Photorheologically reversible micelle composed ofpolymerizable cationic surfactant and 4-phenylazo benzoic acid☆
Jie Chen,Bo Fang*,Hao Jin,Licheng Yu,Meng Tian,Kejing Li,Leiping Jin,Mo Yang
Shanghai Key Laboratory of Multiphase Materials Chemical Engineering,Lab of Chemical Engineering Rheology,Research Center of Chemical Engineering,East China University ofScience and Technology,Shanghai200237,China
1.Introduction
Photorheological fluids(PR fluids)as intelligent materials[1]were first discovered by Wolff et al.[2]in 1989.PR fluids are light-sensitive through specific wavelength causing cis–trans isomerizations, dimerizations,photoscission,,and polymerizations or polarity changes[3–8].Compared to electro-rheological fluids[9]and magnetic fluids[10],PR fluids have more stable rheological properties as well as environmental safety.It has potential applications in microreactor, microscale actuators and valves[11–13].In such applications,the use oflight as a modulator can be particularly advantageous since lightcan be directed at a precise spot with resolution of a few micrometers[14].Ithas been demonstrated impressive rheology-modulation with complex synthesized light-sensitive molecules[15–22].It is a new trend to create PR fluids from simple and existing molecules[23].Ketner et al.have[24]found that that trans-orthomethoxycinnamic acid and cetyltrimethyl ammonium form irreversible light-responsive viscoelastic wormlike micelles without complex synthetic method.Irreversible counter-ions comprising styrene group have been reported,such as para-coumaric acid[25],orthomethoxycinnamic acid[26,27],and cinnamic acid(CA)[28,29].Azobenzene compounds have obvious advantages in terms of the reversibility and a series of fluids based on 4-phenylazo benzoic acid(ACA)that exhibits dramatic,reversible changes in fluid viscosity are reported.Shi et al.have reported a photoresponsive micellar solution oleyl bis(2-hydroxyethyl)methyl ammonium chloride/ACA,a promising working fluid for district heating/cooling systems[30].The drag reduction is advantageous during fluid transport,and efficient heat transfer mode is favored when the fluid passes through heat exchangers.
Suitable surfactants and counter-ions are the keys for photosensitive fluids.In this study,we use a polymerizable cationic surfactant n-cety l dimethyl ally l ammonium chloride(CDAAC)[31]and mix it with counter ion of ACA to obtain a new reversible photosensitive micellar solution with optimum mixture ratio of CDAAC/ACA at 45°C.ACA has both cis and trans structures,and trans-ACA can convert to cis-ACA with UV irradiation and is reversed under visible light[32].Its isomerization process wil be veri fied by UV–Vis spectroscopy,and the rheologicalprocess of CDAAC/trans-ACA micelle is studied with UV irradiation to prove that viscosity,elastic modulus G′and viscous modulus G″of this micelle system reduce quickly.These results will enrich the field of lightrheological micellar solution.
2.Experimental
2.1.Materials and sample preparation
Trans-4-phenylazo benzoic acid(trans-ACA)(98.0%)was purchased from J&K Scienti fic Ltd.and used as received.The salt solutions(trans-ACA-Na)were prepared by adding NaOH at a 12%molar excess relative to trans-ACA.The polymerizable cationic surfactant CDAAC used was synthesized as reference[31].26.95 g(0.1 mol)N,N-dimethyl hexadecyl amine(DMA16≥95%),22.96 g(0.3 mol)allyl chloride and 49.91 g ethyl acetate were mixed in a dry flask with stirrers,and the reactive system was kept at 45°C and re fluxed for 24 h.The crude productcooled to room temperature was recrystallized four times from dehydrated acetone and filtered to obtain white powder product(CDAAC,Fig.1),which is easily soluble in water.
