鴨呼腸孤病毒人工感染SPF雞胚的病理學研究
劉曉麗,劉 婷,劉 波,程國富,谷長勤,張萬坡,胡薛英
(華中農業大學動物醫學院,武漢 430070)
鴨呼腸孤病毒人工感染SPF雞胚的病理學研究
劉曉麗,劉 婷,劉 波,程國富,谷長勤,張萬坡,胡薛英
(華中農業大學動物醫學院,武漢 430070)
【目的】前人研究表明禽呼腸孤病毒可以經卵垂直傳播,禽呼腸孤病毒能引起雛禽的病毒性關節炎、呼吸道和腸道疾病、肝炎、心肌炎、免疫抑制等。以實驗室分離的鴨呼腸孤病毒HP080421株為研究對象,該毒株引起病變主要以鴨軟腳為主要臨床特征,病鴨肝臟和脾臟表面有大量的白色壞死灶、腎臟腫大、出血為主要病變特點。鴨呼腸孤病毒對雞胚的致病性研究,旨在通過雛雞的病理變化特點,探討所分離的HP080421鴨呼腸孤病毒株是否也可感染雞群,為雞場鴨呼腸孤病毒感染的防控提供理論依據。【方法】運用華中農業大學獸醫病理學實驗室分離鑒定的HP080421株鴨呼腸孤病毒通過尿囊腔接種SPF雞胚,建立SPF雞胚鴨呼腸孤病毒感染模型,培養雞胚至雛雞出殼,運用臨床觀察、病理剖檢、H.E染色和免疫組織化學染色等病理組織學檢查方法,對鴨呼腸孤病毒感染SPF雞胚進行病理學研究和致病性分析。【結果】研究發現病毒感染雞胚孵化培養至22—23日齡均啄殼,但不能自主出殼,需人工剝殼。病理剖檢可見雛雞的肝臟、脾臟腫脹質脆,表面及實質分布有黃白色壞死灶;腦部組織腫脹,并可見出血點和出血斑。H.E染色的組織病理學觀察可見接毒組雛雞肝臟、脾臟的壞死灶周圍有大量淋巴細胞浸潤;法氏囊的濾泡髓質部和胸腺的髓質部淋巴細胞減少、缺失;腦、肺臟、腎臟存在不同程度的淤血、出血和水腫;免疫組織化學染色結果表明:病毒陽性信號主要分布于肝臟、脾臟、肺臟和法氏囊等器官,并且位于上皮細胞和巨噬細胞的細胞質和胞核內。【結論】鴨呼腸孤病毒株HP080421 能夠在SPF雞胚中復制增殖,并使雛雞主要器官發生明顯的病理變化,病理變化主要集中在肝臟和淋巴器官,因此鴨呼腸孤病毒可通過垂直傳播感染雞群,還可導致雛雞免疫抑制。本研究通過SPF雞胚鴨呼腸孤病毒感染模型,闡述了鴨呼腸孤病毒感染雞群的可能性,因此在實際養殖過程中,養殖戶要防止鴨呼腸孤病毒對雞胚的污染,切斷傳播途徑,以達到預防雞群感染鴨呼腸孤病毒的目的。
鴨呼腸孤病毒;SPF雞胚;病理剖檢;免疫器官;H.E染色
0 引言
