Biodieselproduction fromgreen seaweed Ulva fasciata catalyzed by novel waste catalysts from Pakistan Steel Industry
Abdul Majeed Khan,Noureen Fatima,Muhammad Shoukat Hussain,Kousar Yasmeen
Research Laboratory of Bioenergy(RLB),Department of Chemistry,Federal Urdu University of Arts,Science and Technology,Gulshan-e-Iqbal Campus,University Road,Karachi-75300,Pakistan
1.Introduction
Presently,most of the energy demands are fulfilled by fossil fuels that are environmentally damaging due to the release of harmful gases into the environment[1-3].In addition,the increasing energy consumption has resulted in the unbalance of the energy management across the globe[4].The massive energy demands coupled with the limited fossil fuel reserves and global environmental concerns have stimulated the search of alternative forms of energy that are environment friendly[5-9].Biodiesel is one of the leading candidates as an alternative of petro-diesel fuel.It is the mixture of fatty acid alkyl esters and produced by the transesterification ofoils with methanolor ethanolin the presence of catalyst[9,10].Itemits less greenhouse gases(GHGs)like CO2,SOx,NOxand volatile organic compounds as compared to petro-diesel[11].
The edible oils such as sun flower,palm,soybean,rapeseed,canola,jatropha and cottonseed oils are usually used in biodiesel synthesis.However,these edible oils have higher prices than the diesel fuel,thus non-edible vegetable oils,waste cooking oil and algal oil are considered as a better option for biodiesel synthesis[6].Algae are the photosynthetic and non-vascular plants[12].They are composed of carbohydrates,proteins,lipids and others.Both marine microalgae and macroalgae have been used as a source for biodiesel production by several researchers[12-14].Ulva fasciatais a chlorophytan marine macroalga,found in the rocky ledge of Bulleji coast,Karachi.It consists of about twenty three fatty acids,including palmitic acid,parinaric acid,oleic acid and others.A number of metals including K,Ca,Na,Mg,Pb,Cd,Cr,Fe,Co,Cu and Zn have also been reported from this alga[15].
The homogeneous strong acids and strong bases are commonly used as catalysts to produce biodiesel.However,the acid catalysts have a slower rate of reaction and corrode the equipment while the base catalysts form soap with the free fatty acids presentin oil that decreases the biodiesel yield.Furthermore,the homogeneous catalyzed biodiesel needs water washing,hence the large quantity of waste water is generated that must be properly disposed due to its toxicity[11,13,16].In contrast,heterogeneous catalysts are favorable because they are noncorrosive,easily separated and reusable.The metal oxides like CaO,BaO,SrO,MgO,ZnO,TiO2,ZrO2and CdO are the most active heterogeneous catalysts for biodiesel synthesis[16-18].
The steelmaking industries generate some waste dusts as byproducts that are generally discarded as waste into the environment.These waste dusts are highly toxic for the environment due to the presence of heavy metals like Ti,Zn,Mn,Fe,Pb,Cu and Ni.Therefore,it is necessary to search for the alternative consumption of these waste dusts.The metals presentin the dusts are converted into metal oxides due to their thermal treatmentat high temperature[19,20].In this way,these waste materials become highly reactive and can be used in biodiesel production as catalysts.The dolomite has been used as lowcost and highly effective catalyst in biodiesel production[21].
2.Materials and Methods
2.1.Collection and pre-treatment of U.fasciata
The marine macroalgaU.fasciata(5 kg)was collected from the Bulleji coast,Karachi(Fig.1).The alga was washed with water to remove dust particles and other impurities,dried and ground into powdered form by grinding machine(Black and Decker Company;FX3508).The powdered alga was soaked inn-hexane(5 L)for five days and this process was repeated three times in order to extract the maximum oily content of algai.e.2.8%with respect to the dry algal powder.Then-hexane extract was concentrated by rotavapor(Buchi,Germany)under reduced pressure(Fig.3).

Fig.1.Pictorial representation of marine macroalga Ulva fasciata growing on the sea rocks within the water.
