基于3-氧醚乙酸-鄰苯二酸和N-輔助配體的鋅和鎘配合物的合成、晶體結構及熒光性質
范會濤 李 波 趙 強 陳寶寬 馮超強
(南陽師范學院化學與制藥工程學院,南陽473061)
基于3-氧醚乙酸-鄰苯二酸和N-輔助配體的鋅和鎘配合物的合成、晶體結構及熒光性質
范會濤 李 波*趙 強 陳寶寬 馮超強
(南陽師范學院化學與制藥工程學院,南陽473061)
水熱法合成得到2個配合物,{[Zn7(L)4(bpe)2(μ3-OH)2(H2O)8]·4H2O}n(1),和{[Cd3(L)2(bpy)2.5(H2O)]·5.5H2O}n(2)(H3L=3-(carboxymethoxy)benzene-1,2-dioic acid,bpe=1,2-bis(4-pyridyl)-ethene,bpy=4,4′-bipyridine),并采用元素分析、紅外光譜、熱重和X-射線單晶衍射對其結構進行表征。配合物1中,配體L3-和bpe連接[Zn5(μ3-OH)2]中心形成一維鏈,這樣的鏈通過氫鍵連接成三維的超分子結構。配合物2呈現(3,3,6)連接的網狀結構。此外,對配合物1和2的熒光性質進行了研究。
3-氧醚乙酸-鄰苯二酸;晶體結構;熒光性質;拓撲
0 Introduction
In recent years,the rational design and assembly of coordination polymers have received remarkable attention in order to develop new function materials with various potential applications[1-5].The construction of coordination polymers is highly influenced by many factors such as the metal coordination preferences and multidentate organic ligands geometries,etc[6-13]. Usually,multidentate organic ligands may potentially provide various coordination modes and favor the construction of polynuclear metal clusters[14].Newaromatic tricarboxylic acid 3-(carboxymethoxy) benzene-1,2-dioic acid(H3L),with two rigid carboxylic groups and one flexible group of-OCH2-COO-at adjacent positions on the aromatic ring,has greater conformational freedom,which can change their conformations to meet the coordination requirement of the metal ion give rise to polynuclear metal clusters, and result in unique networks.In addition,only some coordination polymers based on bifunctional carboxylic acid ligand containing both rigid and flexible carboxylate coordinating groups have sporadically appeared in the literatures[15-17].In the other hand,the use of auciliary ligands is also an effective method for the construction of coordination polymers owing to the fact that they can mediate the coordination needs of the metal center[18-19].
In this paper,we have engaged in the research of coordination polymers based on H3L with d10metal centers(Zn,Cd)to understand the coordination chemistry of H3L and prepared new materials with interesting structural topology.Herein,we would like to present two coordination polymers:{[Zn7(L)4(bpe)2(μ3-OH)2(H2O)8]·4H2O}n(1),{[Cd3(L)2(bpy)2.5(H2O)]·5.5H2O}n(2)(bpe=1,2-bis(4-pyridyl)-ethene,bpy=4,4′-bipyridine),in which the L3-ligand exhibits different coordination modes and makes the complexes interesting 3D framework structures.
1 Experimental
1.1 Materials and measurements
The ligand H3L was synthesized according to the literatures[15,17].All other starting materials were of analytical grade and obtained from commercial sources without further purification.Elemental analysis for C,H,and N were performed on a Perkin-Elmer 240 elemental analyzer.The FTIR spectra were recorded from KBr pellets in the range from 4 000 to 400 cm-1on a Bruker VECTOR 22 spectrometer. Luminescence spectra for the solid samples were recorded on a Hitachi 850 fluorescence spectrophotometer.Thermal analyses were performed on a SDT 2960 thermal analyzer from room temperature to 800℃at a heating rate of 20℃·min-1under nitrogen flow.