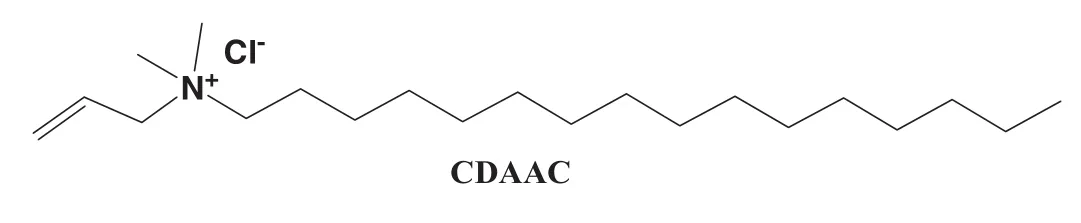
Fig.1.Chemical structure of polymerizable cationic n-cetyl dimethylallyl ammonium chloride.
The CDAAC/trans-ACA solutions were prepared by mixing the two solutions of surfactant CDAAC and trans-ACA-Na.The aqueous mixtures were stirred at45 °C for 1 h and keptin a the rmostatat45 °C for 24 h to reach equilibrium.
2.2.Characterization
2.2.1.UV and visible-irradiation
CDAAC/trans-ACA solutions(20 ml)were irradiated with UV light from a Spectorline UV lamp(broad band centered at 365 nm),and then followed by a 15 WLED lamp(radiation peaked at 460 nm)to irradiate samples for reversible process.To reduce the effect of atmospheric moisture,samples were placed in a sealed Petridish(7.5 cm diameter)during the irradiation process.UV–Vis spectroscopy study before and after UV-irradiation was carried out using a SHIMADZU UV-1102 spectrophotometer.
2.2.2.Rheological measurements
Relative viscosity(ηr)was measured with a Ubbelohde viscometer(Inner diameter 0.9–1.0 mm).Steady shear viscosity(η)was measured using an Anton Paar MCR-101 rheometer ata shear rate of200 s-1with a 25 mm cone-and-plate con figuration.Shear thinning property was studied in the shear rate range from 0.1 to 100 s-1.An Anton Paar MCR-702 rheometer with an inside UV lamp(broad band centered at 365 nm)was used to measure the change of steady shear viscosity and viscoelasticity during the irradiation,the steady shear rate was 200 s-1,and the UVlightwas turned on at t=100 s.In the viscoelasticity experiment,the frequency(f)was 1 Hz and the shear strain(γ)was 10%.Allmeasurements were performed at 45°C.
3.Results and Discussion
3.1.Effects ofconcentration and molar ratio
Steady shear viscosityηof CDAAC/trans-ACA samples at 45°C was tested with different molar ratios of CDAAC and trans-ACA([CDAAC]/[ACA]),with the trans-ACA concentration kept constant at 10 mmol·L-1(10 mmol·L-1).At the molar ratio of CDAAC/trans-ACA less than 1.2,the samples present low viscosity.As CDAAC concentration increases and the molar ratio of CDAAC/trans-ACA reaches 1.4,ηincreases obviously and reaches maximum,implying the formation and growth of worm like micelles.However,further addition of CDAAC makes η decrease and the sample become turbid.Thus,the peak of viscosity in Fig.2 indicates the optimum molar ratio([CDAAC]/[ACA])of1.4.

Fig.2.Steady shear viscosity versus molar ratio([CDAAC]/[ACA])at trans-ACA concentration of10 mmol·L-1 at 45 °C.
Fig.3 shows the photosensitivity of micelle solutions at the optimum molar ratio of CDAAC/trans-ACAof1.4.With the increase ofmicelle concentration,the viscosity of micelle solution increases gradually.With 1 h UV irradiation,all the micelle solutions revert to low viscosity and the reduction percentage of viscosity is around 85%–95%.
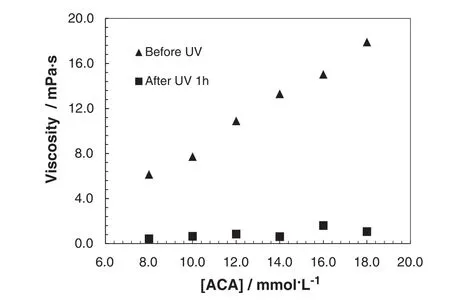
Fig.3.Before and after UV irradiation,steady shear viscosity versus trans-ACA concentration([ACA])at molar ratio([CDAAC]/[ACA])of1.4,45°C,and shear rate of200 s-1.