【研究意義】研究鴨呼腸孤病毒(duck reovirus,DRV)對雞胚的致病作用,同時探討DRV在垂直傳播中的情況,旨在對雞場DRV感染的防護與控制提出積極有效的意見。【前人研究進展】禽呼腸孤病毒的易感動物包括雞、鵝、火雞、鴨等[1]。禽呼腸孤病毒能引起雛禽的病毒性關節炎(腱鞘炎)、呼吸道和腸道疾病、肝炎、心肌炎、免疫抑制等[2-9],另外有研究表明,禽呼腸孤病毒可以經卵垂直傳播[10]。2003年至今鴨呼腸孤病毒陸續在中國被報道,感染鴨呼腸孤病毒相關的疾病歸結為4種,即鴨多臟器壞死癥、多臟器出血癥、肝壞死癥和脾壞死癥[11-15]。而華中農業大學獸醫病理學實驗室對 DRV多年研究結果證實,DRV引起病變主要以造成鴨軟腳為主要臨床特征,肝臟和脾臟表面有大量的白色壞死灶、腎臟以腫大、出血為主要病變特點[16-21]。【本研究切入點】盡管禽呼腸孤病毒的宿主范圍廣泛,雞、鵝、火雞、鴨、鴿子、鸚鵡、鴕鳥等家養禽類與野生禽類均可感染,但本試驗所分離的鴨呼腸孤病毒是否也可感染雞群等其他動物一直未予驗證,為此擬建立SPF雞胚DRV感染模型。【擬解決的關鍵問題】建立SPF雞胚DRV感染模型,通過尿囊腔接種鴨呼腸孤病毒,培養雞胚至雛雞出殼,運用臨床觀察、病理剖檢、組織病理學觀察等技術研究鴨呼腸孤病毒對雞胚的致病性。
1 材料與方法
1.1試驗時間、地點
本研究室內試驗于 2015年在華中農業大學獸醫病理學實驗室進行。
1.2試驗材料
1.2.1病毒 接種材料為華中農業大學動物醫學院獸醫病理學實驗室分離鑒定并保存的HP080421株鴨呼腸孤病毒。
1.2.2試驗動物 SPF雞胚12只,購自武漢市畜牧科技研究所。
1.3試驗方法
1.3.1試驗動物分組 10日齡SPF雞胚12枚分為兩組,接毒組與對照組。接毒組SPF雞胚經尿囊腔注射鴨呼腸孤病毒HP080421株(ELD50=10-5.75/0.2mL),0.2mL/枚;對照組SPF雞胚尿囊腔接種等量滅菌生理鹽水0.2mL/枚。37℃溫箱培養,適時觀察。
1.3.2臨床觀察及尸體剖檢 每天照蛋觀察,第一天死亡的雞胚棄去。待雞胚啄殼、出殼,處死雛雞,剖檢觀察。剖檢雞胚,接毒組與對照組對比觀察。對試驗感染組、對照組雞胚剖檢時,對比切取雛雞肝臟、脾臟、腦、法氏囊、胸腺、肺臟、腎臟、腺胃及小腸有病變或疑似有病變的部位,4%多聚甲醛固定。
1.3.3病理切片制作及顯微觀察 常規石蠟切片制作[22-24],切片厚4μm,H.E染色和IHC染色。IHC染色方法為SABC法,AEC顯色,鼠抗鴨呼腸孤病毒單克隆抗體(中國農業大學蘇敬良教授贈送)工作濃度是1∶400,染色結果在生物光學顯微鏡(Nikon80i)觀察,采集圖片(NIS-Elements高清晰度彩色圖文分析系統),記錄結果。
