尿路上皮癌伴鱗狀分化對TURBT術后pT1期患者預后的影響
何 振,徐 勇,齊士勇,杜 娥,沙 楠,朱 冰
(天津醫科大學第二醫院泌尿外科,天津市泌尿外科研究所,天津300211)
論著
尿路上皮癌伴鱗狀分化對TURBT術后pT1期患者預后的影響
何 振,徐 勇,齊士勇,杜 娥,沙 楠,朱 冰
(天津醫科大學第二醫院泌尿外科,天津市泌尿外科研究所,天津300211)
目的:探討尿路上皮癌伴鱗狀分化對初次經尿道膀胱腫瘤電切術(TURBT)術后pT1期患者預后的影響。方法:回顧性分析初次經TURBT手術、術后病理診斷為T1期的531例膀胱尿路上皮癌患者的臨床病理資料。根據患者的術后病理診斷將患者分為2組:A組為尿路上皮癌(單純型)441例,B組為尿路上皮癌伴鱗狀分化90例,應用SPSS 20.0統計軟件,運用Kaplan-Meier法分析兩種臨床病理特點對無復發生存期(RFS)和無進展生存期(PFS)的影響,并用Log-rank檢驗比較生存曲線;運用COX回歸模型單因素和多因素分析膀胱尿路上皮癌伴鱗狀分化與初次TURBT術后pT1期患者預后之間的關系,評估影響其RFS和PFS的因素。結果:A組單純尿路上皮癌441例(83.05%),B組尿路上皮癌伴鱗狀分化90例(16.95%)。B組與A組比較更易具有高級別腫瘤(P<0.001),同時B組較A組有較高的復發率(P=0.018)、較短的無復發生存期(P<0.001)以及較高的進展率(P=0.001)、較短的無進展生存期(P<0.001)。B組與A組比較,年齡(P=0.185)、性別(P=0.135)、吸煙(P=0.728)、腫瘤大小(P=0.436)、腫瘤數目(P=0.112)和膀胱灌注(P=0.054)等差異均無統計學意義。COX多因素生存分析顯示:吸煙(HR 1.34, 95%CI 1.00-1.79,P=0.048)、鱗狀分化的伴發情況(HR 1.43,95%CI 1.02-2.00,P=0.040)以及病理分級(HR 1.51,95% CI 1.13-2.01,P=0.005)等因素顯著增加TURBT術后pT1期患者的復發風險;同時,吸煙(HR 1.80,95%CI 1.17-2.76, P=0.008)、鱗狀分化的伴發情況(HR 2.07,95%CI 1.32-3.24,P=0.001)以及病理分級(HR 1.90,95%CI 1.24-2.92,P=0.003)等因素顯著增加TURBT術后pT1期患者的進展風險。結論:尿路上皮癌伴鱗狀分化是TURBT術后pT1期患者的預后獨立影響因素,復發率及進展率較高,需密切隨訪。
膀胱;尿路上皮癌;鱗狀分化;TURBT;復發;進展
膀胱癌是最常見的惡性腫瘤之一,全球每年新發病例中膀胱癌位于第6位,因癌癥死亡的病例中位于第9位[1]。據文獻報道,75%的膀胱癌患者診斷為非肌層浸潤性,這些患者首次經尿道膀胱腫瘤電切術(TURBT)術后1年內復發、進展率分別為15%~70%、7%~40%[2-3]。膀胱尿路上皮癌(UCB)是膀胱腫瘤中主要的組織類型,約占90%[4],但尿路上皮癌(UC)具有明顯向不同病理類型分化的傾向,文獻報道7%~81%的UC病例伴有變異型[5-6],最常見的組織學分化就是鱗狀分化[7-8]。最新報道,在16.8%~22.1%的UCB患者中會發現鱗狀分化[9-11]。研究發現,UCB伴鱗狀分化治療方法及預后與單純UCB有較大差異[7],因此伴有鱗狀分化是否預示著不良預后,這一問題目前仍存在爭議[5,7,12]。本研究回顧性分析531例初次經TURBT手術、術后病理診斷為T1期的尿路上皮癌患者的臨床資料,探討UCB伴鱗狀分化對患者預后的影響。
1 對象與方法
1.1 病例選擇 收集2006年1月—2008年12月天津醫科大學第二醫院收治的初次經TURBT術后病理分期T1期的膀胱尿路上皮癌患者531例,納入標準:(1)患者均為首發膀胱尿路上皮癌;(2)手術方式均為TURBT;(3)病理診斷為T1期。排除標準:(1)病理診斷中合并腺樣分化或伴有其他尿路上皮癌分型的患者;(2)存在上尿路腫瘤或是轉移性尿路上皮癌的患者;(3)手術時已發生遠處轉移的患者;(4)術前和(或)圍手術期放化療的患者;(5)病歷信息不全,隨訪資料不完整的患者。其中男433例,女98例,年齡27~90歲,中位數65歲。按研究目的分為兩組:A組單純尿路上皮癌441例(83.05%),B組尿路上皮癌伴鱗狀分化90例(16.95%)。
