艾滋病疫苗
?
艾滋病疫苗
·編者按·
艾滋病又稱獲得性免疫缺陷綜合癥(Acquired Immunodeficiency Syndrome,AIDS),由感染人免疫缺陷病毒(Human Immunodeficiency Virus,HIV)引起。機體感染HIV后,免疫系統受到破壞,抗感染能力下降,成為許多伺機性疾病的攻擊目標,促成多種臨床癥狀,伴有機會感染、惡性腫瘤及神經障礙等多種癥候群,是一種危害性極大的傳染病。
自20世紀80年代初首例報道艾滋病病例以來,艾滋病在全球范圍迅速感染和蔓延。2015年12月1日是第28個世界艾滋病日,全球現有大約3690萬人感染艾滋病毒,其中約有200萬人是2014年新增感染者。迄今為止,估計有3400萬人左右因艾滋病毒或艾滋病死亡,其中120萬人死于2014年。因而,盡管國際社會在防治艾滋病方面取得了重要進展,艾滋病和艾滋病病毒依然是人類健康最嚴峻的挑戰之一。
目前尚未發現對艾滋病有效的治愈方法,研制艾滋病疫苗是控制艾滋病流行甚至根除艾滋病的理想途徑,疫苗免疫接種也是控制傳染病的最經濟有效的措施。自1987年第一個艾滋病疫苗進入臨床試驗以來,按照研究的側重點劃分,艾滋病疫苗研究進程大致分為3個階段:體液免疫的中和抗體階段、刺激CD8 T細胞介導的細胞免疫階段以及體液免疫和細胞免疫兩者結合階段。尚在研究的HIV疫苗包括HIV滅活疫苗、HIV減毒活疫苗、亞單位疫苗、活載體病毒蛋白疫苗和DNA疫苗等。
雖然有效的HIV疫苗還未研發出來,但新免疫策略不斷涌現,泰國進行的RV-144Ⅱ期臨床試驗,證明了保護性的HIV疫苗的可能性,為HIV疫苗的研發帶來了希望。一些醫藥公司也對外宣稱其研發取得的新進展,比如法國生態健康科技公司稱其研發出可望徹底治愈艾滋病的疫苗,2016年將確定疫苗的最終配方,從2017年起開始全球市場營銷,最終的效果令人期待。
本專題得到江文正教授(華東師范大學生命科學學院)的大力支持。
·熱點數據排行·
截至2015年12月1日,中國知網(CNKI)和Web of Science(WOS)的數據報告顯示,以“艾滋病疫苗”為詞條可以檢索到的期刊文獻分別為716與829條,本專題將相關數據按照:研究機構發文數、作者發文數、期刊發文數、被引用頻次進行排行,結果如下。
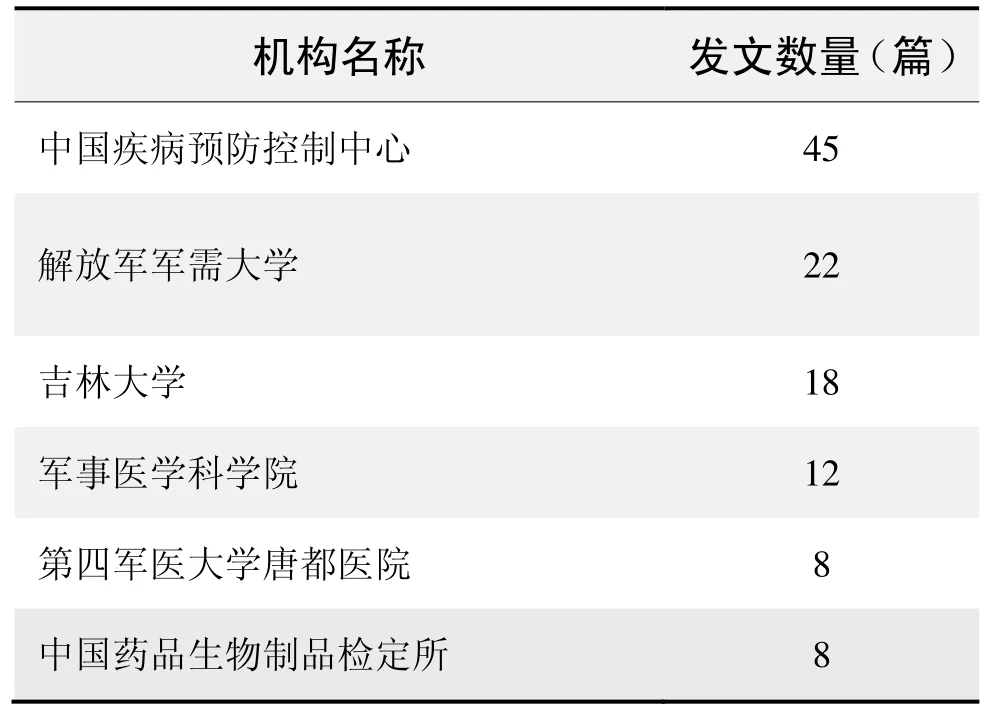
研究機構發文數量排名(CNKI)

研究機構發文數量排名(WOS)
(數據來源:中國知網、Web of Science,檢索時間:2015-12-01)
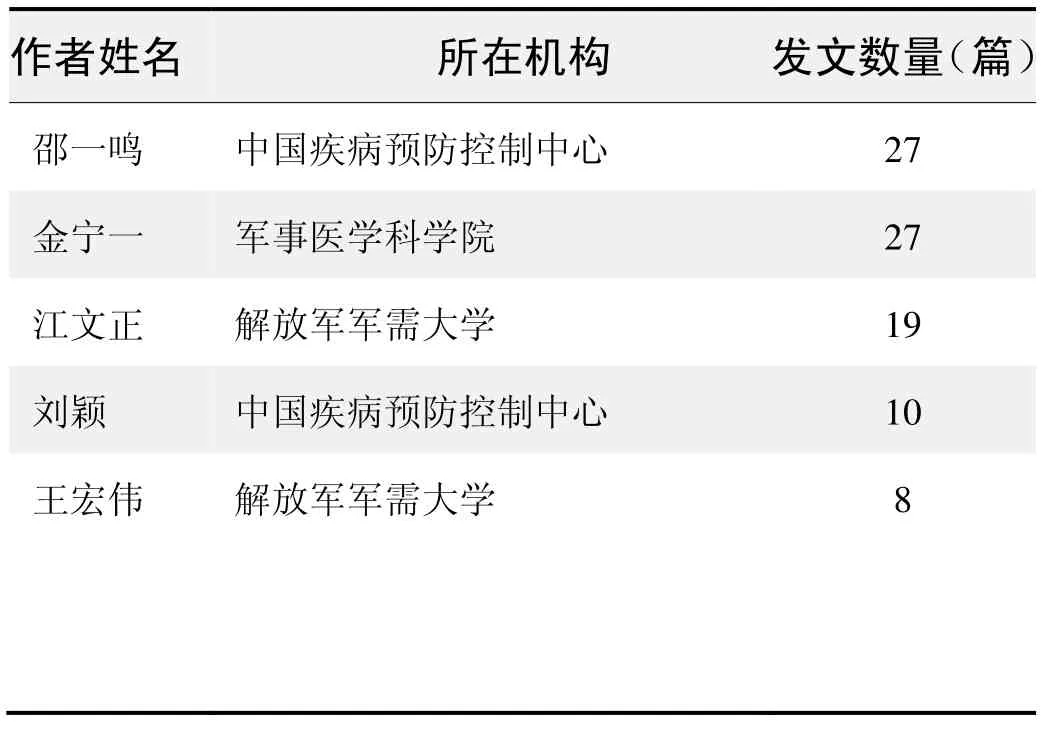
作者發文數量排名(CNKI)

作者發文數量排名(WOS)
(數據來源:中國知網、Web of Science,檢索時間:2015-12-01)

期刊發文數量排名(CNKI)
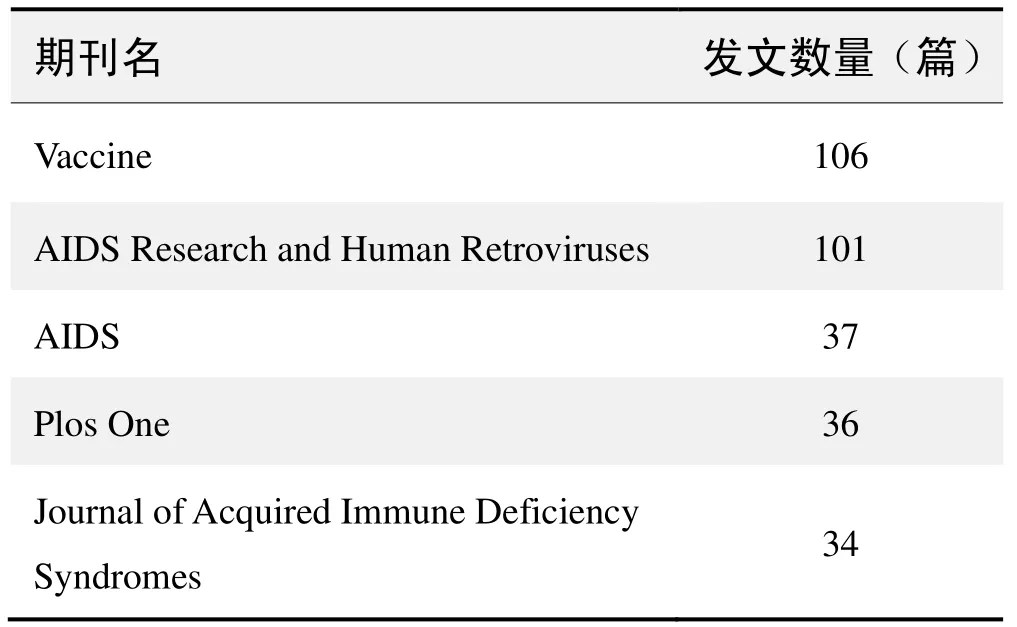
期刊發文數量排名(WOS)
(數據來源:中國知網、Web of Science,檢索時間:2015-12-01)
根據中國知網(CNKI)數據報告,以“艾滋病疫苗”為詞條可以檢索到的高被引論文排行結果如下。

國內數據庫高被引論文排行

(續表)
(數據來源:中國知網,檢索時間:2015-12-01)
根據Web of Science統計數據,以“艾滋病疫苗”為詞條可以檢索到的高被引論文排行結果如下。
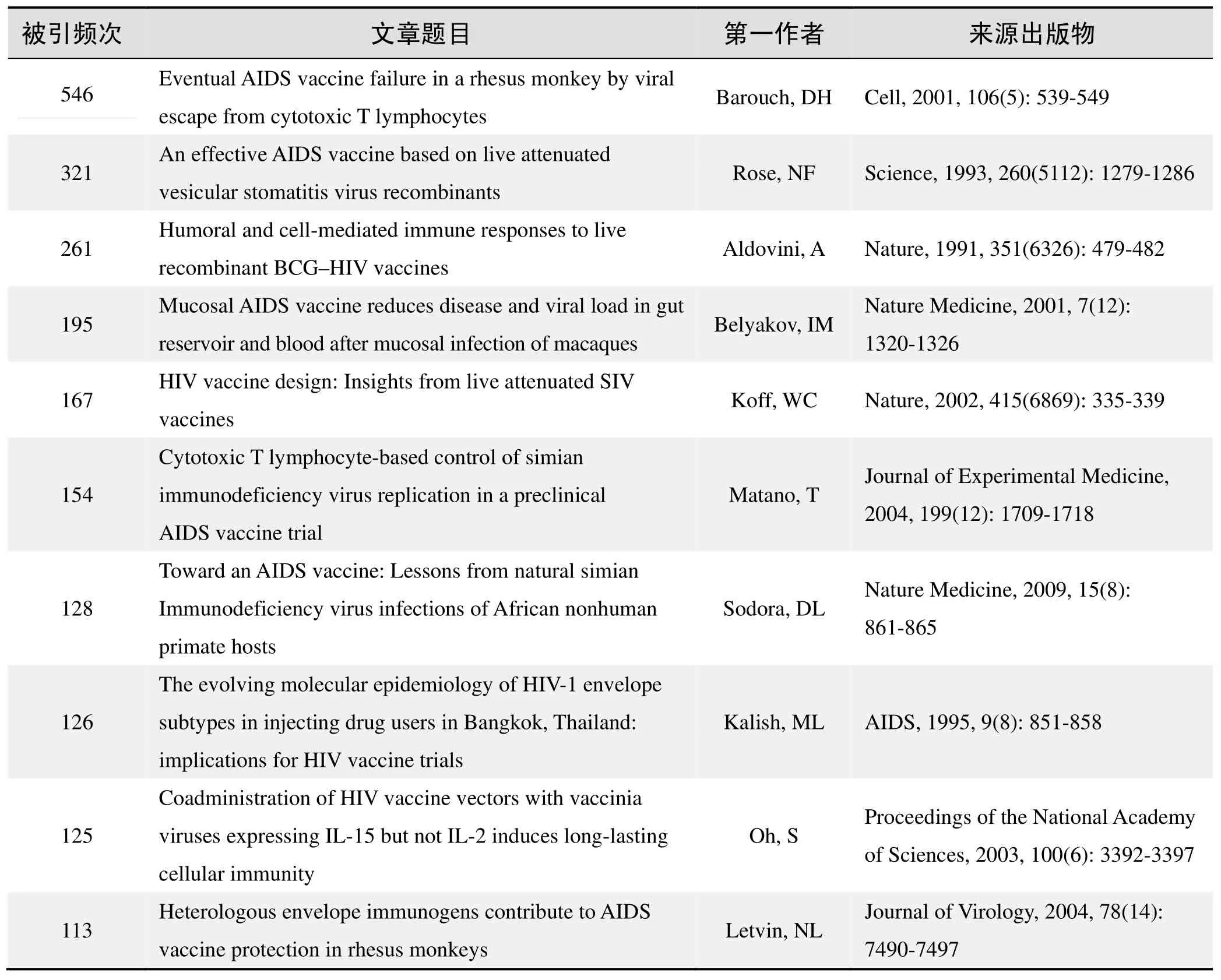
國外數據庫高被引論文排行
(數據來源:Web of Science,檢索時間:2015-12-01)
·經典文獻推薦·
基于Web of Science檢索結果,利用Histcite軟件選取LCS(Local Citation Score,本地引用次數)TOP 30文獻作為節點進行分析,得到本領域推薦的經典文獻如下。
Development and testing of AIDS vaccines
Cleaver, JE