2 結果
2.1感染鴨呼腸孤病毒后雞胚的存活狀況
對照組6枚全部存活,孵化培養至20—21d陸續啄殼并出殼;試驗組6枚全部存活,孵化培養至22—23日齡均啄殼,但不能自主出殼,于是人工剝殼并處死雛雞。
2.2剖檢病理變化
肝臟腫大、質脆、表面及實質分布有大量肉眼可見的大小不等的黃白色壞死灶(圖1-A)。脾臟腫脹近球形、質脆、表面及實質分布有大量肉眼可見的綠豆大小的黃白色壞死灶(圖1-B)。腦組織腫脹,表面分布有大小不一的出血點和出血斑。對照組雛雞各組織器官眼觀病理變化不明顯。

圖1 雛雞剖檢病理變化Fig.1 Necropsy pathologic changes of infected chicken
2.3組織病理學變化
肝細胞不同程度變性壞死崩解,其間夾雜出血灶,并見大量炎性細胞浸潤,肝臟壞死灶機化形成包囊,(圖2-A);脾臟白髓內淋巴細胞凋亡、數量減少,部分淋巴細胞空泡變性、核碎裂、崩解壞死,壞死灶周圍可見多核巨細胞、炎性細胞浸潤形成肉芽腫(圖2-B);胸腺皮質部淋巴細胞排列疏松,髓質部組織細胞間充滿紅細胞,淋巴細胞變性壞死崩解,數量減少,可見胸腺小體(圖2-C);法氏囊肌層與黏膜間距增大,組織水腫,濾泡的皮質、髓質部細胞排列疏松,濾泡髓質部有大量細胞核碎片,淋巴細胞減少、凋亡(圖2-D);大腦血管、神經細胞與周圍組織細胞間隙增大(圖2-E);腺胃和小腸黏膜固有層中有大量炎性細胞浸潤(圖2-F);腎小管周圍毛細血管擴張,充滿了紅細胞,部分腎小管上皮細胞變性并與基底膜分離,脫落至管腔(圖2-G);肺臟小靜脈、毛細血管充滿紅細胞,肺小葉間隔增寬(圖2-H)。對照組未見明顯的組織病理變化。
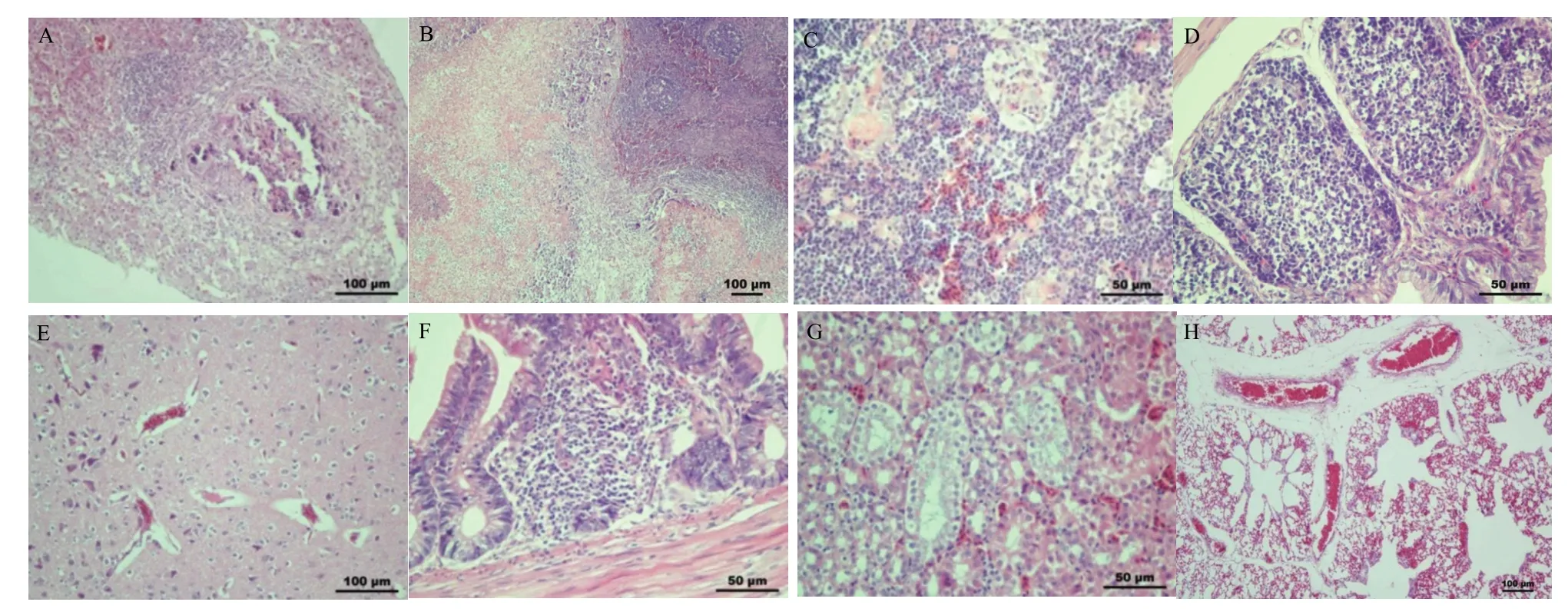
圖2 感染雛雞病理組織學變化Fig.2 Histopathological changes of infected chicken
免疫組織病理學變化表明:陽性信號主要位于肝臟、脾臟的壞死灶周圍的巨噬細胞的細胞質內,另外法氏囊和肺支氣管粘膜上皮細胞內亦可見陽性信號(圖3)。

圖3 感染雛雞免疫組織化學變化Fig.3 Immunohistochemical changes of infected chicken (200×)
3 討論
3.1鴨呼腸孤病毒感染后雞胚存活狀況
