordination networks.The coordination environment of the Cd(Ⅱ)ion is shown in Fig.5 and the relevant parameters are summarized in Table 3.The unique Cd(Ⅱ)ion resides at the crystallographic 2/m position and adopts transoctahedral[N2O4]coordination geometry,completed by four equivalent carboxylate oxygen atoms(O1,O1B, O1C and O1E)and two equivalent nitrogen atoms(N1 and N1D).Different from that in 1,the L ligand, having the C2vpoint symmetry,serves as a syn-syn-μ4bridge using its two carboxylate groups to connect four Cd(Ⅱ)ions(Cd-O,0.229 5(2)nm,Table 3),and due to the C2vsymmetry,the pyridyl nitrogen atom(N4)and one(C5)of the benzene carbon atoms are disordered. The azide ion adopts end-on μ-1,1 mode to bridge neighboring Cd(Ⅱ)ions(Cd-N,0.215 8(2)nm).Thus the adjacent Cd(Ⅱ)ions are triply bridged by two carboxylate groups and an azide ion to generate a 1D uniform[Cd(N3)(OCO)2]nchain running along the a direction.The Cd…Cd distance separated by the triple bridges is 0.373(1)nm,and the bridging angle Cd…N…Cd is 109.86(1)°.

Fig.5Coordination environments in 2

Fig.6(a)Top view of the 2D network;(b)side view of the space-filling diagram along a direction showing the 1D rhombic channels
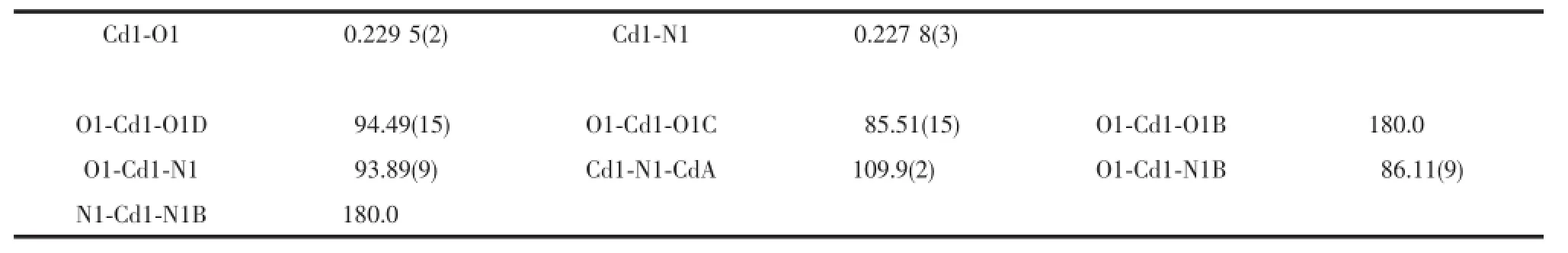
Table 3Selected bond(nm)and angles(°)for compound 2
Thecompoundconsistsof2Dcoordination network parallel to the ab plane,in which identical anionic[Cd(N3)(OCO)2]nchains are further interlinkedthrough the cationic N-benzylpyridinium backbones of the L ligands,with Cd…Cd distance of 1.419 12(7) nm,which is equal to the b dimension of the unit cell(Fig.6a).Due to the V-shaped conformation of the L ligand,the two aromatic rings are titled to each other with an dihedral angle of 64.01(15)°and the∠C5-C6-N4 angle is 112.46(23)°,and both angles are also smaller than those in the free HL ligand.The layer is tunneled through by 1D rhombic channels alongthechaindirection(Fig.6b).PLATON calculations revealed that the guest-accessible volume is 0.626 3 nm3per unit cell[13],comprising 33.7%of the crystal volume.The free spaces in the framework are occupied by the structural disordered H2O guest molecules.