2.2.Collection of catalysts
The waste industrial dusts produced as by-products during different stages of steel manufacturing,were obtained from Pakistan Steel Industry,Karachi.The industrial dusts were directly used as catalysts without any pre-treatment.However,they had been passed through the furnace at 1500-1700°C during manufacturing processes in Pakistan Steel Industry that oxidized the metals to metal oxides.Therefore,they are already activated catalysts for biodiesel synthesis and no further treatment is required that will reduce the cost and time.These industrial dusts include waste brown dust from the steel converter(A),waste gray dust from the slab casting machine(B),waste gray dustfrom the steel converter(C),waste light pink lining broken dust of the steel converter(D),waste black lining broken dust of the steel ladle(E)and waste limestone(F).In addition to these waste industrial dusts,the left over waste off white paint(G)of Nelson Paint Industry was also used as catalyst(Fig.2).
2.3.Catalyst characterization
2.3.1.Flame atomic absorption spectroscopy(FAAS)
The catalysts were digested by Wet Digestion Method[22]to analyze themby the flame atomic absorption spectroscopy(FAAS).The catalyst(5 g)and conc.HNO3(5 ml)were taken in a 50 ml beaker and the contents were heated and stirred with the help of glass rod on the hotplate then,hydrogen peroxide(2 ml)was added to decolorize the catalyst.Now,the solution was filtered,the filtrate was transferred into 50 ml volumetric flask and the de-ionized water was added upto the mark.The concentration of different metals in mg·kg-1of waste catalyst was determined by flame atomic absorption spectrophotometer(PE-A Analyst 700)in the wavelength range of 190-900 nm,fuel flow 10 L·min-1and flame type Air-C2H2.
2.3.2.Chemical analysis
The chemical analysis of waste catalysts was performed by following the method of Svehla[23]to identify the major metals present in the waste catalysts.For this purpose,20%(catalyst/hot distilled water)solution of each catalyst was prepared and filtered.The filtrate of each catalyst was used as original solution.The mixture of original solution(2 ml)and HCl(2 mol·L-1;0.5 ml)was shaken in the test tube but no precipitates were formed as a result.In another test,the original solution was mixed with the saturated aqueous solution of H2S but no colored precipitates were observed.In addition,the original solution was mixed with the saturated aqueous solution of H2S in the presence ofNH3and NH4Clbutno colored precipitates were formed.The negative results of these three tests con firmed that the metals of groups I,II and III of cations were absent in all the catalysts.On the other hand,when the original solution,(NH4)2CO3,NH4Cl(s)and NH4OH were mixed in a test tube,white precipitates were formed that dissolved in CH3COOH.In another test,original solution and ammonium oxalate solution were mixed together that also formed white precipitates.In addition,flame test was performed in which each catalyst was heated on the non-luminous Bunsen flame,all of them burned with the red flame.These three tests con firmed the presence of Ca2+in all the catalysts.Furthermore,the original solution was dissolved in NaOH solution that formed white precipitates.The catalysts also formed white precipitates with the(NH4)2CO3.These two tests con firmed the presence of Mg2+in all the catalysts.Other metals did not show the positive tests because they were present in the trace amounts in catalysts.
2.3.3.Basicity test
摘 要:隨著互聯(lián)網(wǎng)技術(shù)的發(fā)展,其應(yīng)用已經(jīng)與眾多領(lǐng)域發(fā)展融合,可以說(shuō)“互聯(lián)網(wǎng)+”時(shí)代已經(jīng)來(lái)臨。將互聯(lián)網(wǎng)技術(shù)與小學(xué)數(shù)學(xué)教學(xué)課堂設(shè)置結(jié)合,能夠提升小學(xué)數(shù)學(xué)教學(xué)質(zhì)量,并且能夠?yàn)樾W(xué)數(shù)學(xué)教學(xué)工作的規(guī)劃奠定基礎(chǔ)。
The basicity of catalysts was determined by the method described by Fraileet al.[24].To perform the basicity test,20%(catalyst/hot distilled water)solution of each catalyst was prepared and the solution was subjected to filtration.The phenolphthalein solution in toluene(0.5 ml;1.5 mg·ml-1)was added to the sample filtrate(1 ml)and titrated with the benzoic acid solution(0.01 mol·L-1)prepared in toluene.The volume of benzoic acid consumed was noted to determine the basicity in milli moles of benzoic acid per gram of catalysts.