已知在孵化正常的情況下,健雛出殼時間比較一致,通常在孵化第20日齡開始出雛,滿21日齡出雛結束;而病雛、弱雛會過早或過遲出雛,出雛時間延至 22日齡以上[25]。在培養條件一致的情況下,對照組孵化培養至20—21日齡陸續啄殼并出殼,而接毒組孵化培養至22—23 d啄殼卻不能出殼。垂直傳播指感染母雞通過卵將病原體傳給子代的過程,垂直傳播病是危害養禽業健康發展的全球性的一大類重要疾病;尤其是蛋用種雞,因其可以將病原體通過卵直接傳給商品代蛋雞,引起生殖系統損傷,表現為發病率高、產蛋率低,疾病在雞場內長期存在并難以消除和經濟損失嚴重等特點[26]。本研究結果表明:鴨呼腸孤病毒尿囊腔接種SPF雞胚后,能夠在雞胚體內增殖并垂直傳播,且對雞胚有致弱作用,導致其不能正常出殼,影響生長發育。
3.2鴨呼腸孤病毒感染SPF雞胚后剖檢變化
對照組雛雞組織器官未見明顯病理變化,接毒組雛雞的主要病理變化集中在肝臟和脾臟,表面和實質分布有肉眼可見大小不一的黃白色壞死灶;而其他組織器官如心臟、腦組織、腺胃、小腸、腎臟、肺臟、胸腺等未出現壞死,僅出現出血、水腫等輕微病理變化。這些病理變化與DRV感染引起雛鴨的病理變化具有相似性[16-21],表明 HP080421株鴨呼腸孤病毒(DRV)對SPF雞胚有致病性,且能引起與鴨感染DRV后相似的病理變化。
3.3鴨呼腸孤病毒感染SPF雞胚后組織病理學變化
脾臟、肝臟壞死灶內細胞壞死,周圍有炎性細胞浸潤;法氏囊濾泡髓質部、胸腺髓質部組織排列疏松、呈空洞狀、淋巴細胞數量減少,細胞核碎裂或溶解消失;而肺臟、腎臟、腺胃及小腸、腦組織存在不同程度的水腫和出血、淤血。從結果中可以看出,雛雞的病理變化主要集中在肝臟和淋巴器官,病毒的陽性信號主要出現在肝、肺、脾和法氏囊,并且位于上皮細胞和巨噬細胞的細胞質和胞核內。鴨呼腸孤病毒侵染胚體免疫器官后,引起淋巴細胞凋亡,繼而免疫器官內淋巴細胞數量減少,提示鴨呼腸孤病毒可能會通過損傷免疫器官,減弱免疫系統的功能,引起雛雞的免疫抑制,與李爽得出的鴨源禽呼腸孤病毒感染能引起雛鴨機體免疫抑制的結論具有一致性[18]。
4 結論
鴨呼腸孤病毒株HP080421能夠在SPF雞胚中復制增殖,并使雛雞產生明顯的病理變化。因此本研究表明鴨呼腸孤病毒存在交叉感染,而且鴨呼腸孤病毒能夠通過雞胚垂直傳播,因此在實際養殖過程中,要防止病毒對雞胚的污染,切斷傳播途徑。鴨呼腸孤病毒感染引起雞胚免疫器官損傷,推測雞場感染鴨呼腸孤病毒,雞群免疫力低下,可能會加重其他病原對雞群的致病性,更易發生繼發感染。
References
[1] ROBERTSON M D,WILCOX G E. Avian reovirus. Veterinary Bulletin,1986,56: 155-174.