The 2D coordination sheets are stacked in an offset ABAB fashion(Fig.7),with an interlayer separation of 0.915 4(2)nm.Interlayer interdigitation occurs with the methylene CH groups of a layer protruding towards the mesh centers of the two neighboring layers,and this allows the formation of interlayer C-H…N hydrogen bonds:each N3 atom from one layer interact with six CH groups from the neighboring layer,including two equivalent methylene C6-H groups and four equivalent aromatic C4-H groups)(H…N 0.274~0.295 nm,C…N 0.362~0.378 nm,∠C-H…N 144°~158°)and the nearest interlayer Cd…Cd distance is 1.129 4(3)nm. Additionally,the 3D supramolecular structure are further sustained by π-π stacking interactions between the parallel aromatic rings(benzene/pyridyl)from different layers,with the center-to-center(0.349 8(1) nm)and interplanar distances(0.374 79(6)nm).

Fig.73D structure constructed via the hydrogen bonds and π-π interactions between the ABAB stacking sheets

Fig.8(a)solid-state emission spectra and excitation spectra(inset)of HL ligand and compounds 1,2 at room temperature;(b)The CIE chromaticity diagrams
2.3 Luminescent properties
CPs with d10metal centers are desirable candidates for fluorescent materials due to their ability to affect the emission wavelength of the organic materials by metal coordination[7a,14].The luminescent measurements of the HL ligand and corresponding compounds 1 and 2 in the solid state were carries out at room temperature.As can be seen from Fig.8,compounds 1 and 2 exhibit blueemissions at 427 and 453 nm,respectively,in the similar excitation(about 350 nm).Meanwhile,the maximum emission of the free HLligand in solid state is observed at 384 nm(λex=319 nm).In comparison with the emission peak of free HL ligand,the obvious redshift observed in compounds 1 and 2 can be tentatively assigned to the cooperative effects of intraligand π-π* transitions and ligand-to-metal charge transfer(LMCT), which is not rare for the complexes of d10metal like Cd(Ⅱ)[7a,14-15].The bathochromic shifts ofemission occurring in 1 and 2 are possibly assigned to different coordinationeffectbetweentheligandsandmetalions[16].
Acknowledgements:We are thanking for the financial support of Key Discipline Grant for Composite Materials from Shanghai Institute of Technology(No.10210Q140001)and the Foundation of Science and Technology Development of Shanghai (No.14ZR1447900).
[1]Furukawa H,Cordova K E,Okeeffe M,et al.Science, 2013,341:974-986
[2]Batten S R,Neville S M,Turner D R.Coordination Polymers: Design,Analysis,Application.London:Royal Society of Chemistry,2009:273-372
[3]Zhou H C,Long J R,Yaghi O M,et al.Chem.Rev.,2012, 112:673-1268
[4](a)Langmi H W,Ren J,North B,et al.Eletrochim.Acta, 2014,128:368-392
(b)Li J R,Ma Y,McCarthy M C,et al.Coord.Chem.Rev., 2011,255:1791-1823
[5](a)Wang Z,Chen G,Ding K.Chem.Rev.,2000,109:322 -359
(b)Dhakshinamoorthy A,Garcia H.Chem.Soc.Rev.,2012, 41:5262-5284
[6](a)Kreno L E,Leong K,Farha O K,et al.Chem.Rev., 2012,112:1105-1125
(b)Lin X,Gao G,Zheng L,et al.Anal.Chem.,2014,86: 1223-1228
[7](a)Cui Y J,Yue Y F,Qian G D,et al.Chem.Rev.,2012, 112:1126-1162
(b)Heine J,Muller-Buschbaum K.Chem.Soc.Rev.,2013, 42:9232-9242
[8](a)Kong G Q,Wu C D.Cryst.Growth Des.,2010,10:4590-4595
(b)MAO Jiang-Gao(毛江高).Chinese J.Struct.Chem. (結構化學),1998,17(5):353-360
(c)ZHOU Ye-Fang(周業芳),LI Song-Lin(李松林),LIU Xue-Jia(劉雪佳).Chinese J.Inorg.Chem.(無機化學學報),2014,30(9):2189-2196
[9](a)Zhang X M,Wang Y Q,Gao E Q,et al.Chem.Commun., 2011,47:1815-1817
(b)Sun W W,Tian C Y,Gao E Q,et al.Chem.Commun., 2009,4741-4743
(c)Wen Y Q,Ma Y,Gao E Q,et al.Inorg.Chem.Commun., 2012,20:46-49
[10]Wang X B,Dacres J E,Yang X,et al.J.Am.Chem.Soc., 2003,125:296-304
[11]Sheldrick G M.Program for Empirical Absorption Correction of Area Detector Data,University of G?ttingen,G?ttingen, Germany,1996.