1.2 Syntheses of the complexes
1.2.1 Synthesis of{[Zn7(L)4(bpe)2(μ3-OH)2(H2O)8]· 4H2O}n(1)
A mixture of H3L(12.0 mg,0.05 mmol),bpe(9.1 mg,0.05 mmol),Zn(NO3)2·6H2O(15.0 mg,0.05 mmol), and KOH(8.4 mg,0.15 mmol)in distilled water(7 mL) was placed in a Teflon-lined stainless steel container, heated to 120℃for 3 days.After cooling to room temperature,the colorless block crystals of 1 were obtained in 67%yield based on zinc.Anal.Calcd.for C64H64N4O41Zn7(2 002.78)(%):C,38.38;H,3.22;N, 2.80.Found(%):C,38.41;H,3.28;N,2.72.IR(KBr, cm-1):3 396(m),1 608(s),1 560(s),1 418(s),1 363 (s),1 230(m),1 097(w),1 069(w),915(w),812(m), 772(m),689(w),621(w),589(w).
1.2.2 Synthesis of{[Cd3(L)2(bpy)2.5(H2O)]·5.5H2O}n(2)
Complex 2 was synthesized by analogy to 1 except that Cd(NO3)2·4H2O(15.4 mg,0.05 mmol)and bpy (11.7 mg,0.075 mmol)were used instead of Zn(NO3)2· 6H2O and bpe.Colorless block crystals of 2 were obtained in 72%yield based on cadmium.Anal. Calcd.for C90H86Cd6N10O41(2 638.09)(%):C,40.97;H, 3.29;N,5.31.Found(%):C,41.02;H,3.28;N,5.49. IR(KBr,cm-1):3 422(m),1 617(s),1 399(m),1 256 (w),1 236(w),1 208(w),1 094(w),1 070(w),1 033 (w),839(w),760(m),617(w).
1.3 Structure Determ inations