1.2 術后治療方法 病理分期為T1期患者術后膀胱內灌注表柔比星50 mg(參考2014版《中國泌尿外科疾病診斷治療指南》:每周1次,連續8次,后改為每2周1次,連續8次,最后改為每月1次,連續8次)。
1.3 術后隨訪 患者均在術后1年內每3個月行泌尿系B超、尿脫落細胞學檢查及膀胱鏡檢查1次,術后第2年每6個月行膀胱鏡檢查1次,隨后每年行膀胱鏡檢查1次。
1.4 納入項目 本研究中分析的因素包括性別、年齡、吸煙、腫瘤數量和大小(術中確定腫瘤大小及數量,腫瘤數量為1個者定義為單發腫瘤,2個及以上者定義為多發腫瘤;直徑小于或等于3 cm者定義為較小腫瘤,大于3 cm者為較大腫瘤)、病理分級(WHO,2004)、膀胱灌注。本研究觀察終點是復發和進展。研究結果是無復發生存期(RFS)和無進展生存期(PFS),其中RFS定義為首次診斷為pT1期UCB術后到首次復發的時間,未復發者截止到末次隨訪的日期;PFS被定義為首次診斷為pT1期UCB到首次腫瘤進展(腫瘤分級、分期的提高)的時間,未進展者截止到末次隨訪的日期。
1.5 病理診斷 病理切片由我院兩位泌尿外科病理學專家進行診斷。尿路上皮癌伴鱗狀分化定義為腫瘤組織中存在角質化或細胞間橋等鱗狀細胞成分,同時免疫組化染色指標p40表現為陽性。
1.6 統計學方法 采用SPSS 20.0對數據進行分析。獨立樣本采用t檢驗,組間各因素的比較采用χ2檢驗,運用Kaplan-Meier法分析兩種臨床病理特點對無復發生存期和無進展生存期的影響,并用Logrank檢驗比較生存曲線。采用COX回歸模型單因素和多因素分析預測影響無復發生存期和無進展生存期的危險因素,同時計算出95%置信區間(CI)內的風險比率(HR),以P<0.05為差異有統計學意義。
2 結果
2.1 臨床和病理特征 表1顯示了531例患者的臨床、病理特征。性別、年齡、吸煙、腫瘤數量及大小、膀胱灌注等組間比較差異均無統計學意義(P≥0.05),然而B組較A組在病理分級上更易伴有高級別(64.44%/37.41%,P<0.001)。患者隨訪中位數87.0個月,在隨訪期間內,A組患者復發比例占37.41%(165/441),B組患者復發比例占51.11%(46/ 90)。同樣,A組患者進展比例占15.65%(69/441),B組患者進展比例占32.22%(29/90)。B組患者的復發率和進展率均高于A組患者,差異均有統計學意義(P=0.018,P=0.001)。
2.2 生存分析
2.2.1 累積生存分析 Kaplan-Meier法繪圖分析A組與B組在無復發生存期和無進展生存期的差異見圖1、2,同時,表1結果顯示B組與A組比較有較短的無復發生存期(P<0.001)以及較短的無進展生存期(P<0.001)。
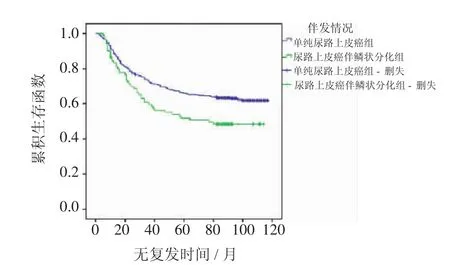
圖1 兩組患者RFS曲線(P<0.001)Fig 1 Kaplan-Meier curve of the recurrence-free survival rates for the two groups(P<0.001)
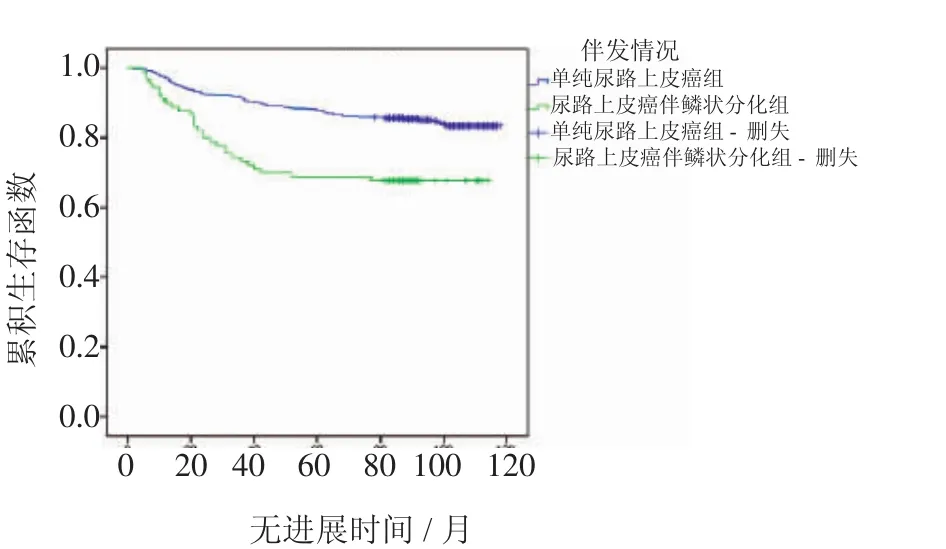
圖2 兩組患者PFS曲線(P<0.001)Fig 2 Kaplan-Meier curve of the progression-free survival rates for the two groups(P<0.001)
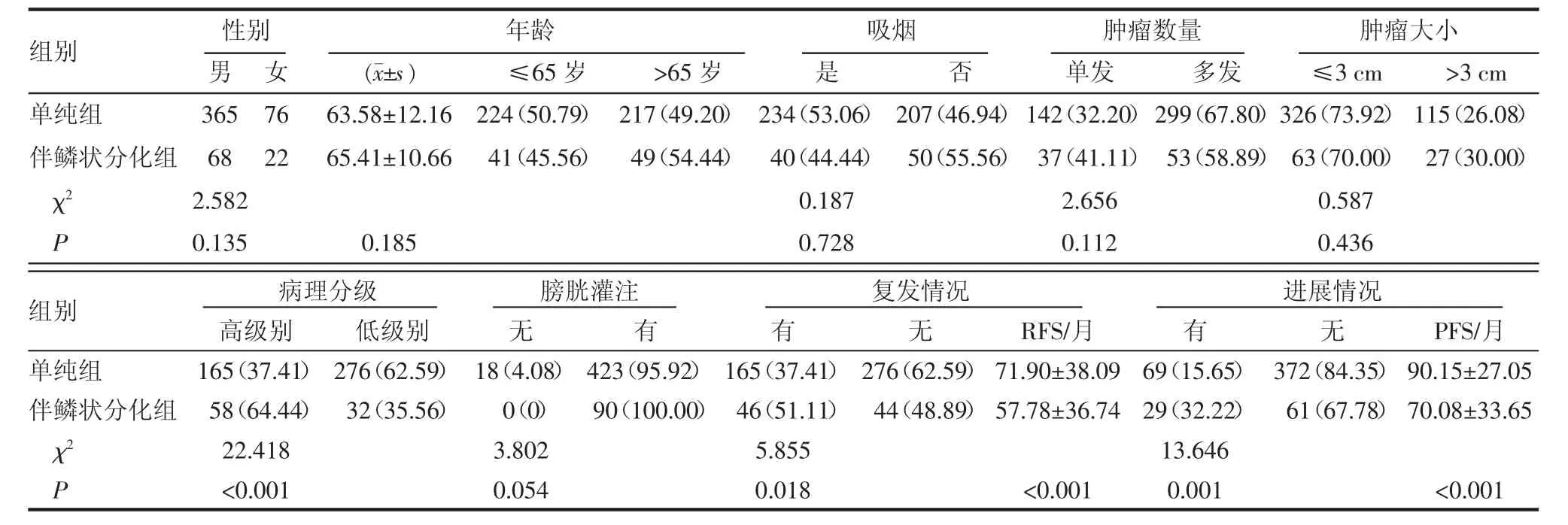
表1 單純尿路上皮癌組和尿路上皮癌伴鱗狀分化臨床特點的比較 [n(%)]Tab 1 Comparison of clinical characteristics between pure UCB and UCB with squamous differentiation [n(%)]