3.2.Effectof UV irradiation time on viscosity ofmicelle solutions
With 20 ml CDAAC/trans-ACA(14 mmol·L-1/10 mmol·L-1),viscosity curves with different UV irradiation time are showed in Fig.4.Without irradiation,the viscosity ofmicelle solution keeps almost constant at low shear rate less than 1 s-1,and drops significantly as shear rate increases.With the increase of UV irradiation time,the viscosity of micelle solution decreases obviously.The viscosity curve of micelle solution with 60 min UV irradiation is almost same as that with 75 min UV irradiation,and the viscosity is decreased by nearly 95%at the shear rate of100 s-1.Therefore,we consider 60 min as a suitable UV irradiation time for 20 ml CDAAC/trans-ACA solutions.
3.3.Rheological properties during UV irradiation process
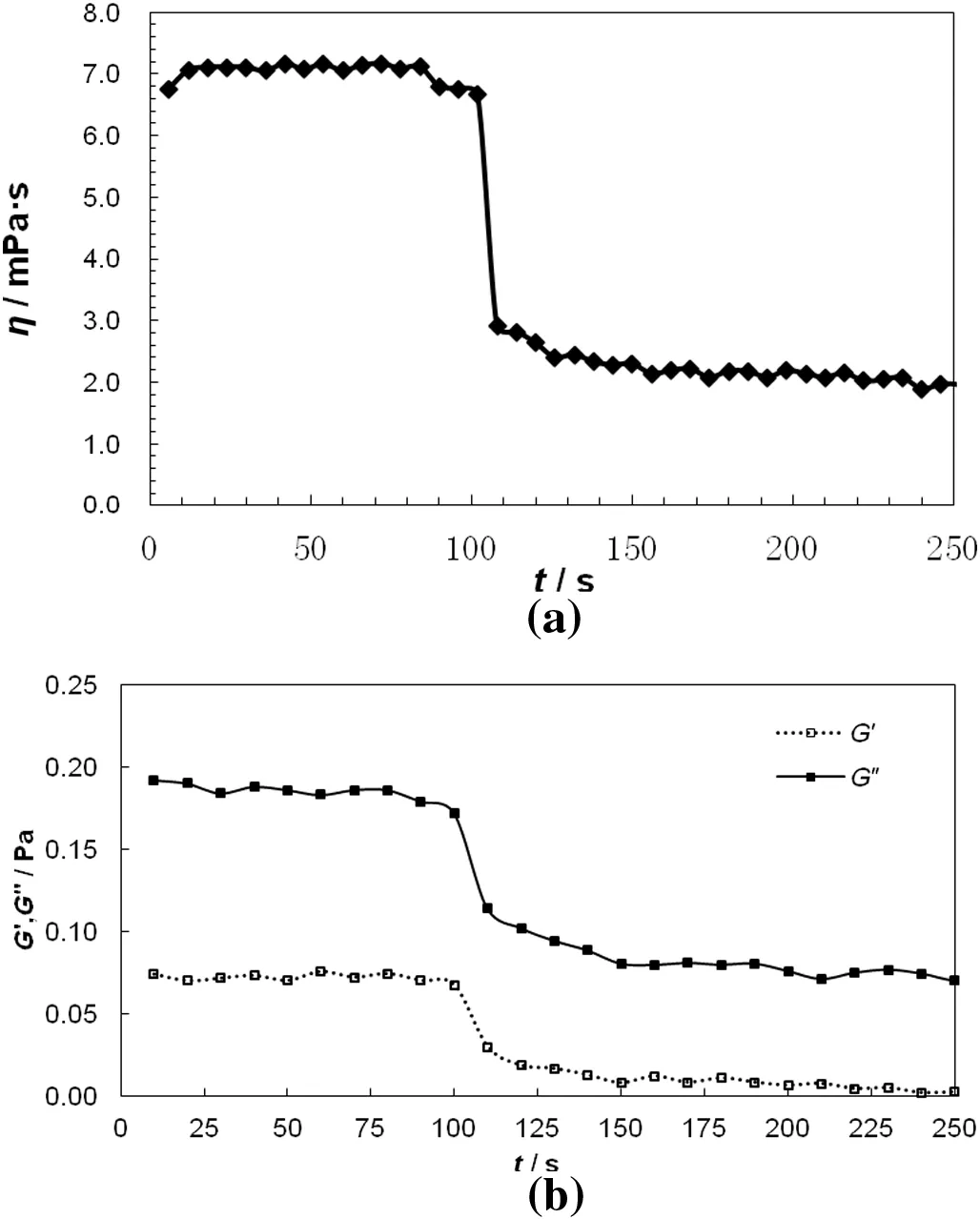
Fig.5.The rheological process of0.3 mlCDAAC/trans-ACA(14 mmol·L-1/10 mmol·L-1)solution under UVirradiation with UVlightturned on at t=100 s.(a)steady shear viscosityη;(b)viscoelasticity.