2.4.Production of biodiesel
Methanol(17 ml),U.fasciataoil(10 g)i.e.methanol/oil(9:1)and catalyst(2 g)were added to an Erlenmeyer flask.The mixture was stirred and re fluxed on the hot plate stirrer(Lab Tech?Daihan Labtech Co.,Ltd)for 6 h at80-100°C.After the reaction,the flask was allowed to cool down at room temperature and the layers were separated by the separating funnel.The upper most layer was collected as biodieseli.e.fatty acid methyl ester(FAME),the middle layer contained glycerol,un-reacted oil and un-reacted methanol while the lowest layer contained catalyst that was recovered for reuse.Biodiesel was also produced using other six catalysts(Fig.3).The FAME yield was calculated by the following formula:

2.5.Recovery and reusability of catalysts
After the completion of reaction,the catalysts were filtered from the product mixture and washed withn-hexane:chloroform(1:1)to separate the impurities then,they were dried at 100°C in the oven for an hour.The catalysts were re-used for biodiesel synthesis with methanol usingU.fasciataoil as raw material in order to check the catalytic activity of the reused catalysts.About 85-90%catalysts were recovered and they showed the same potential towards the biodiesel synthesis as used for the first time.

Fig.2.Waste industrial catalysts collected from Pakistan Steel Industry.

Fig.3.Pictorial representation of the biodiesel production from Ulva fasciata.
2.6.Characterization of oil and biodiesel
The crude oil and biodiesel were purified by the column chromatography usingn-hexane:toluene:chloroform(7:2:1)as mobile phase and silica gelas stationary phase.After purification,the oiland biodiesel were characterized by thin layer chromatography(TLC),using the same mobile phase as used for the column chromatography and iodine crystals as TLC visualizing reagent.The standard free fatty acid(stearic acid)was compared with the triglycerides(algal oil)by the TLC examination usingn-hexane:toluene:chloroform(7:2:1)as mobile phase and silica gel as stationary phase but both of them showed the spots at the same Rf value.It was difficult to separate them by the TLC examination therefore,the free fatty acid content in biodiesel was determined using the formula:Free fatty acid content(%)=0.503×acid value[25].In addition,the oil was also characterized by gas chromatography(GC)by means of gas chromatogram(Shimadzu,Model No.GC-8A,Japan).The fuel properties like density,kinematics viscosity,acid value,free fatty acid content,cloud point,pour point,ash content and total contamination of oil and biodiesel were investigated and compared to the ASTM standard limits of biodiesel(Fig.3).
3.Results and Discussion
The oily contentin marine macroalgaU.fasciatawas found to be 2.8%with respectto the dry algal powder.The pigments and impurities from the crude oil were separated by column chromatography.The TLC examination of oil showed the presence of a large number of free fatty acids and other compounds.The GC analysis of oil showed the presence of numerous fatty acids in the retention time range of 6-49 min.The renewable feedstocki.e.U.fasciataoil was used to produce green,renewable and sustainable biodiesel.CO2is the major greenhouse gas emitted by fossil fuels which will be reduced by using biodiesel.In addition,U.fasciatadoes not need any useful land area for its cultivation.It grows rapidly on the rocky ledge along the seashore in bulk.In addition,the toxic metals of this alga can be harmfulFor the sea life.Therefore,It is the best way to use this alga to produce biodiesel.