Single-crystal X-ray diffraction data of complexes 1 and 2 were collected on a Bruker Smart Apex CCD diffractometer[20]equipped with graphite monochromatized Mo Kα radiation(λ=0.071 073 nm)at room temperature using the ω-scan technique.Empirical absorption corrections were applied to the intensities using the SADABS program[21].The structure was solved by direct methods using SHELXS-97[22]computer program and refined by full-matrix least-squares methods on F2with the SHELXL-97[23]program package. All nonhydrogen atoms were subjected to anisotropic refinement.The hydrogen atoms of the organic ligands were included in the structure factor calculation at idealized positions using a riding model and refined isotropically.The crystallographic data and selectedbond distances and angles for 1 and 2 are listed in Table 1 and Table 2,respectively.
CCDC:965303,1;965304,2.

Table1 Crystal data and structure refinement for com pounds 1 and 2
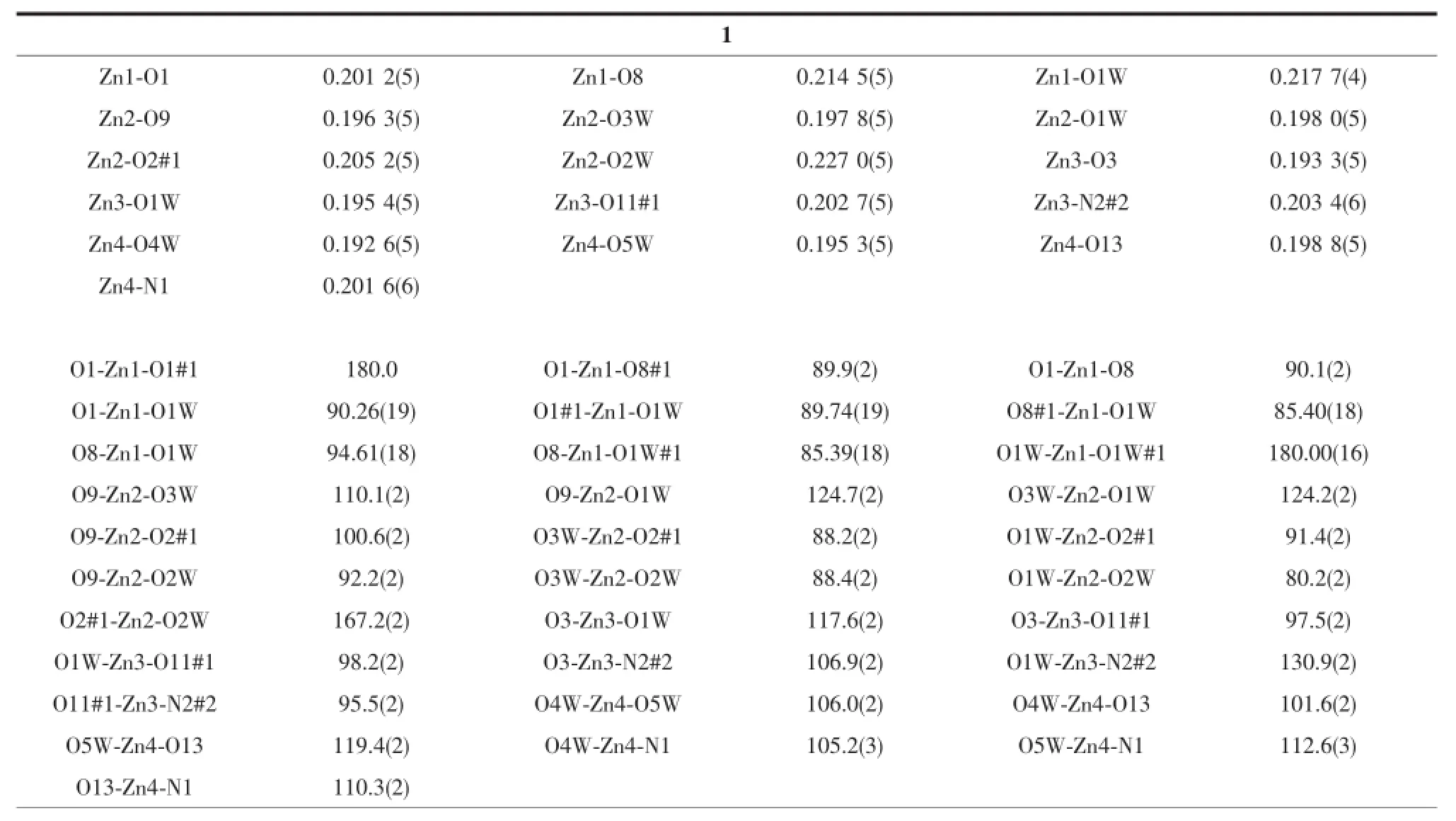
Table2 Selected bond lengths(nm)and angles(°)for compounds 1 and 2
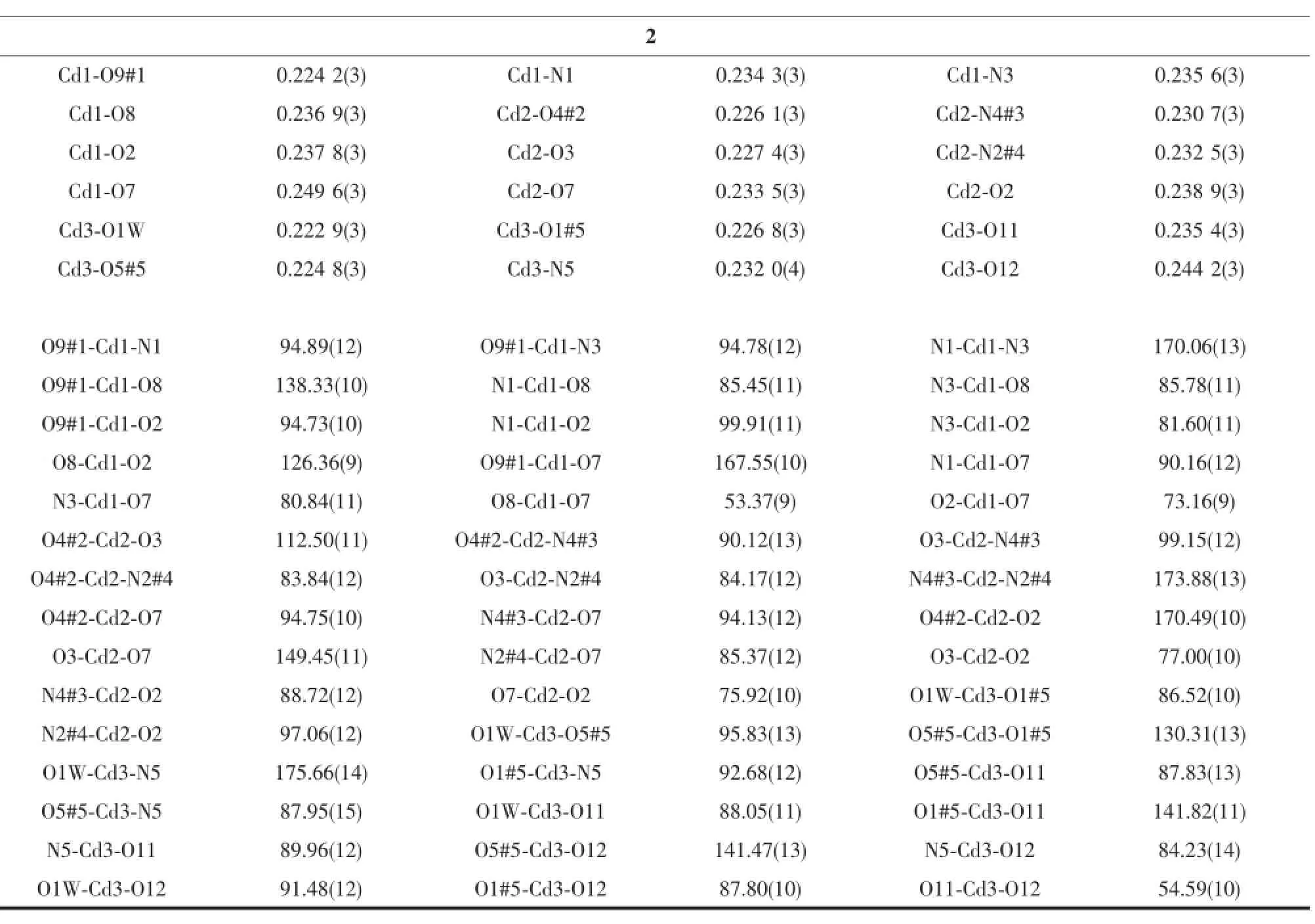
Continued Table 2
2 Results and discussion
2.1 Description of the Structure
2.1.1 {[Zn7(L)4(bpe)2(μ3-OH)2(H2O)8]·4H2O}n(1)
Single crystal X-ray diffraction revealed that complex 1 crystallized in the triclinic system with space group P1,and can be formulated as{[Zn7(L)4(bpe)2(μ3-OH)2(H2O)8]·4H2O}n(1).There are four crystallographically independent Znions,two