Fig.5 shows the photosensitivity of CDAAC/trans-ACA(14 mmol·L-1/10 mmol·L-1)solution during UV irradiation at 45 °C.Before UV irradiation,the viscosityηof 0.3 mlsample is around 7–8 mPa·s,then drops rapidly to 2.9 mPa·s with the UV light turned on at t=100 s,and decreases gradually to 1.8 mPa·s with UV irradiation in next150 s.This process indicates that the solution is sensitive to UV irradiation.The viscoelasticity of the solution is also sensitive to UV irradiation.With the irradiation started at t=100 s,elastic modulus G′decreases from 0.07 Pa to 0.03 Pa in 10 s and viscous modulus G″drops rapidly from 0.2 Pa to 0.1 Pa.With UV irradiation for next 150 s,both G′and G″reach stability.The rapid response of rheologicalproperties of micelle to UV light is bene ficialfor viscoelastic drag reduction to enhance heat transfer[27,30].
3.4.Photoisomerization analysis by UV-Vis spectra
Fig.6 shows the UV–Vis spectroscopy for photoisomerization of two solutions,0.04 mmol·L-1trans-ACA solution and 0.056 mmol·L-1CDAAC+0.04 mmol·L-1trans-ACA solution.In the wavelength range from 200 to 500 nm,their results are similar.Before UV irradiation there are three peaks around 231,321 and 427 nm;after1 h UV irradiation,the first and second peaks shift to 251 and 298 nm,respectively,with the second peak decreased significantly and the third peak increases slightly.These data con firm those in reference[30]and the UV–Vis spectra for the two solutions correspond to the photoi somerization of trans- of trans-ACA to cis-ACA.
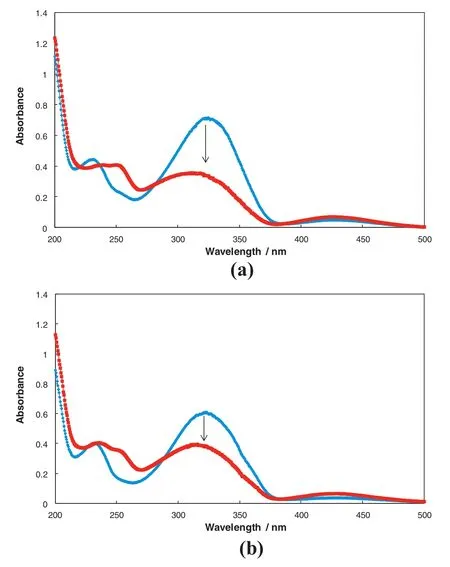
Fig.6.UV–Vis spectra of two solutions for fresh sample(blue)and after 1 h UVirradiation(red)at 45 °C.(a)0.04 mmol·L-1 trans-ACA;(b)0.056 mmol·L-1 CDAAC+0.04 mmol·L-1 trans-ACA.(Please refer to the on line version for the colorized figures).
