兼具高負載量和高性能的碳/硫復合材料的低成本合成
季恒星RUOFFRodney S.
(1中國科學技術大學材料科學與工程系,中國科學院能量轉換材料重點實驗室,iChEM,合肥230026;2韓國蔚山科學技術大學(UNIST)化學與材料科學學院及韓國基礎科學研究所(IBS),多維碳材料中心(CMCM),韓國蔚山689-798)
兼具高負載量和高性能的碳/硫復合材料的低成本合成
季恒星1RUOFFRodney S.2
(1中國科學技術大學材料科學與工程系,中國科學院能量轉換材料重點實驗室,iChEM,合肥230026;2韓國蔚山科學技術大學(UNIST)化學與材料科學學院及韓國基礎科學研究所(IBS),多維碳材料中心(CMCM),韓國蔚山689-798)
單質硫能夠與鋰離子在正極發生多電子反應,使得鋰硫電池具有高達2567W?h?kg-1的理論能量密度,因而鋰硫電池也成為了目前鋰離子電池的研究焦點1。然而,要實現鋰硫電池的大規模應用仍面臨諸多挑戰,包括:鋰枝晶的形成導致電池安全性降低,硫的低導電能力(5×10-30S?cm-1,25°C)使得電池倍率性能不夠,充放電過程中形成的可溶性多硫化鋰(Li2Sx,4≤x≤8)產生“穿梭”效應降低電池壽命,另外在充放電過程中硫也會發生體積膨脹和收縮(~80%)影響電池穩定性2。碳材料由于其結構多樣性、優異的導電能力、卓越的化學穩定性以及廉價易得,被普遍做為單質硫的載體用于解決鋰硫電池正極材料的問題3。負載硫納米顆粒的碳可以傳輸電化學反應過程中產生的電子,限制多硫化物的溶出,容納硫的體積變化,從而提高電池比容量,改善倍率和循環性能。然而,這些性能還與硫在碳硫復合物中的質量比及單位面積電極中硫的負載量密切相關。更高的硫含量和高單位面積硫負載量對于鋰硫電池的實際應用具有極其重要的意義,但通常也伴隨著硫的低利用率,從而降低電極材料的比容量、倍率性能與循環性能。
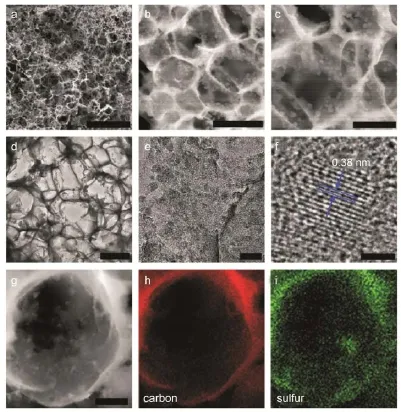
圖1 含硫質量分數達90%的碳/硫復合電極材料的形貌Fig.1 Morphology of the porousgraphitic carbon loaded w ith su lfurw ith a sulfur contentof 90% The SEMimages(a,b,c)and TEMimages(d,e,f)show the porousstructure of the carbonw ith sulfurnanoparticlesuniform ly distributed on the pore surface,which is confirmed by the EDAX study(g,h,i).Scale bars in a,b,and c:20;1;and 0.5μm.Scalebars in d,e,f,and g:500;50;2;and 200nm.Fig.1 from reference4.CopyrightNature Publishing Group.
中國科學院理化技術研究所耿建新團隊在最近的Nature Communications上發表研究論文報道他們通過一種新型的合成路徑獲得了硫含量高達90%(w,質量分數)的碳硫復合材料。當以這種復合材料作為電極材料,在硫的面負載量為~2.3mg?cm-2時,鋰硫電池仍顯示出卓越的電化學性能。他們應用了低成本的原料,例如氯化鈉、硫化鈉、硝酸鐵、葡萄糖和溫和的合成條件,例如常壓、氬氣保護下750°C煅燒4。所獲得這種復合物(如圖1所示)作為鋰硫電池正極時,在0.5C、1C、2C倍率下的比容量分別為1382、1242和1115mAh?g-1,并且長時間循環測試表明:在2C倍率下充/放電循環1000次的容量衰減率僅為每圈0.039%。復合材料的優異性質主要源于合成路徑所導致的特殊結構:原位化學沉積過程保證高含量硫納米顆粒的均勻負載;硫納米顆粒與碳材料表面形成的碳―硫鍵限制了單質硫的聚集與多硫化物的溶出;多孔碳材料載體的高導電性與良好的結構穩定性。
作者同時也提出設計碳硫復合物的幾個關鍵因素:大的孔體積及合適孔徑的碳材料載體以確保硫的負載量和電解液的浸漬;高石墨化的碳結構以促進電子傳輸從而提高導電性;硫納米顆粒與碳材料表面的強力集合,以及低成本的合成路線。文中提出的這一碳/硫復合材料的合成方法為進一步提升鋰硫電池性能以及采用其他碳材料制備鋰硫電池提供了可能。
Lithium-sulfur(Li-S)batteriesoffer ahigh theoreticalenergy density of 2567W?h?kg-1by themulti-electron-transfer cathode reaction between elementalsulfur and lithium ions,and area focus of post lithium-ion batteries technology1.Yet,therearechallenging obstacles standing in theway of the large-scaleapplication of the Li-S technology in themarket,which include the potential safety risky of Li-dendrite formation,low electrical conductivity of sulfur(5×10-30S?cm-1at25°C),the dissolution of the charge/ discharge intermediates,polysulfides(Li2Sx,4≤x≤8)in the electrolyte,and the volume change of the sulfur during lithiation/ delithiation(~80%)2.Carbonmaterialsare commonly used as the host to accommodate sulfur to address the issues relevant to the sulfur-cathode ow ning to their diversity,conductivity,robust stability and chemistry,and their ready abundance and cost3.A carbon hostw ith sulfurnanoparticlesadhered can conductelectrons generated by sulfur lithiation/delithiation,limit the dissolution of polysulfides,and w ithstand the volume change of the sulfur;it therefore can provide an improved specific capacity,rate capability,and cyclic life.However,these improvements are closely connected to both themass ratio of sulfur in such a sulfur/ carbon composite and the areal loading density of sulfur in the cathode.Highermass ratio and areal loading density of sulfurare favored for practical Li-S battery application,but are usually accompanied by lower‘sulfur utility’thatyields reduced specific capacity,rate capability,and cycle life.