L3-ligands,one bpe ligand,oneμ3-OH anion,four coordinated water molecules,and two free water molecules in the asymmetric unit.Zn1 ion was refined with an occupancy factor of 0.5.As illustrated in Fig. 1,Zn1 is six-coordinated with a distorted octahedral geometry with four oxygen atoms from four L3-ligands, and two hydroxyl groups.Zn2,Zn3,and Zn4 display square-pyramidal[O5]and[O4N]geometries,respectively.The Zn-N bond length is 0.201 6(6),0.203 4(6) nm and the Zn-O bond lengths are in the range of 0.192 6(5)~0.227 0(5)nm,which are in agreement with those observed in other structures[24-25].The N-Zn-O and O-Zn-O angles are in the range of 95.5(2)°~130.9(2)°and 80.2(2)°~180.0°,respectively.Clearly, five Zncenters form a[Zn5(μ3-OH)2]cluster linked by two3-hydroxyl oxygen(O1W)atom(Fig.2).All the flexible and rigid carboxyl groups of the L3-ligand are fully deprotonated and adopt the μ3-η2:η1:η0and μ4-η2: η1:η1bridging modes(Scheme 1a,1b).The remaining Zn4 ions are linked by the adjacent pentanuclear [Zn5(μ3-OH)2]subunits to produce infinite chain(Fig. 3).Such chains are associated through hydrogen bonding(O3W-O6#8,O3W-O13#6,O4W-O3#4, O4W-O7#4,O5W-O14#5,O5W-O11#5)to give rise to a three-dimensional supramolecular framework(Fig.4). The geometric parameters of hydrogen bonds are summarized in Table 3.