Abstract:Recent advances in delineating the molecular biology of human immunodeficiency virus type 1 (HIV-1) have led to innovative approaches to development of a vaccine for acquired immunodeficiency syndrome (AIDS). However, the lack of understanding of mechanisms of protective immunity against HIV-1, the magnitude of genetic variation of the virus, and the lack of effective animal models for HIV-1 infection and AIDS have impeded progress. The testing of AIDS vaccines also presents challenges. These include liability concerns over vaccine-related injuries; identification of suitable populations for phase 3 efficacy studies; balancing the ethical obligation to counsel research subjects to avoid high-risk behavior with the necessity to obtain vaccine efficacy data; and the effect of vaccine-induced seroconversion on the recruiting and welfare of trial volunteers. Several candidate AIDS vaccines are nevertheless currently under development, and some are undergoing phase 1 clinical trials. Rapid progress will depend on continued scientific advancement in conjunction with maximum use of resources, open information and reagent exchange, and a spirit of international collaboration. Issue: Several investigators are preparing to conduct efficacy trials of human immunodeficiency virus (HIV) vaccines in the developing world. Failure to adequately address the unique ethical, behavioral, and social issues that surround vaccine testing in that setting will jeopardize the success of these trials and future acquired immunodeficiency syndrome (AIDS) research in the host nation. Description of the Project: Twelve investigators from Africa, Asia, North America, and South America reviewed previous experience with HIV trials in developing countries and explored potential solutions to these issues. Conclusions: Host country scientists, government officials, and