GENG Jian-Xin and coauthors demonstrated that90%(w, mass fraction)sulfur content in the sulfur/carbon composite and~2.3mg?cm-2sulfurareal loading density in the cathode can be achieved w ithoutsignificantly harm ing the overall performance of the sulfur/carbon cathode;thiswas achieved,per the recent paper published by theauthors,by using anew synthesis route for their sulfur/carbon composite.They used low cost chem icals including sodium chloride,sodium sulfide,iron nitrate,and glucose and moderate conditions of 750°C at atmospheric pressure in argon gas to generate thecompositematerialshown in Fig.14.This com positematerial,when tested as a cathode in the Li-S battery, delivered a specific capacity of 1382,1242,and 1115mAh?g-1at 0.5C,1C,and 2C,and a long cycle life demonstrated by a small capacity decay of 0.039%per cycle over 1000cyclesat2C.These resultswere,per theauthors,due to the in situ chemicaldeposition process that allowed for the formation of sulfur nanoparticles uniformly distributed in the porous carbonhostwith ahigh content of up to 90%S;the C―Sbonds between the sulfur nanoparticles and carbon surface are stated to lim it theagglomeration of sulfur and the dissolution of polysulfides;and the high electrical conductivity and mechanical flexibility of the porous carbon host favorbetterbattery performance.
Theauthorshaveaddressed several critical factors for rationally designed sulfur/carbon composites:large pore volume w ith suitable pore size of the carbon host for high sulfur loading and electrolyte impregnation,good conductivity by achieving a relatively high degree of‘graphitic carbon’to facilitate electronconduction,uniform ly distributed sulfur nanoparticleswith strong adhesion to the carbon surface,and a stated opportunity for scaled and low cost production.Onemay expect further improvements in Li-Sbatteries including through theuseof this typeof approach, and also perhapswith other carbonmaterials.
References
(1)Bruce,P.G;Freunberger,S.A.;Hardw ick,L.J.;Tarascon,J.M. Nat.Mater.2012,11,19.doi:10.1038/NMAT3237
(2)Manthiram,A.;Fu,Y.;Su,Y.S.Accoutns Chem.Res.2013,46, 1125.doi:10.1021/ar300179v
(3)Evers,S.;Nazar,L.F.Accoutns Chem.Res.2013,46,1135.doi: 10.1021/ar3001348
(4)Li,G.;Sun,J.;Hou,W.;Jiang,S.;Huang,Y.;Geng,J.Nat. Commun.2016,7,10601.doi:10.1038/ncomms10601
Low-Cost Synthesis Route for High-Performance S/C Composite with 90%S Content
JIHeng-Xing1RUOFFRodney S.2
(1DepartmentofMaterials Science and Engineering,CASKey Laboratory ofMaterialsfor Energy Conversion,iChEM(Collaborative Innovation CenterofChemistry for EnergyMaterials),University ofScience and Technology ofChina, Hefei230026,P.R.China;2Center forMultidimensional Carbon Materials(CMCM),Institute for Basic Science(IBS)Center at the Ulsan National Institute ofScience&Technology(UNIST)Campus;and DepartmentofChemistry and SchoolofMaterials Science,UNIST,Ulsan 689-798,Republic ofKorea)
10.3866/PKU.WHXB201602192