The waste industrial dusts(Table 1)have been thermally treated at high temperature(1500-1700°C)during different stages of the steel manufacturing process in Pakistan Steel Industry that converted the metals to metal oxides.The major metals present in the waste industrial catalysts were identified by the chemical analysis.The results showed that these waste catalysts mainly consist of Ca2+and Mg2+.In order to know the presence of other metals in catalysts, flame atomic absorption spectroscopy(FAAS)was performed that detected Ca2+,Mg2+,Cd2+,Cu2+,Fe3+,Pb2+,Zn2+,Na+and K+in different quantities in the waste catalysts.The waste brown dust from the steel converter possesses the highest quantity of Ca2+(121.7 mg·kg-1).The waste gray dust from the slab casting machine and waste gray dust from steel converter showed the highest amount of Fe3+i.e.50.170 and 50.530 mg·kg-1respectively as detected by FAAS(Tables 2,3).
The results showed that the waste catalysts were composed of toxic metals that were harmful to the living system(Tables 2,3).Hence,either they should be dumped properly or some alternative uses of these materials should be explored.One of the interesting uses of these catalysts can be their utilization in biodiesel production.Due to the thermal treatment,the metals present in these catalysts were already converted to metal oxides.The results showed that CaO,MgO and ZnO that are active heterogeneous catalyst for biodiesel synthesis were present in these waste catalysts therefore they showed high reactivity towards transesterification of algal oil.
The catalysts were easily recovered after the reaction and re-used for the next coming batch of reaction.Almost,85-90%catalysts were recovered after the first reaction and they showed the similar reactivity for the next reaction.They were found to be stable,active and reusable for several times.The use of these catalysts in biodiesel synthesis was economical because they were easily available from Pakistan Steel Industry.Waste brown dust from the steel converter gave 88%conversion while waste black lining broken dust of the steel ladle gave 82%conversion of oil to biodiesel.The overall reactivity of the catalysts was decreased in the order of A>E>D>B>C>F>G(Fig.5).
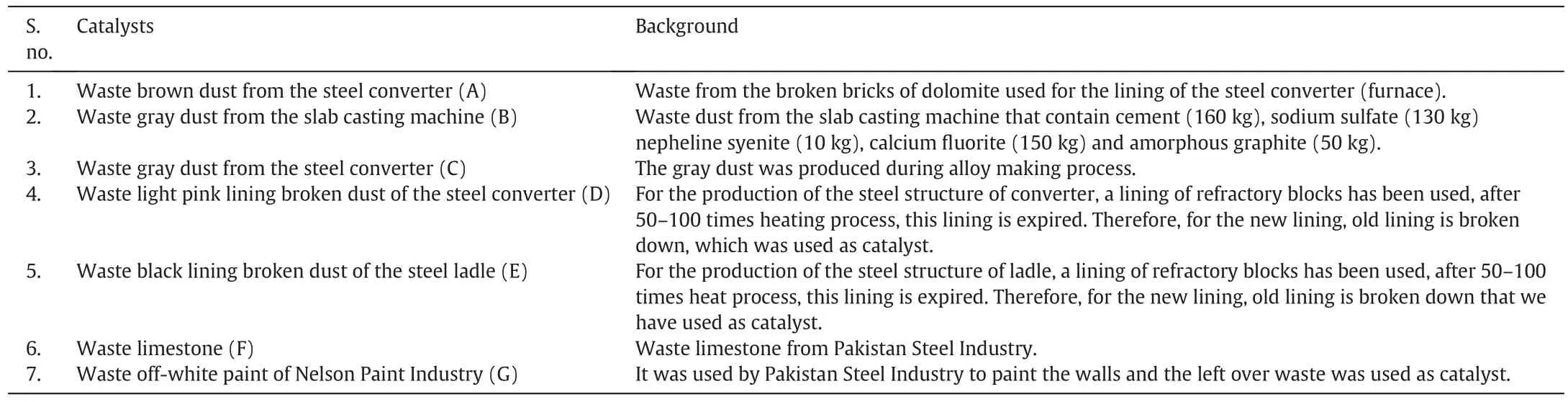
Table 1Background of the waste industrial catalysts obtained from Pakistan Steel Industry

Table 2Characterization of waste industrial catalysts obtained from Pakistan Steel Industry

Table 3Determination of metal content(mg·kg-1)in waste industrial catalysts by flame atomic absorption spectroscopy

Fig.4.transesterification of oil with methanol in the presence of waste industrial catalysts.