media must be actively involved in all aspects of the trials. Minimum prerequisites for conducting the trial include the following: (1) researching vaccines active against developing world HIV isolates; (2) establishing and maintaining an adequate technological infrastructure; (3) assessing the feasibility of recruitment in countries where the existence of HIV may be denied; (4) designing methods to obtain informed consent from each individual subject, rather than exclusively from family members or community elders; (5) creating locally appropriate instruments to measure risk behavior; (6) identifying a behavioral intervention for placebo and treatment groups; (7) making available laboratory methods to distinguish between natural HIV infection and vaccine-induced seropositivity; and (8) guaranteeing that an effective vaccine is available free of charge to the placebo group and at affordable prices to other host country residents. Omitted. An anonymous cross-sectional paper-and-pencil survey was used to assess incentives and disincentives to participate in a Phase I preventive human immunodeficiency virus (HIV) vaccine trial in a potential Thai target population. A total of 255 persons employed in health care service and research settings completed questionnaires after attending informational briefings regarding the proposed vaccine product and the planned trial procedures, Willingness to participate was related to self-perceived benefits from joining a preventive vaccine trial, as well as to concerns about product safety and social discrimination that might result from participation. The distinction between positive results of enzyme-linked immunosorbent assay from vaccine administration and positivity from HIV infection was unclear for many participants. Men were