2.2.2 COX比例風險模型分析單因素、多因素(臨床特征)對復發和進展的影響 表2顯示了臨床特征對復發的影響,其中單因素結果表明吸煙、腫瘤數量、腫瘤大小、鱗狀分化的伴發情況以及病理分級等影響因素與腫瘤的復發相關。然而,多因素結果顯示僅有吸煙、鱗狀分化的伴發情況以及病理分級證明是復發的重要獨立預測指標。
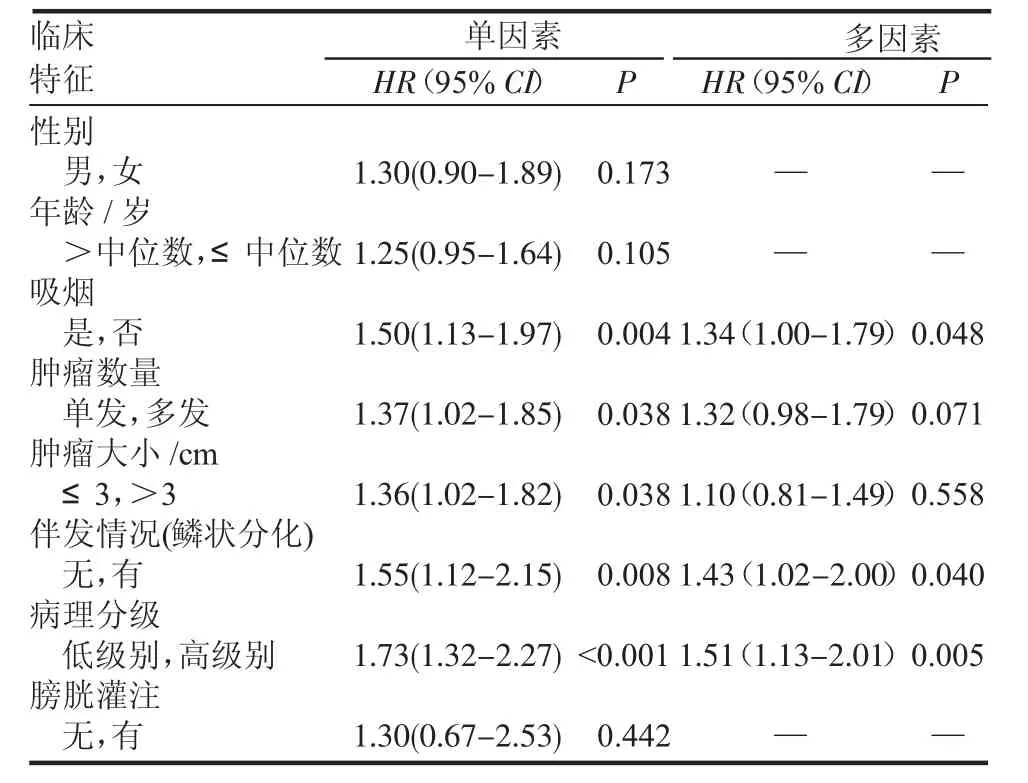
表2 單因素和多因素分析各臨床特征對TURBT術后pT1期患者RFS的影響Tab 2 Univariableandmultivariableanalysesaccordingtorecurrence
同樣,表3分析了臨床特征對進展的影響。其中單因素結果表明年齡、吸煙、鱗狀分化的伴發情況以及病理分級等影響因素與腫瘤的進展相關。多因素結果顯示僅有吸煙、鱗狀分化的伴發情況以及病理分級證明是進展的重要獨立預測指標。
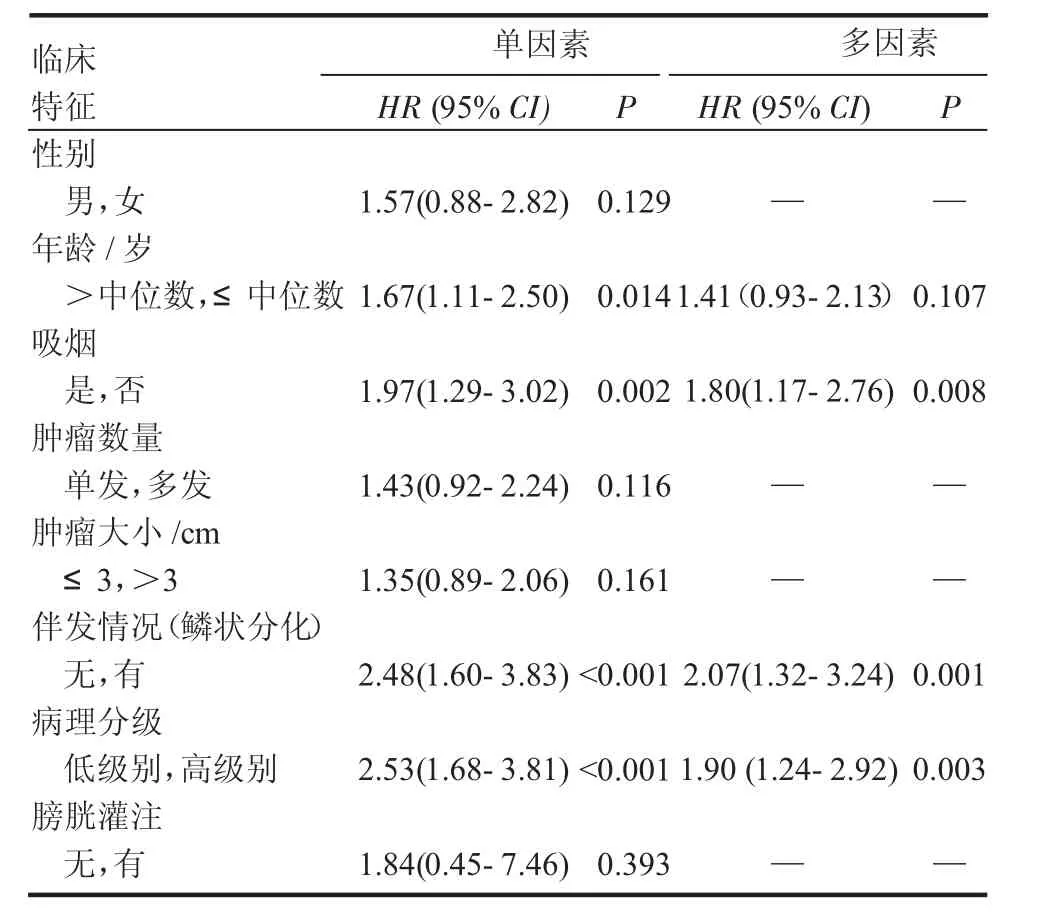
表3 單因素和多因素分析各臨床特征對TURBT術后pT1期患者PFS的影響Tab 3 Univariableandmultivariableanalysesaccordingtoprogression
3 討論
膀胱癌具有廣泛的組織學類型,其中膀胱癌患者中超過90%的病理結果是來源于尿路上皮的UC[13]。尿路上皮具有多向分化的能力,可以向多種分化形式及變異亞型變化。2004年WHO將尿路上皮腫瘤伴異向分化分為13種[13],約占UC的15%。其中UC伴鱗狀分化,在臨床上也常見,但由于目前仍沒有統一的診療標準,所以其臨床意義仍不確定[7,14-16]。
UCB伴鱗狀分化在患者的癥狀及影像學檢查上表現并無特異性,其診斷仍需進一步的組織學檢查,鏡下往往表現為:腫瘤組織中除了伴有尿路上皮成分外,局部還伴有鱗狀上皮的成分,在鱗狀上皮的區域可以看到細胞角化或伴有細胞間橋,必要時仍需免疫組化進行鑒別。p40作為鱗狀細胞癌敏感性指標,當鱗狀分化區域較大時可以依靠p40加以明確診斷;但當鱗狀分化的區域較小時,單純依靠組織染色無法辨別時,則需依靠鱗狀分化的特異性指標CK1、L1抗原加以甄別[17-18]。此外,有報道橋粒膠蛋白2也是鱗狀分化區域的特異性指標[19]。由于治療方法的不同,需與膀胱鱗狀細胞癌進行鑒別。但有文獻報道,UCB伴鱗狀分化與膀胱鱗狀細胞癌的預后差異無統計學意義[20]。
目前由于缺乏隨機的前瞻性對照研究,鱗狀分化能否作為UCB預后不良特點仍存在爭議。既往的觀點認為單純UCB和UCB伴鱗狀分化在預后方面沒有差異[7,21-22]。Mitra等[23]回顧性分析了2 444例行根治性膀胱切除術的患者,發現單純UCB與伴鱗狀分化的患者預后無統計學意義,但結果顯示伴鱗狀分化可能是預后不良的指標。Kim等[7]也回顧性分析了1 013例根治性膀胱切除術的患者,發現伴有鱗狀分化或(和)腺樣化生的患者更可能有膀胱外腫瘤和淋巴結陽性,但多因素分析臨床病理在膀胱癌死亡風險上沒有差異。然而,也有報道否定上述發現,由于UCB伴鱗狀分化往往表現為高級別腫瘤,可以作為不良預后的因素[24],本研究符合該觀點。Antunes等[11]發現伴鱗狀分化在患者根治性膀胱切除術后可作為腫瘤特異性生存的獨立的預測因素。另外,有研究證實組織中鱗狀細胞腫瘤成分的存在對根治性膀胱切除術后局部復發起到重要作用[15]。
對UCB伴鱗狀分化仍沒有統一的治療標準。以往的報道認為UCB伴變異型對放化療不敏感[25-27],尤其是Martin等[28]認為UCB伴鱗狀分化對放療缺乏敏感性。但是,Scosyrev等[21]報道UCB伴鱗狀分化或腺樣分化的患者對新輔助化療反應較好。仍需要大量研究評價放化療對UCB伴變異型的療效。