Fig.5.Comparative yield of biodiesel using different waste catalysts.
The presence of the reactive metals including Ca2+,Mg2+and Zn2+in the form of metal oxides made the waste catalysts reactive for transesterification.The yield depends upon the concentration and reactivity of the metal oxides.The highest yield of biodiesel was obtained in case of A due to the highest concentration of reactive metals(Ca2+121.7 and Mg2+7.566).The concentration of Mg2+was high in case of E therefore it gave 82%yield of biodiesel.The concentration of Ca2+and Mg2+both were high in case of D therefore,ityielded 77%biodiesel.The catalytic activity is highly dependenton the basicity of catalysts.The basicity of solid catalysts has been described in Table 2.The results were quite similar to each other due to the presence of similar basic oxides in these catalysts.
The pure metal oxides have already been reported for biodiesel synthesis and the waste industrial catalysts have been compared with the pure metal oxide.The literature showed that CaO yielded 98%biodiesel within 3 h when the transesterification reaction was carried outat70°C using very high molar ratio of methanol/oil(30:1).Similarly,MgO gave 60%yield at 130°C and 55:1 molar ratio within 7 h[26]while ZnO produced 95%biodiesel using methanol/oil M ratio 40:1 at 250°C and 10.5 MPa after 10 min of reaction[17].In the present work,only 9:1 molar ratio of methanol/oil using waste catalysts yielded 48-88%biodiesel within 6 h at 80-100°C.The biodiesel was purified by column chromatography thatseparated pigments and other impurities.The TLC examination of biodiesel provided the clear evidence of biodiesel production.The amountofoilconverted to biodieselcould also be observed by the TLC of biodiesel produced by each catalyst in comparison with the oil.The Rf value of biodiesel(FAME)was found to be 0.76(Fig.6).The oil and biodiesel were analyzed by a number of fuel properties like acid value,free fatty acid content,density,kinematics viscosity,cloud point,pour point,ash content and total contamination.The properties of oil were found to be greater than the ASTM standard limits of biodiesel whereas the biodiesel values were found to be close to the ASTM limits(Table 4).

Fig.6.TLC examinations of oil and FAME produced by waste catalysts.

Table 4Characterization of oil and FAME
This research will play a vital role in the development of green and sustainable globe.The marine macroalgaU.fasciataabsorbsthe sunlight that is utilized for photosynthesis and biosynthesis that resulted in the formation of triglycerides which are the basic precursors of biodiesel.It can be consumed by the same engines as currently used for petrodiesel combustion.The petro-diesel combustion releases the greater amount of CO,CO2,NOx,SOxand volatile hydrocarbons that pollutes our environment to a larger extent.On the other hand,biodiesel combustion will mitigate the emission of these hazardous gases.In addition,the sources of the biodieselare renewable thatabsorb CO2therefore It is referred to as carbon neutral.Furthermore,CO2is the major greenhouse gas and its mitigation is highly significant for the development of green and sustainable globe.