more willing to participate than women, and there was a trend toward greater willingness to participate in those who were less educated, Preparations for preventive vaccine trials may be more successful if they emphasize personal benefits of trial participation, clearly address safety issues, and consider ways to prevent social discrimination against participants. Objective: To determine the willingness of populations at high risk of HIV-1 infection to participate in HIV vaccine efficacy trials, determine factors influencing decisionmaking, and evaluate knowledge levels of vaccine trial concepts. Design: Cross-sectional study. Methods: HIV-1-negative homosexual men, male and female injecting drug users and non-injecting women at heterosexual risk were recruited in eight cities in the United States (n= 4892). Results: A substantial proportion of the study population (77%) would definitely (27%) or probably (50%) be willing to participate in a randomized vaccine efficacy trial. Increased willingness was associated with high-risk behaviors, lower education level, being uninsured or covered by public insurance, and not having been in a previous vaccine preparedness study. Altruism and a desire for protection from the vaccine were major motivators for participation. Major concerns included positive HIV-1 antibody test due to vaccine, safety of the vaccine, and possible problems with insurance or foreign travel. Baseline knowledge of vaccine trial concepts was low. Conclusions: It is likely that high-risk volunteers will be willing to enroll in HIV vaccine efficacy trials. A variety of participant and community educational strategies are needed to address participant concerns, and to ensure understanding of key concepts prior to giving consent for participation.