目前對TURBT術后UCB伴鱗狀分化的預后影響依舊不明確。Billis等[29]總結了165例TURBT的臨床病理特征,發現腫瘤伴有鱗狀或(和)腺樣分化在臨床分級上有明顯的統計相關性,并表現為更有侵襲性腫瘤。Erdemir等[12]認為UCB伴變異型與單純UCB比較在TURBT術后具有更高的復發、進展風險和較低的生存率。目前仍沒有更多的研究pT1期UCB伴鱗狀分化對腫瘤預后的影響。我們的研究重點就放在pT1期UCB患者首次TURBT術后伴鱗狀分化對復發和進展的影響。入組病例均為首次TURBT,術后病理診斷為T1期UCB。結果分析顯示伴鱗狀分化的患者比單純UCB的患者有較高的復發率和較短的無復發生存期,同時有較高的進展率和較短的無進展生存期。COX多因素回歸分析吸煙、伴鱗狀分化以及病理分級證明為無復發生存期和無進展生存期的獨立預后因素,尤其是腫瘤變異與病理分級的分布,可以明顯發現UCB伴鱗狀分化更可能伴有高級別腫瘤,這些發現暗示鱗狀分化的存在與腫瘤的侵襲性息息相關,術后患者需密切隨訪,以防復發和進展。但我們的研究仍存在缺陷,本研究僅將我院pT1期病例納入,仍需多中心、多樣本研究來證實。
總之,膀胱尿路上皮癌伴鱗狀分化,發病率高,惡性程度高,復發、進展率高,可能為預后不良的指標,術后需密切隨訪并采取積極的治療方式,對改善預后起到重要作用。
[1]Torre L A,Bray F,Siegel R L,et al.Global cancer statistics,2012[J]. CA Cancer J Clin,2015,65(2):87
[2] Kurth K H,Denis L,Bouffioux C,et al.Factors affecting recurrence and progression in superficial bladder tumours[J].Eur J Cancer,1995, 31A(11):1840
[3] Allard P,Bernard P,Fradet Y,et al.The early clinical course of primary Ta and T1 bladder cancer:a proposed prognostic index[J]. Br J Urol,1998,81(5):692
[4]SamaratungaH,DelahuntB.Recentlydescribedand unusual variants of urothelial carcinoma of the urinary bladder[J].Pathology,2012,44 (5):407
[5] Xylinas E,Rink M,Robinson B D,et al.Impact of histological variants on oncological outcomes of patients with urothelial carcinoma of the bladder treated with radical cystectomy[J].Eur J Cancer,2013,49(8):1889
[6] Shah R B,Montgomery J S,Montie J E,et al.Variant(divergent)his tologic differentiation in urothelial carcinoma is under-recognized in community practice:impact of mandatory central pathology review at a large referral hospital[J].Urol Oncol,2013,31(8):1650
[7] Kim S P,Frank I,Cheville J C,et al.The impact of squamous andglandular differentiation on survival after radical cystectomy for urothelial carcinoma[J].J Urol,2012,188(2):405
[8] Abd E A,Watts K E,Elson P,et al.The sensitivity of initial transurethral resection or biopsy of bladder tumor(s)for detecting bladder cancer variants on radical cystectomy[J].J Urol,2013,189(4): 1263