4.Conclusions
The marine macroalgaU.fasciatawasutilized to produce biodieselas the green and renewable source.The extraction of alga in non-polar solvents produced 2.8%oily content with respect to the dry algal powder.The transesterification of algal oil with methanol was carried out using waste industrial dusts as catalysts that were produced as by-products during different processes in the Pakistan Steel Industry.The catalysts were analyzed by different chemical tests and flame atomic absorption spectroscopy(FAAS)that identified the metal ions such as Ca2+,Mg2+,Cd2+,Cu2+,Fe+3,Pb2+,Zn2+,Na+and K+.The metals were present in the form of metal oxides in these catalysts due to their calcinations at high temperature during the steel manufacturing process.These metal oxides(especially CaO,MgO and ZnO)are highly reactive for biodiesel production.The basic strength of catalysts was calculated by their basicity test.The highest biodiesel yield(88%)was obtained using waste brown dust from the steel converter as catalyst.As the catalysts were heterogeneous in nature,therefore they were easily separated from the product and re-used for the transesterification of algal oil.The fuel properties of biodiesel such as the acid value,free fatty acid content,density,kinematics viscosity,cloud point,pour point,ash content and total contamination were found to be within the ASTM standard limits.The TLC examination showed the clearspots of biodiesel and hence verified the production of biodiesel.This research article will be helpful to develop the cheapest approach of the biodiesel synthesis on a large scale that will reduce the energy crisis,global warming,loss of biodiversity,pollution,international political stress and dependence on fossil fuels producing countries as well as it will play a significant role to improve the public health and global economy.
Acknowledgments
The authorsare highly thankful to the Higher Education Commission of Pakistan for the provision of scholarship to Noureen Fatima through Indigenous Ph.D.5000 Fellowship Program(117-3083-PS7-208,50018488).In addition,authors are also greatly thankful to the administration of Pakistan Steel Industry for the provision of waste materials that have been used as catalysts for biodiesel synthesis.
[1]A.M.Khan,Ataullah,A.Shaheen,I.Ahmad,F.Malik,H.A.Shahid,Correlation of COD and BOD of domestic wastewater with the power output of bioreactor,J.Chem.Soc.Pak.33(2)(2011)269-274.
[2]A.M.Khan,M.Obaid,R.Sultana,Production of biodiesel from marine algae to mitigate environmental pollution,J.Chem.Soc.Pak.37(3)(2015)612-620.
[3]A.M.Khan,A.Shaikh,I.Khan,S.Kanwal,Comparative production of biodiesel from waste chicken fats and cooking oil,J.Biofuels5(1)(2014)32-40.
[4]A.M.Khan,Electricity generation by microbial fuel cells,Adv.Nat.Appl.Sci.3(2)(2009)279-286.
[5]A.M.Khan,M.M.Ali,S.Naz,M.Sohail,Generation of bioenergy by the aerobic fermentation of domestic wastewater,J.Chem.Soc.Pak.32(2)(2010)209-214.
[6]S.P.Singh,D.Singh,Biodiesel production through the use of different sources and characterization of oils and their esters as the substitute of diesel:A review,Renew.Sust.Energ.Rev.14(2010)200-216.
[7]A.M.Khan,S.Ali,Production of biodiesel and bioethanol from the legumes ofLeucaena leucocephala,J.Biofuels5(2)(2014)76-82.
[8]A.M.Khan,S.Naz,Biopower generation from kitchen wastewater using a bioreactor,Water Environ.Res.86(1)(2014)3-12.
[9]J.Boro,A.J.Thakur,D.Deka,Solid oxide derived from waste shells ofTurbonilla striatulaas a renewable catalyst for biodiesel production,Fuel Process.Technol.92(2011)2061-2067.
[10]A.M.Khan,S.Khaliq,R.Sadiq,Investigation of waste banana peels and radish leaves for their biofuels potential,Bull.Chem.Soc.Ethiop.29(2)(2015)239-245.
[11]P.Zhang,Q.Han,M.Fan,P.Jiang,A novel wastewater scale-derived solid base catalyst for biodiesel production,Fuel124(2014)66-72.
[12]A.M.Khan,Phytochemical and structural studies on the chemical constituents ofTaxus wallichiana,Tanacetum gracile,Jolyna laminarioidesand other marine algae(Ph.D.Thesis)H.E.J Research Institute of Chemistry,University of Karachi,Pakistan,2000(Retrieved from http://eprints.hec.gov.pk/979/1/711.html.htm).
[13]A.M.Khan,M.S.Hussain,Production of biofuels from marine macroalgaeMelanothamnus afaqhusainiiandUlvafasciata,J.Chem.Soc.Pak.37(2)(2015)371-379.