3.5.Reversible process
Fig.7 shows the change inηrfor a mixture of CDAAC/trans-ACA(14 mmol·L-1/10 mmol·L-1)upon repetitive irradiation of UV and visible light.Before UV irradiation,the sample exhibits higher viscosity(ηr=5.7);after 1 h UV irradiation,the viscosity is lower(ηr=2.6);with subsequently exposed to 4 h visible light,it restores to its initial high viscosity.It indicates that this photorheological micelle can be cycled between high and low viscosity states.
4.Conclusions
In this study,a new reversible photorheological micelle composed of polymerizable cationic surfactant n-cetyl dimethylallyl ammonium chloride(CDAAC)and 4-phenylazo benzoic acid(ACA)was obtained.
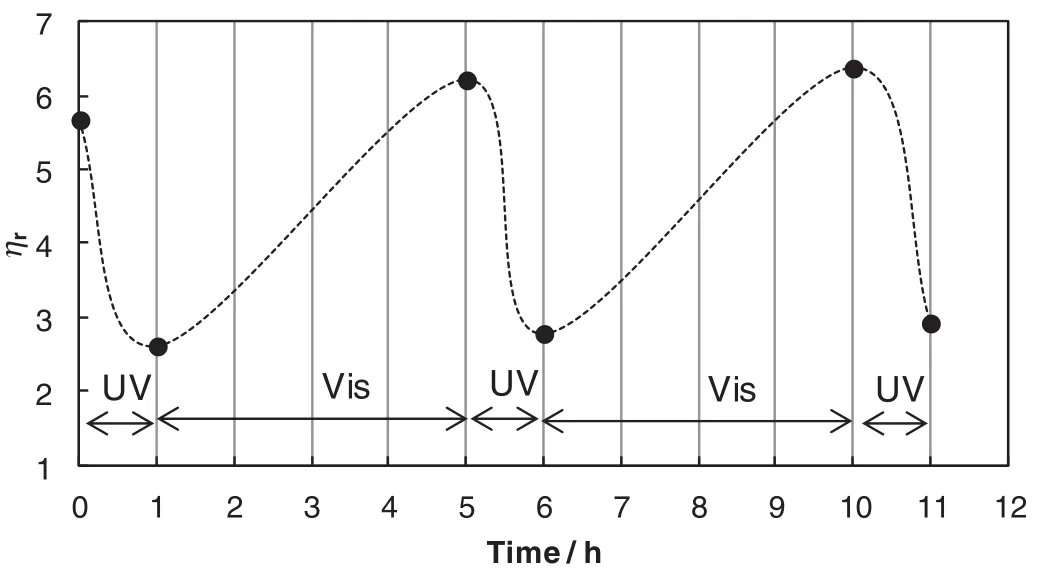
Fig.7.The relative viscosity of CDAAC/trans-ACA(14 mmol·L-1/10 mmol·L-1)with repetitive UV and visible light irradiations at 45°C.
The viscoelastic CDAAC/trans-ACA micelle formed with the optimum molar ratio of CDAAC/trans-ACA of1.4 at 45°C.For CDAAC/trans-ACA(14 mmol·L-1/10 mmol·L-1)micelle system,the viscosity is decreased by 90%after 1 h UV irradiation at 365 nm,closely related to the photoisomerization of trans-ACAto cis-ACA.The rheological process during UV irradiation for CDAAC/trans-ACA(14 mmol·L-1/10 mmol·L-1)micelle proves that viscosity,elastic modulus G′and viscous modulus G″are reduced rapidly with the UV light,which will be beneficialto enhance heat transfer with photosensitive drag reducing micelle.Furthermore,it is con firmed that CDAAC/trans-ACA(14 mmol·L-1/10 mmol·L-1)micelle is reversible photorheologicalmicelle by UV and visible light irradiations.It is expected that the reversible photosensitive micelle will enrich the new PR fluids system and applications.
[1]A.M.Al-Dhafeeri,J.J.Xiao,N.S.Al-Habib,Smart fluids—Their role in exploration and production(E&P),Saudi Aramco J.Technol.(2008)72–77.
[2]T.Wolff,C.S.Emming,T.A.Suck,G.V.Bunau,Photorheologicaleffects in micellar solutions containing anthracene derivatives—A rheologicaland static low angle lightscattering study,J.Phys.Chem.93(12)(1989)4894–4898.