[9] Yang M H,Yen C C,Chen P M,et al.Prognostic-factors-based risk-stratification model for invasive urothelial carcinoma of the urinary bladder in Taiwan[J].Urology,2002,59(2):232
[10]Lopez-Beltran A,Requena M J,Alvarez-Kindelan J,et al. Squamous differentiation in primary urothelial carcinoma of the urinary tract as seen by MAC387 immunohistochemistry[J].J Clin Pathol,2007,60(3):332
[11]Antunes A A,Nesrallah L J,Dall’Oglio M F,et al.The role of squamous differentiation in patients with transitional cell carcinoma of the bladder treated with radical cystectomy[J].Int Braz J Urol, 2007,33(3):339
[12]Erdemir F,Tunc M,Ozcan F,et al.The effect of squamous and/or glandular differentiation on recurrence,progression and survival in urothelial carcinoma of bladder[J].Int Urol Nephrol,2007,39(3):803 [13]Chalasani V,Chin J L,Izawa J I.Histologic variants of urothelial bladder cancer and nonurothelial histology in bladder cancer[J].Can Urol Assoc J,2009,3(6 Suppl 4):S193
[14]Mazzucchelli L,Bacchi M,Studer U E,et al.Invasion depth is the most important prognostic factor for transitional-cell carcinoma in a prospective trial of radical cystectomy and adjuvant chemotherapy [J].Int J Cancer,1994,57(1):15
[15]Honma I,Masumori N,Sato E,et al.Local recurrence after radical cystectomy for invasive bladder cancer:an analysis of predictive factors[J].Urology,2004,64(4):744
[16]Jozwicki W,Domaniewski J,Skok Z,et al.Usefulness of histologic homogeneity estimation of muscle-invasive urinary bladder cancer in an individual prognosis:a mapping study[J].Urology,2005,66(5): 1122
[17]Gaisa N T,Braunschweig T,Reimer N,et al.Different immunohistochemical and ultrastructural phenotypes of squamous differentiation in bladder cancer[J].Virchows Arch,2011,458(3):301