[2] LIU H J,LIN P Y,LEE J W,HSU H Y,SHIH W L. Retardation of cell growth by avian reovirus p17 through the activation of p53 pathway. Biochem BiophysRes Commun,2005,336: 709-715.
[3] BENAVENTE J,MARTINEZ-COSTAS J. Avian reovirus: structure and biology. Virus Research,2007,123: 105-119.
[4] 陳華美,曹三杰,文心田. 雞病毒性關節炎診斷方法研究進展. 動物醫學進展,2006,27(3): 43-46. CHEN H M,CAO S J,WEN X T. Research progress of diagnosis methods of chicken viral arthritis. Animal Veterinary Progress,2006,27(3): 43-46(in Chinese).
[5] 殷震,劉景華. 動物病毒學. 2版. 北京: 科學出版社,1997: 510-511. YIN Z,LIU J H. Animal Viruses. Second Edition. Beijing: Scientific Press,1997: 510-511. (in Chinese)
[6] KUNTZ-SIMON G,LE GALL-RECULé G,JESTIN V. Muscovy duck reovirus σC protein is atypically encoded by the smallest genome segment. Journal of General Virology,2002,83: 1189-1200. [7] GAUDRY D,CHARLES J,TEKTOFF J. A new disease expressing itself by a viral pericarditis in Barbary ducks. Comptees Rendus Hebdomadaires des Seances de L’Academie des Sciences Serie D,1972,274(21): 2916-2929. (in French)
[8] KUNTZ-SIMON G,LE GALL-RECULé G,JESTIN V. Muscovy duck reovirus σC protein is atypically encoded by the smallest genome segment. Journal of General Virology,2002,83: 1189-1200.
[9] MARIUS-JESTIN V,LAGADIC M,LE MENEC Y. Histological data associated with muscovy duck reovirus infection. Veterinary Record,1988,123: 32-33.
[10] 韓天龍,王敏,李清泉,張廣和,張英浩,石劍華,李志明. 種雞垂直傳播病的病原學研究進展. 黑龍江畜牧獸醫,2012,2: 44-46. HAN T L,WANG M,LI Q Q,ZHANG G H,ZHANG Y H,SHI J H,LI Z M. Research Progress of Pathogeny related to vertical Infected Disease of Breeding Chickens. Livstock and Veterinary of Hei Longjiang,2012,2: 44-46. (in Chinese)
[11] 程安春,汪銘書,陳孝躍,周毅,劉菲,宋涌,郭宇飛,韓曉英,袁桂萍,徐超,廣永宏,方鵬飛,劉兆宇. 一種新發現的鴨病毒性腫頭出血癥的研究. 中國獸醫科技,2003,33 (10): 32-34. CHEN A C,WANG M S,CHEN X Y,ZHOU Y,LIU F,SONG Y,GUO Y FI,HAN X Y,YUAN G P,XU C,GUANG Y H,FANG P F,LIU Z Y. Research of a novel viral swollen head hemorrhagic disease. Chinese Veterinary Sciences,2003,33 (10): 32-34. (in Chinese)
[12] LIU Q,ZHANG G,HUANG Y,REN G,CHEN L,GAO J,ZHANG D,HAN B,SU W,ZHAO J,HU X,SU J. Isolation and characterization of a reovirus causing spleen necrosis in Pekin ducklings. Veterinary Microbiology,2011,148: 200-206.