Scheme 1 Coordination modes of the L3-anion in 1 and 2
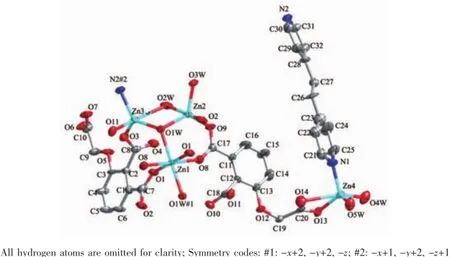
Fig.1 Metal coordination and atom labeling in complex 1(thermal ellipsoids at 50%probability level)
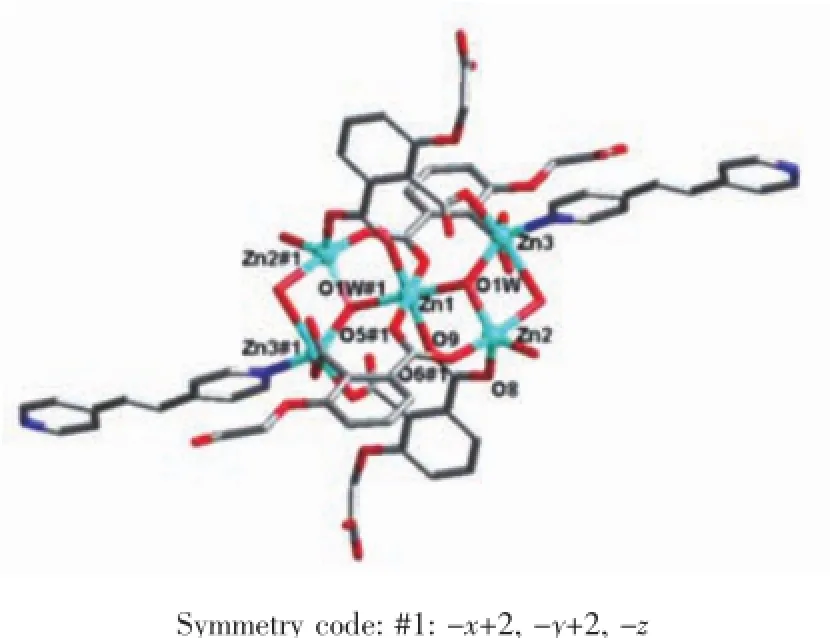
Fig.2 Coordination environment of[Zn5(OH)2]cluster in 1
2.1.2 {[Cd3(L)2(bpy)2.5(H2O)]·5.5H2O}n(2)
In complex 2,the asymmetric unit consists of three crystallographically independent Cdcenters, two L3-ligands,two and a half bpy ligands,one coordinated water molecule,and five and a half free water molecules(Fig.5).Three independent Cdions exhibiting slightly distorted octahedral[O4N2]and [O5N1]coordination geometries,respectively.The Cd-O and Cd-N bond distances are in the ranges of 0.223 0(3)~0.249 6(3)nm and 0.230 7(5)~0.235 7(5)nm, respectively.The coordination modes of μ4-η3:η1:η1and μ4-η3:η2:η1for L3-is found in complex 2(Scheme 1c,1d).As shown in Fig.6,Cd1 and Cd2 centers are bridged by a pair of carboxylate groups into a dimer unit.Neighboring dimer units are further linked by L3-ligands to form an infinite metal-carboxylate ribbon.Such ribbons are associated together by Cd3 and one kind of bpy(N5,N5#6)to generate a 2D sheet.As illustrated in Fig.7,adjacent layers are linked through the remaining two kinds of bpy ligands(N1-N2,N3-N4) to produce an overall three-dimensional coordination polymer encapsulating free water molecules.Among the water molecules,O1W,O2W and O4W-O6W show tricoordinated,while all other water molecules are mono-or di-coordinated modes with the O…O distance ranging from 0.261 5(18)to 0.318 1(12)nm.Notably, there are rich hydrogen bonds between the free water molecules and the coordinated carboxylic oxygen atoms, which bring further stability for the structure.The geometric parameters of hydrogen bonds are summarized in Table 4.Better insight into such an intricate framework can be achieved by using the topological approach,if the L3-anion can be considered as a 3-connected node,Cd1 and Cd2 as a 6-connected node,Cd3 as a 3-connected node,respectively.Thus,a (3,3,6)-connected network is formed with a Schl?fli

Fig.3 Infinite chain in 1

Fig.4 3D framework of 1 forming through hydrogen bonding

Table3 Selected hyd rogen-bonding geometries for 1

Fig.5 Metal coordination and atom labeling in complex 2(thermal ellipsoids at 50%probability level)

Fig.6 View of a 2D layer connecting bpy ligands

Fig.7 3D framework of 2 connecting bpy ligands

Table4 Selected hyd rogen-bonding geometries for 2
symbol of{4.5.7}2{42.5.68.72.82}{52.8}(Fig.8).