[18]Cao L,Zhou X D,Sens M A,et al.Keratin 6 expression correlates to areas of squamous differentiation in multiple independent isolates of As(+3)-induced bladder cancer[J].J Appl Toxicol,2010,30(5):416 [19]Hayashi T,Sentani K,Oue N,et al.Desmocollin 2 is a new immunohistochemical marker indicative of squamous differentiation in urothelial carcinoma[J].Histopathology,2011,59(4):710
[20]Ehdaie B,Maschino A,Shariat S F,et al.Comparative outcomes of pure squamous cell carcinoma and urothelial carcinoma with squamous differentiation in patients treated with radical cystectomy [J].J Urol,2012,187(1):74
[21]Scosyrev E,Ely B W,Messing E M,et al.Do mixed histological features affect survival benefit from neoadjuvant platinum-based combination chemotherapy in patients with locally advanced bladder cancer?A secondary analysis of Southwest Oncology Group-Directed Intergroup Study(S8710)[J].BJU Int,2011,108(5):693
[22]Kastritis E,Dimopoulos M A,Antoniou N,et al.The outcome of patients with advanced pure squamous or mixed squamous and transitionalurothelialcarcinomas following platinum-based chemotherapy[J].Anticancer Res,2006,26(5B):3865
[23]Mitra A P,Bartsch C C,Bartsch G J,et al.Does presence of squamous and glandular differentiation in urothelial carcinoma of the bladder at cystectomy portend poor prognosis?An intensive case-control analysis[J].Urol Oncol,2014,32(2):117
[24]Rogers C G,Palapattu G S,Shariat S F,et al.Clinical outcomes followingradicalcystectomyforprimarynontransitionalcell carcinoma of the bladder compared to transitional cell carcinoma of the bladder[J].J Urol,2006,175(6):2048
[25]Coulson W F.Clinical importance of squamous metaplasia in invasive transitional cell carcinoma of the bladder[J].J Clin Pathol,1989,42 (11):1227
[26]Miller J S,Epstein J I.Noninvasive urothelial carcinoma of the bladderwithglandulardifferentiation:reportof24cases[J].Am J Surg Pathol,2009,33(8):1241