[13] 劉紅,郁宏偉,朱朝輝,吳志新,李蘇芝,廖明. 鴨源呼腸孤病毒DRV-GZ株 S2基因的序列分析及其表達. 中國獸醫科學,2008,38(3): 224-226. LIU H,YU H W,ZHU C H,WU Z X,LI S Z,LIAO M. S2 gene sequences analysis and expression of duck reovirus DRV-GZ strain. Chinese Veterinary Sciences,2008,38(3): 224-226. (in Chinese)
[14] 張 穎,刁有祥,高 明,葛平萍,孫曉艷,魯愛玲. 鴨源呼腸孤病毒對雛鴨致病性研究. 中國獸醫學報,2015,35(3): 350-354. ZHANG Y,DIAO Y X,GAO MI,GE P P,SUN X Y,LU A L. Pathogenic research of ducklings infected by duck reovirus. Chinese Academic of Veterinary,2015,35(3): 350-354. (in Chinese)
[15] 黃瑜,蘇敬良,施少華,傅光華,程龍飛,林芳,林建生,陳紅梅.我國鴨呼腸孤病毒感染相關的疫病. 中國獸醫雜志,2009,45(7): 57-58. HUANG Y,SU J L,SHI S H,FU G H,CHENG L F,LIN F,LIN J S,CHEN H M. Deseases related with duck reovirus infection in China. Chinese Journal of Veterinary,2009,45(7): 57-58. (in Chinese)
[16] 張寶來. 鴨源呼腸孤病毒的分離與鑒定[D]. 武漢: 華中農業大學,2009. ZHANG B L. Isolation and identification of duck reovirus[D]. Wuhan: Huazhong Agricultural University,2009. (in Chinese)
[17] 李爽. 鴨源禽呼腸孤病毒感染雛鴨的免疫病理學研究[D]. 武漢:華中農業大學圖書館,2010. LI S. Immunological and pathological research of ducklings infected by duck reovirus[D]. Wuhan: Library of Huazhong Agricultural University,2010. (in Chinese)
[18] 李 爽,谷長勤,白家媛,張 紅,張萬坡,程國富,胡薛英. 鴨源呼腸孤病毒感染引起雛鴨免疫損傷的觀察. 中國農業科學,2010,43(21): 4514-4520. LI S,GU C Q,BAI J Y,ZHANG H,ZHANG W P,CHENG G F,HU X Y. Observation of immunological damage of duckings infected by duck reovirus. Chinese Agricultural Sciences,2010,43(21): 4514-4520. (in Chinese)
[19] 李美霞,楊軍,艾博,谷長勤,劉曉麗,張萬坡,程國富,胡薛英.呼腸孤病毒與鏈球菌混合感染雛鴨的病理學研究. 湖北農業科學,2014,53(8): 1848-1850. LI M X,YANG J,AI B,GU C Q,LIU X L,ZHANG W P,CHENG G F,HU X Y. Pathological research of ducklings infected by duck reovirus and streptococcus. Hubei Agricultural Sciences,2014,53(8):1848-1850. (in Chinese)
[20] 趙 赫,何 莉,李美霞,谷長勤,張萬坡,程國富,胡薛英. 鴨源呼腸孤病毒與沙門菌共感染雛鴨的免疫器官的動態病理學觀察. 中國獸醫科學,2014,44(6): 650-655. ZHAO H,HE L,LI M X,GU C Q,ZHANG W P,CHENG G F,HU X Y. Dynamic pathology observation of imunological organs of ducklings infected by duck reovirus and salmonellae. Chinese Veterinary Sciences,2014,44(6): 650-655. (in Chinese)
[21] 趙赫,李爽,谷長勤,張萬坡,程國富,劉曉麗,胡薛英. 鴨呼腸孤病毒自然感染肉鴨的病毒分布與病理學觀察. 畜牧獸醫學報,2015,46(1): 119-123. ZHAO H,LI S,GU C Q,ZHANG W P,CHENG G F,LIU X L,HU X Y. Study on pathology and distribution of duck reovirus in naturally infected duck. Acta Veterinaria et Zootechnica Sinic,2015,46(1): 119-123. (in Chinese)
[22] 丁偉,王德田. 簡明病理學技術. 1版. 杭州: 浙江科學技術出版社,2014: 2-5. DING W,WANG D T. Brief Technology of Pathology. First Edition. Hangzhou: Scientific Technology Press of Zhejiang,2014: 2-5. (in Chinese)
[23] 麥兆煌.病理組織標本制作技術.1版. 北京: 人民衛生出版社,1964: 10. MAI Z H. Manufacturing Technique of Pathological Tissue Specimen. First Edition. Beijing: People’s Press of Sanitary,1964: 10. (in Chinese)
[24] 劉增輝.病理染色技術.1版. 北京: 人民衛生出版社,2006,1-10. LIU Z H. Technique of Pathological Staining[ First Edition. Beijing: People’s Press of Sanitary,2006: 1-10. (in Chinese)
[25] 賈汝敏. 羽速基因對雛雞出殼速度、出殼時間與性比關系的研究.第八屆優質雞的改良生產暨發展研討會論文集.南寧: 2006: 141-146. JIA R M. Study on the relationship between the hatching rate and the time to sex ratio of the hatching rate of the chicken. The eighth session of the Symposium on the improvement of production and development of high quality chicken. Nanning: 2006: 141-146. (in Chinese)
[26] CALLICOTT K A,FRIETHRIKSDOTTIR V,REIERSEN J. Lack of evidence for vertical transmission of Campylobacter spp.In chickens. Applied Environmental Microbiology,2006,72(9): 5794-5798.