[3]J.Eastoe,A.Vesperinas,Self-assembly oflight-sensitive surfactants,Soft Matter 1(5)(2005)338–347.
[4]M.Bonini,D.Berti,J.M.Meglio,M.Almgren,J.Teixeira,P.Baglioni,Surfactant aggregates hosting a photoresponsive amphiphile:Structure and photoinduced conformational changes,Soft Matter 1(16)(2005)444–454.
[5]D.L.Zhao,B.Ren,S.Liu,X.Liu,Z.Tong,A novelphotoreversible poly(ferrocenylsilane)with coumarin side group:Synthesis,characterization,and electrochemical activities,Chem.Commun.32(2)(2006)5737–5740.
[6]L.C.Chien,L.Cada,Photo-cross-linkable and optically active side-chain liquid crystalline copolymers,Macromolecules 27(14)(1994)3721–3726.
[7]G.Pouliquen,C.Tribet,Light-triggered association of bovine serum albumin and azobenzene-modified poly(acrylic acid)in dilute and semidilute solutions,Macromolecules 39(1)(2006)373–383.
[8]Y.Lin,X.Cheng,Y.Qiao,C.Yu,Z.Li,Y.Yan,J.Huang,Creation of photo-modulated multi-state and multi-scale molecular assemblies via binary-state molecular switch,Soft Matter 6(5)(2010)902–908.
[9]Y.Kakizawa,H.Sakai,A.Yamaguchi,Electrochemical control of vesicle formation with a double-tailed cationic surfactant bearing ferrocenyl moieties,Langmuir 17(26)(2001)8044–8048.
[10]J.S.Jiang,Z.F.Gan,Y.Yang,B.Du,M.Qian,P.Zhang,A novelmagnetic fluid based on starch-coated magnetite nanoparticles functionalized with homing peptide,J.Nanoparticle Res.11(6)(2009)1321–1330.
[11]S.W.Bates,“Quantifying the stimuli of photorheological fluids”,master's degree,Massachusetts Institute of Technology,America,2010.
[12]H.Komatsu,S.Matsumoto,S.Tamaru,K.Kaneko,M.Ikeda,I.Hamachi,Supramolecular hydrogel exhibiting four basic logic gate functions to fine-tune substance release,J.Am.Chem.Soc.131(15)(2009)5580–5585.
[13]S.Sugiura,A.Szilágyi,K.Sumaru,K.Hattori,T.Takagi,G.Filipcsei,M.Zrinyi,T.Kanamori,On-demand micro fluidic control by micropatterned light irradiation of a photoresponsive hydrogel sheet,Lab Chip 9(2)(2009)196–198.
[14]G.Pouliquen,C.Amiel,C.Tribet,Photoresponsive viscosity and host–guest association in aqueous mixtures of poly-cyclodextrin with azobenzene-modified poly(acrylic)acid,J.Phys.Chem.B 111(20)(2007)5587–5595.
[15]T.Wolff,K.J.Kerperin,In fluence of solubilized 2,2,2-tri fluoro-1-(9-anthryl)-ethanol and its photodimerization on viscoelasticity in dilute aqueous cetyltrimethy–lammonium bromide solutions,J.Colloid Interface Sci.157(1)(1993)185–195.
[16]X.L.Yu,T.Wolff,Rheologicaland photorheological effects of 6-alkyl coumarins in aqueous micellar solutions,Langmuir 19(23)(2003)9672–9679.
[17]C.Lehnberger,T.Wolff,Photorheological effects in aqueous micellar tetramethylammoniumhydrogen-2-dodecylmalonate via photodimerization of acridizinium bromide,J.Colloid Interface Sci.213(1)(1999)187–192.
[18]R.Kumar,A.M.Ketner,S.R.Raghavan,Nonaqueous photorheological fluids based on light-responsive reverse wormlike micelles,Langmuir 26(8)(2010)5405–5411.
[19]R.Kumar,S.R.Raghavan,Photogelling fluids based on light-activated growth of zwitterionic wormlike micelles,Soft Matter 5(4)(2009)797–803.