[14]S.G.Musharraf,M.A.Ahmed,N.Zehra,N.Kabir,M.I.Choudhary,Atta-ur-Rahman,Biodiesel production from microalgal isolates of southern Pakistan and quantification of FAMEs by GC-MS/MS analysis,Chem.Cent.J.6(149)(2012)1-10.
[15]E.E.Valeem,M.A.Rizvi,M.Shameel,Bioactivity,elementology and fatty acid composition ofUlva fasciataDelile from a rocky ledge of Buleji,Pakistan,Int.J.Phycol.Phycochem.7(1)(2011)81-90.
[16]B.Salamatinia,I.Hashemizadeh,A.Z.Abdullah,Alkaline earth metal oxide catalysts for biodiesel production from palm oil:Elucidation of process behaviors and modeling using response surface methodology,Iran.J.Chem.Chem.Eng.32(1)(2013)113-126.
[17]S.J.Yoo,H.S.Lee,B.Veriansyah,J.Kim,J.D.Kim,Y.W.Lee,Synthesis of biodiesel from rapeseed oil using supercritical methanol with metal oxide catalysts,Bioresour.Technol.101(2010)8686-8689.
[18]M.B.Alves,F.C.M.Medeiros,P.A.Z.Suarez,Cadmium compounds as catalyst for biodiesel production,Ind.Eng.Chem.Res.49(16)(2010)7176-7182.
[19]T.C.S.Girisun,C.Babeela,V.Vidhya,Extraction of nanostructured metal oxides from the furnace dust for the realization of low cost solar applications,Phys.Procedia49(2013)79-83.
[20]C.Z.Rizescu,Z.Bacinschi,E.V.Stoian,A.A.Poinescu,Characterization of steel mill electric-arc furnace dust,advances in waste management,University of Sfax,Kantaoui,Tunisia,2010 139-143.
[21]R.Wang,H.Li,F.Chang,J.Luo,M.A.Hanna,D.Tan,D.Hu,Y.Zhang,B.Song,S.Yang,A facile,low-cost route for the preparation of calcined porous calcite and dolomite and their application as heterogeneous catalysts in biodiesel production,Catal.Sci.Technol.3(2013)2244-2251.
[22]H.Matusiewicz,in:Z.Mester,R.E.Sturgeon(Eds.),Wet digestion methods,Elsevier,Amsterdam 2003,pp.193-233.
[23]G.Svehla,Vogel's qualitative inorganic analysis,seventh ed.Pearson Education,India,2008.
[24]J.M.Fraile,N.García,J.A.Mayoral,E.Pires,L.Roldán,The basicity of mixed oxides and the influence of alkaline metals:The case of transesterification reactions,Appl.Catal.A Gen.387(2010)67-74.
[25]A.M.Khan,N.Fatima,Synthesis of biodiesel from the oily content of marine green algaUlva fasciata,J.Chem.Soc.Pak.37(05)(2015)1040-1045.
[26]L.T.Thanh,K.Okitsu,L.V.Boi,Y.Maeda,Catalytic technologies for biodiesel fuel production and utilization of glycerol:A review,Catalysts2(2012)191-222.
 Chinese Journal of Chemical Engineering2016年8期
Chinese Journal of Chemical Engineering2016年8期
- Chinese Journal of Chemical Engineering的其它文章
- Synthesis of hierarchical dendritic micro-nano structure ZnFe2O4 and photocatalytic activities for water splitting☆
- Simultaneous desulfurization and denitrification of sintering flue gas via composite absorbent☆
- Non-catalytic conversion of wheat straw,walnut shell and almond shell into hydrogen rich gas in supercritical water media
- Synthesis and characterization of poppy seed oil methyl esters
- Increasing isobutanol yield by double-gene deletion of PDC6 and LPD1 in Saccharomyces cerevisiae☆
- Modeling and simulation of urea-water-solution dropletevaporation and thermolysis processes for SCR systems☆