(責任編輯 林鑒非)
The Pathogenicity of Duck Reovirus on SPF Chicken Embryo
LIU Xiao-li,LIU Ting,LIU Bo,CHENG Guo-fu,GU Chang-qin,ZHANG Wan-po,HU Xue-ying
(College of Veterinary Medicine,Huazhong Agricultural University,Wuhan 430070)
【Objective】Previous studies showed that avian reovirus could infect vertically through egg,and avian reovirus can cause avian viral arthritis,respiratory and intestinal disease,myocarditis,hepatitis,immune suppression and so on. HP080421 strain of duck reovirus(DRV) isolated in the authors’ laboratory can cause soft duck feet as the main clinical features,and sick duck showed that a lot of white necrotic stove was on the surface of liver and spleen,and also kidney swelling and bleeding as the main pathological features. In this study,the pathogenicity of DRV to chicken embryo was investigated and whether the isolated HP080421 strain could infect chickens through the pathological changes of chicken was discussed,in order to provide a theoretical basis for the prevention and control of DRV infection.【Method】The infection model of SPF chicken to DRV was established through allantoic cavity inoculation SPF chicken embryos by using the isolated and identified HP080421 strains of DRV isolated in the lab. After chicks hatched from embryos,pathological examination methods such as clinicalobservation,pathological section examination,HE staining and immunohistochemical staining were used to study the pathobiology and pathogenicity of the SPF chicken embryo infected by DRV.【Result】Clinical observation found that chicken embryos were able to peck the shell after 22 - 23 days,but could not go out from the eggshell by themselves compared to the control group. At necropsy,liver and spleen were breakable and slightly swelling,many different size and yellow-white necrotic foci were consistently observed in the spleen and liver of the experimental group; the brain tissue was slightly swelling with few bleeding spots covered on it. Histopathological examination of H.E staining revealed necrotic foci in spleen and liver,which consisted of a necrotic center with lymphocytes infiltration at the periphery; in the Bursa of Fabricus,as well as in the thymus,lymphocyte depletion was apparent and cavities had developed in medulla. Besides,other organs such as lung,brain and kidney,showed different degrees of congestion and edema. Immunohistochemical detection showed that liver,lung,spleen and bursa of fabriciusa had positive signals,and were located in the cytoplasm and nucleus of epithelial cells and macrophages.【Conclusion】The results showed that the virus strain HP08421 could infect SPF chicken embryo and cause some specific pathologic changes. The pathological changes mainly focus on the liver and lymphoid organs,so DRV can be infected by vertical transmission of chickens,and can also lead to immune suppression. This study has expounded the possibility of infection of chickens by DRV,through the infection model of SPF chicken to DRV,as a matter of fact,in the actual process of production,farmers should prevent chicken embryo pollution by DRV to cut off the route of transmission,to achieve the purpose of preventing DRV infection.
duck reovirus; SPF chicken embryo; necropsy; immune organs; hematoxylin-eosin staining
2015-11-04;接受日期:2016-06-06
教育部培育專項(新興領域)(2011PY081)
聯系方式:劉曉麗,E-mail:xiaoliliu1983@mail.hzau.edu.cn。通信作者胡薛英,Tel:027-87280282;E-mail:hxying@mail.hzau.edu.cn