Fig.8 Schematic representation of the(3,3,6)-connected topology of 2
2.2 TG analysis

Fig.9 TG curves for compounds 1 and 2
Thermogravimetric analyses were carried out for the two compounds and corresponding TG curves are shown in Fig.9.For complex 1,a weight loss of 10.28%is detected in the range of 121~181℃ corresponding to the loss of six water molecules (Calcd.10.78%),and then,a plateau region follows. The further weight loss is observed at about 321℃due to the decomposition of overall framework. According to the type of water in compound 2,the loss of coordination water takes place at temperature above 283℃(Obsd.8.83%;Calcd.8.87%).Rapid weight loss is observed from 316℃,which indicated collapse of the whole structure.
2.3 Photolum inescence properties
Emission behaviors of complexes 1 and 2 were investigated in the solid-state at room temperature and shown in Fig.10.It was observed that the emission peaks occurred at ca.458(λex=368 nm)and 343 nm (λex=274 nm)for complexes 1 and 2,respectively.In order to understand the nature of these emission bands,the photoluminescence properties of free H3L ligand was measured,upon excitation at ca.274 nm, which shows similar emission at ca.345 nm. Compared to the emission peak for free,the emission of 1 with the significant red-shift of luminescence may be mainly attributed to the coordination interactions of the L3-ligand,which effectively increases the rigidity of the ligand and reduces the loss of energy by radiationless decay[26-28].The emission of complex 2 can probably be assigned to the intraligand(π-π*)fluorescent emission because similar emission is observed for the free H3L ligand.

Fig.10 Solid-state emission spectra of complexes 1 and 2,and free H3L ligand at room temperature
3 Conclusions
In summary,two new coordination polymers based on the assembly of H3L and co-ligands with different divalent zinc and cadmium salts under hydrothermal conditions have been synthesized and characterized.Complex 1 shows metal-organic chain structure built from[Zn5(μ3-OH)2]subunit,and the adjacent chains are further linked through hydrogen bonding to form a 3D supramolecular structure. Complex 2 exhibits a three-dimensional metal-organic framework,which is also consolidated by the hydrogen bonding.The study reveals that the multi-flexible ligand can be used as a good multidentate bridging ligand to construct novel coordination polymers based on polynuclear clusters.