[27]Frazier H A,Robertson J E,Dodge R K,et al.The value of pathologic factors in predicting cancer-specific survival among patients treated with radical cystectomy for transitional cell carcinoma of the bladder and prostate[J].Cancer,1993,71(12):3993 [28]Martin J E,Jenkins B J,Zuk R J,et al.Clinical importance of squamous metaplasia in invasive transitional cell carcinoma of the bladder[J].J Clin Pathol,1989,42(3):250
[29]Billis A,Schenka A A,Ramos C C,et al.Squamous and/or glandular differentiation in urothelial carcinoma:prevalence and significance in transurethral resections of the bladder[J].Int Urol Nephrol, 2001,33(4):631
(2015-10-27收稿)
Influence of squamous differentiation on the prognosis of patients with pT1 urothelial carcinoma of bladder after TURBT
HE Zhen,XU Yong,QI Shi-yong,DU E,SHA Nan,ZHU Bing
(Department of Urology,The Second Hospital,Tianjin Medical University,Tianjin Institute of Urology,Tianjin 300211,China)
Objective:To evaluate squamous differentiation on prognosis in patients with pT1 urothelial carcinoma of bladder(UCB)after first transurethral resection(TURBT).Methods:The retrospective study based on clinicopathologic data was applied to 531 patients of pTl UCB after first TURBT.The patients were divided into two groups:group A included 441 patients with pure UCB,and group B included 90 patients with squamous differentiation.All the data were calculated by using SPSS 20.0 statistical software(IBM Company,version20.0).Recurrence-free survival(RFS)and progression-free survival(PFS)curves were estimated to study clinicopathologic features of two groups using the Kaplan-Meier method,and the difference was determined by the log-rank test.Univariate and multivariate analyses were performed to study patient prognosis