[20]H.Y.Lee,K.K.Diehn,K.Sun,T.H.Chen,S.R.Raghavan,Reversible photorheological fluids based on spiropyran-doped reverse micelles,J.Am.Chem.Soc.133(22)(2011)8461–8463.
[21]Y.Takahashi,Y.Yamamoto,S.Hata,Y.Kondo,J.,Unusualviscoelasticity behaviour in aqueous solutions containing a photoresponsive amphiphile,J.Colloid Interface Sci.407(10)(2013)504–509.
[22]F.Delbecq,N.Kaneko,H.Endo,T.Kawai,Solvation effects with a photoresponsive two-component 12-hydroxystearic acid-azobenzene additive organogel,J.Colloid Interface Sci.384(1)(2012)94–98.
[23]L.C.Yu,J.Chen,B.Fang,T.Y.Lv,J.S.Wang,H.Jin,Review on research and application ofphotosensitive surfactant micelles,China Surfactant Deterg.Cosmet.44(3)(2014)151–154(in Chinese).
[24]A.M.Ketner,R.Kumar,T.S.Davies,P.W.Elder,S.R.Raghavan,A simple class of photorheological fluid:Surfactant solutions with viscosity tunable by light,J.Am.Chem.Soc.129(6)(2007)1553–1559.
[25]D.Wang,R.Dong,P.Long,J.Hao,Photo-induced phase transition from multilamellar vesicles to wormlike micelles,Soft Matter 7(22)(2011)10713–10719.
[26]P.Baglioni,E.Braccalenti,E.Carretti,R.Germani,L.Goracci,G.Savelli,M.Tiecco,Surfactant-based photorheological fluids:Effect of the surfactant structure,Langmuir 25(10)(2009)5467–5475.
[27]Y.Wang,H.Shi,B.Fang,J.Zakin,B.Yu,Heattransfer enhancementfor drag-reducing surfactant fluid using photorheological counterion,Exp.Heat Transfer 25(3)(2012)139–150.
[28]J.Li,M.Zhao,H.Zhou,H.Gao,L.Zheng,Photo-induced transformation of wormlike micelles to spherical micelles in aqueous solution,Soft Matter 8(30)(2012)7858–7864.
[29]H.Sakai,S.Taki,K.Tsuchiya,A.Matsumura,K.Sakai,M.Abe,Photochemi calcontrol of viscosity using sodium cinnamate as a photoswitchable molecule,Chem.Lett.41(3)(2012)247–248.
[30]H.F.Shi,W.Ge,H.Oh,S.Pattison,J.Huggins,Y.Talmon,D.Hart,S.Raghavan,J.Zakin,Photoreversible micellar solution as smart drag-reducing fluid for use in district heating/cooling systems,Langmuir 29(1)(2013)102–109.
[31]Y.Xie,C.H.Zhang,X.Xu,Study on synthesis of cationic trimeric-type quaternary ammonium salt surfactant,China Adhes.10(16)(2007)10–13(in Chinese).
[32]H.Oh,A.M.Ketner,R.Heymann,E.Kesselman,D.Danino,D.E.Falvey,S.R.Raghavan,A simple route to fluids with photo-switchable viscosities based on a reversible transition between vesicles and wormlike micelles,Soft Matter 9(20)(2013)5025–5033.
 Chinese Journal of Chemical Engineering2016年2期
Chinese Journal of Chemical Engineering2016年2期
- Chinese Journal of Chemical Engineering的其它文章
- Relationship between breakthrough curve and adsorption isotherm of Ca(II)imprinted chitosan microspheres for metaladsorption☆
- Experimental study on the effects of big particles physical characteristics on the hydraulic transport inside a horizontal pipe
- Investigation of extraction fraction in con fined impinging jet reactors for tri-butyl-phosphate extracting butyric acid process☆
- Experimental evaluation and modeling of liquid jet penetration to estimate droplet size in a three-phase riser reactor
- Review on current advances,future challenges and consideration issues for post-combustion CO2 capture using amine-based absorbents☆
- Analysis of drop deformation dynamics in turbulent flow