[12]Sheldrick G M.SHELXL-97,Program for X-ray Crystal StructureSolution,UniversityofG?ttingen,G?ttingen, Germany,1997.
[13]Spek A L.J.Appl.Crystallogr.,2003,36:7-13
[14](a)Bauer C A,Timofeeva T V,Settersten T B,et al.J.Am. Chem.Soc.,2007,129:7136-7144
(b)LIU Hong-Wen(劉宏文),LU Wen-Guan(盧文冠), Chinese J.Inorg.Chem.(無機化學學報),2011,27(1): 152-156
(c)ZHAO Yue(趙越),ZHAI Ling-Ling(翟玲玲),SUN Wei-Yin(孫為銀).Chinese J.Inorg.Chem.(無機化學學報),2014,30(1):99-105
[15](a)Liu F J,Hao H J,Zheng L S,et al.Cryst.Growth Des., 2012,12:2004-2012
(b)Brito I,Vallejos J,Cardenas A,et al.Inorg.Chem. Commun.,2011,14:897-901
[16](a)Tao J,Tong M L,Shi J X,et al.Chem.Commun.,2000, 2043-2044
(b)Feng R,Jiang F L,Hong M C.Chem.Commun.,2009, 5296-5298
Two Low Dimensional Cd(Ⅱ)Coordination Polymers Constructed from Zwitterionic Dicarboxylate Ligand:Syntheses,Structures,and Fluorescent Properties
DING Fang-Fang1ZHANG Na1ZHANG Jian-Yong*,1WANG Min2GAO En-Qing3
(1Shanghai Institute of Technology,Shanghai 200235,China)
(2Science and Technology on Electromagnetic Scattering Laboratory,Shanghai 200438,China)
(3Shanghai Key Laboratory of Green Chemistry and Chemical Processes,Department of Chemistry,East China Normal University,Shanghai 200062,China)
By controllable syntheses,two low dimensional Cd(Ⅱ)complexes with the newly zwitterionic dicarboxylate ligand 1-(4-carboxylatobenzyl)pyridinium-4-carboxylate(HL),{Cd(L)2·4H2O}n(1),and{[Cd(L)(N3)]· 3H2O}n(2)have been synthesized and structurally characterized by IR,elemental analyses,single-crystal X-ray diffraction.In compound 1,the eight-coordinated Cd(Ⅱ)ions are chelated by four equivalent L in a bis(chelating) mode,forming a one-dimensional coordination polymer structure.In 2,six-coordinated Cd(Ⅱ)ions are triple bridged by two carboxylate groups and an azide ion into 1D uniform[Cd(N3)(OCO)2]nchains,which are further interlinked through the cationic N-benzylpyridinium backbones of the L ligands into 2D sheet.Interestingly,the fluorescence measurements show that all compounds exhibit intense blue emission in the solid state,and the emission bands are correlated to the coordination modes of the L ligand.CCDC:1031439,HL;1031440,1; 1031441,2.
zwitterionic ligand;crystal structure;coordination polymers;fluorescence;cadmium(Ⅱ)complex
O614.24+2文獻標示碼:A
1001-4861(2015)10-1929-09
10.11862/CJIC.2015.231
2015-01-27。收修改稿日期:2015-06-04。
上海市自然科學基金(No.14ZR1447900),校復合材料學科建設項目(No.10210Q140001)資助。
*通訊聯系人。E-mail:jianyong1106@163.com