between patients with squamous differentiation and patients with pT1 UCB through using a Cox proportional hazards regression model,and the factors influencing its RFS and PFS were evaluated.Results:In this study,group A included 441 patients with pure UCB,and group B included 90 patients with squamous differentiation.High grade tumors were more common in patients with squamous differentiation than those with pure UCB (P<0.001).Meanwhile,compared with group A,group B had a higher recurrence rate(P=0.018),shorter RFS (P<0.001),and the progress of the higher rate(P=0.001),shorter PFS(P<0.001).Correlations among the age(P=0.185),gender(P=0.135),smoking(P=0.728),tumor size(P=0.436),and tumor count(P=0.112)were not statistically significant.Based on multivariate Cox regression analysis,smoking (HR 1.34,95%CI 1.00-1.79,P=0.048),comorbid conditions of squamous differentiation(HR 1.43,95%CI 1.02-2.00,P=0.040)and pathology classification (HR 1.51,95%CI 1.13-2.01,P=0.005)in patients with pT1 urothelial carcinoma of bladder after first TURBT had a higher risk of recurrence;smoking(HR 1.80,95%CI 1.17-2.76, P=0.008),comorbid conditions of squamous differentiation(HR 2.07,95%CI 1.32-3.24,P=0.001)and pathology classification(HR 1.90, 95%CI 1.24-2.92,P=0.003)in patients with pT1 urothelial carcinoma of bladder after first TURBT had a higher risk of progress.Conclusion:UCB with squamous differentiation is an independent prognostic predictor,and the presence of squamous differentiation couldbe associated with higher recurrence/progress rate,and patients with this variant should be followed up closely.
bladder;urothelial carcinoma;squamous differentiation;TURBT;recurrence;progression
R737.14
A
1006-8147(2016)03-0213-05
何振(1989-),男,碩士在讀,研究方向:泌尿系腫瘤;通信作者:徐勇,E-mail:Drxuyong@126.com。