[1]Halder G J,Kepert C J,Moubaraki B,et al.Science,2002, 298:1762-1765
[2]Tranchemontagne D J,Mendoza-Cortés J L,O′Keeffe M, et al.Chem.Soc.Rev.,2009,38:1257-1283
[3]Ma L F,Wang L Y,Du M,et al.Inorg.Chem.,2010,49:365 -367
[4]Du M,Li C P,Chen M,et al.J.Am.Chem.Soc.,2014,136: 10906-10909
[5]Dong X Y,Li B,Ma B B,et al.J.Am.Chem.Soc.,2013, 135:10214-10217
[6]Gutschke S O H,Price D J,Powell A K,et al.Angew. Chem.,Int.Ed.,2001,40:1920-1923
[7]Li B,Zang S Q,Ji C,et al.Dalton Trans.,2011,40:788-792
[8]Suh M P,Ko J W,Choi H J.J.Am.Chem.Soc.,2002,124: 10976-10977
[9]Pan L,Woodlock E B,Wang X T.Inorg.Chem.,2000,39: 4174-4178
[10]Chen W,Wang J Y,Chen C,et al.Inorg.Chem.,2003,42: 944-946
[11]Zhang Z,Ma J F,Liu Y Y,et al.Cryst.Growth Des.,2013, 13:4338-4348
[12]Xu C Y,Li L K,Wang Y P,et al.Cryst.Growth Des., 2011,11:4667-4675
[13]Aliev S B,Samsonenko D G,Rakhmanova M I,et al.Cryst. Growth Des.,2014,14:4355-4363
[14]Xiong K C,Jiang F L,Gai Y L,et al.Inorg.Chem., 2012,51:3283-3288
[15]Cao X Y,Zhang J,Cheng J K,et al.CrystEngComm, 2004,6:315-317
[16]Cao X Y,Zhang J,Li Z J,et al.CrystEngComm,2007,9: 806-814
[17]Wang G H,Lei Y Q,Wang N,et al.Cryst.Growth Des., 2010,10:534-540
[18]Du M,Li C P,Liu C S,et al.Coord.Chem.Rev.,2013,257: 1282-1305
[19]LaDuca R L.Coord.Chem.Rev.,2009,253:1759-1792
[20]SMART and SAINT,Area Detector Control and Integration Software,Siemens Analytical X-Ray Systems,Inc.:Madison, WI(USA),1996.
[21]Sheldrick G M.SADABS 2.05,University of G?ttingen, Germany,2000.
[22]Sheldrick G M.SHELXS-97,Program for the Solution of Crystal Structures,University of G?ttingen,Gttingen, Germany,1997.
[23]Sheldrick G M.SHELXL-97,Program for the Refinement of Crystal Structures,University of G?ttingen,G?ttingen, Germany,1997.
[24]Zang S Q,Fan Y J,Li J B,et al.Cryst.Growth Des., 2011,11:3395-3405
[25]Zang S Q,Cao L H,Liang R,et al.Cryst.Growth Des., 2012,12:1830-1837
[26]Dai J C,Wu X T,Fu Z Y,et al.Inorg.Chem.,2002,41: 1391-1396
[27]Zhang L Y,Liu G F,Zheng S L,et al.Eur.J.Inorg.Chem., 2003,16:2965-2971
[28]Wang X L,Qin C,Li Y G,et al.Inorg.Chem.,2004,43: 1850-1856
Zinc and Cadm ium Coordination Polymers Based on 3-(Carboxymethoxy)benzene-1, 2-dioic Acid and N-donor Ancillary Ligands:Syntheses,Crystal Structures, and Lum inescent Properties
FAN Hui-Tao LI Bo*ZHAO Qiang CHEN Bao-Kuan FENG Chao-Qiang
(College of Chemistry and Pharmaceutical Engineering,Nanyang Normal University,Nanyang,Henan 473061,China)
Two coordination polymers,{[Zn7(L)4(bpe)2(μ3-OH)2(H2O)8]·4H2O}n(1),and{[Cd3(L)2(bpy)2.5(H2O)]·5.5H2O}n(2)(H3L=3-(carboxymethoxy)benzene-1,2-dioic acid,bpe=1,2-bis(4-pyridyl)-ethene,and bpy=4,4′-bipyridine),have been synthesized under hydrothermal condition.Both complexes were characterized by elemental analysis,IR spectra,thermogravimetric analysis(TGA),and single-crystal X-ray crystallography.Complex 1 features a threedimensional supramolecular architecture linked through hydrogen bonding interactions in which the secondary building unit(SBU)is[Zn5(μ3-OH)2]cluster.Complex 2 features three-dimensional metal-organic framework with (3,3,6)-connected topology.The luminescence behaviors of 1 and 2 were also discussed.CCDC:965303,1; 965304,2.
3-(carboxymethoxy)benzene-1,2-dioic acid;crystal structure;luminescence properties;topology
O614.24+1;O614.24+2
A
1001-4861(2015)04-0848-09
10.11862/CJIC.2015.068
2014-11-21。收修改稿日期:2014-12-16。
河南省教育廳科學技術研究重點項目基礎研究計劃(No.14A150026)、南陽師范學院高層次人才科研啟動費(No.ZX2014041)項目資助。*