來源出版物:Science, 1988, 241(4864): 426-432
Ethical, behavioral, and social aspects of HIV vaccine trials in developing countries
Lurie, P; Bishaw, M; Chesney, MA; et al.
來源出版物:JAMA, 1994, 271(4): 295-301
Participation of homosexual/bisexual men in preventive HIV vaccine trials: Baseline attitudes and concerns and predicted behaviors during trials
Douglas, JM; Judson, FN; Parks, JP; et al.
來源出版物:AIDS Research and Human Retroviruses, 1993, 10: S257-60
Incentives and disincentives to participate in prophylactic HIV vaccine research
Jenkins, RA; Temoshok, LR; Virochsiri, K
Keywords:HIV vaccine; vaccine trials; research participation; incentives vaccine trials; homosexual men; injecting drug users; women; HIV
來源出版物:AIDS, 1998, 12(7): 785-793
·推薦綜述·
來源出版物:Journal of Acquired Immune Deficiency Syndromes, 1995, 9(1): 36-42
Readiness of high-risk populations in the HIV Network for Prevention Trials to participate in HIV vaccine efficacy trials in the United States
Koblin, BA; Heagerty, P; Sheon, A; et al.
文獻編號本領域經典文章題目第一作者來源出版物1 Development and testing of AIDS vaccines Cleaver, JE Science, 1988, 241(4864): 426-432 2Ethical, behavioral, and social aspects of HIV vaccine trials in developing countries Lurie, P JAMA, 1994, 271(4): 295-301 3 Participation of homosexual/bisexual men in preventive HIV vaccine trials: Baseline attitudes and concerns and predicted behaviors during trials Douglas, JM AIDS Research and Human Retroviruses, 1993, 10: S257-S260 4 Incentives and disincentives to participate in prophylactic HIV vaccine research Jenkins, RA Journal of Acquired Immune Deficiency Syndromes, 1995, 9(1): 36-42 5 Readiness of high-risk populations in the HIV Network for Prevention Trials to participate in HIV vaccine efficacy trials in the United States Koblin, BA AIDS, 1998, 12(7): 785-793

