煤焦油在三氯化鋁作用下催化炭化的研究
段 淼, 田永明, 李四中
(1.華僑大學 材料科學與工程學院,福建 廈門361021;2.Materials Department, New Mexico Tech, 801 Leroy Pl, Socorro, NM87801, USA)
?
煤焦油在三氯化鋁作用下催化炭化的研究
段淼1,田永明2,李四中1
(1.華僑大學 材料科學與工程學院,福建 廈門361021;2.Materials Department, New Mexico Tech, 801 Leroy Pl, Socorro, NM87801, USA)
采用AlCl3作為催化劑,在633~1 873 K和一定壓力下對煤焦油進行催化炭化,得到催化炭化焦,并對其進行了高溫處理。利用熱重,X射線衍射,拉曼光譜和偏光顯微鏡表征催化炭化焦的聚合程度,微觀結構和光學織構。結果表明,AlCl3顯著地加速了煤焦油的聚合并使之發生了脫氫反應,在673 K即可以得到具有高殘炭率的焦。其中,AlCl3的存在可以得到具有海島狀結構(即中間相微球分布于各向同性炭中間,且各向同性成分并存)的催化炭化焦。
煤焦油; 三氯化鋁; 催化炭化; 微觀結構; 光學織構
1 Introduction
Carbon materials are known for their high thermal and electrical conductivities, and excellent mechanical strength at high temperature, etc[1]. These unique properties are closely related to their microstructures and textures, which are strongly dependent on the carbonization to convert precursors into carbon. Therefore, carbonization is an important for production of carbon materials. There are three typical carbonization methods, gas phase carbonization, solid phase carbonization and liquid phase carbonization[2-6].
In this work, the catalytic carbonization of coal tar was carried out using AlCl3as catalyst. The effect of AlCl3on polymerization degree, microstructures and optical texture of as-prepared samples were characterized by thermogravimetry (TG), X-ray diffraction (XRD) and polarized light microscopy. Moreover, the relationship between distribution of AlCl3and microstructures of the resultant coke was studied by Raman spectroscopy.
2 Experimental
2.1Preparation of materials
Coal tar (Fujian Sangang Group Co., Ltd) and AlCl3(Sinopharm Chemical Reagent Co., Ltd, >99% purity) were used as carbonaceous precursor and catalyst, respectively. The coal tar mixed with 5% AlCl3was placed in an autoclave. The autoclave was first evacuated, and then pressurized with nitrogen to 4 MPa. The autoclave was raised to a required temperature ranging from 633 to 673 K and maintained for 3 h to carry out polymerization. As-prepared samples at 673 K were further heated to 1 173, 1 623 and 1 873 K in a tube furnace and kept at the final temperature for 2 h to carry out carbonization. The C series samples catalyzed by AlCl3were signed as C-t and the N series samples without AlCl3as N-t, in which t represented the polymerization temperature.
2.2Characterization
The content of carbon, hydrogen and nitrogen in the samples were analyzed by a vario MACRO CHN analyzer (Elementar, Germany). The carbon yield was measured by TG (Shimadzu DTG-60H) under nitrogen atmosphere from ambient temperature to 1 073 K at a heating rate of 10 K /min. The crystal structures were analyzed by XRD (Rigaku SmartLab) operating at 40 kV and 30 mA, using CuKαradiation (k=0.154 18 nm) with a step size of 0.01° and a scanning rate of 8°/min. The interlayer spacing (d002), crystallite size (Lc) and graphitization degree (g) were calculated from the Bragg equation, Scherrer equation and Maire and Merings equation, respectively[22-24]. The internal silicon standard was used to correct the instrumental error. The Raman spectra were obtained by a Renishaw invia Raman Microscope with the 532 nm wavelength of a 50 mW Nd-YAG laser. The samples obtained below 673 K were easily charred, so the exposure time and laser power were different for different samples. Some samples were mounted in epoxy resin, then ground and polished by waterproof sandpaper, and finally their optical textures were observed using a polarized light microscope (Leica DM 2500P).
3 Results and discussion
3.1Polymerization degree
The toluene insoluble (TI) content is related to the polymerization degree[25]. As seen in Fig.1, samples without AlCl3held a low level of TI content (~20%). The TI contents of samples with AlCl3apparently increased with polymerization temperature to 96.4% at 673 K. The elemental analysis results of different samples are listed in Table 1. It could be seen that the value of H/C ratio was low for the samples with AlCl3, indicating that AlCl3promoted dehydrogenation of coal tar during polymerization. In the presence of AlCl3, multi-component aromatic hydrocarbons in coal tar were highly condensed and dehydrogenated, generating numerous high-molecular-weight aromatic hydrocarbons, which resulted in the increase of TI content.
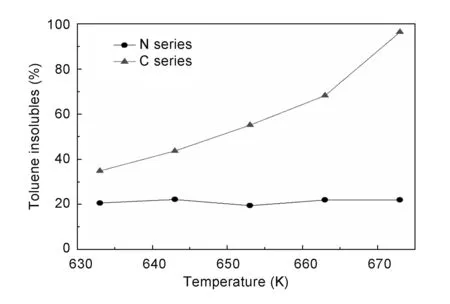
Fig. 1 Variation of TI contents with polymerization temperature.
Fig. 2 shows TG curves of C-673 and N-673 samples.The N-673 sample without AlCl3lost weight sharply from 450 to 800 ℃ with a maximum weight loss reached ~70%. However, the C-673 sample with AlCl3had a high weight of carbonaceous residues of 95.5%, a much lower weight loss than that of the N-673. The AlCl3catalyst could combine the aromatic planes via dehydrogenation, resulting in a sharp increase of the molecular weight.

Table 1 Elemental analysis of different samples.

Fig. 2 TG curves of C-673 and N-673 samples.
3.2Microstructures
The N series samples were black viscous liquid or semisolid. Only XRD patterns of the C series samples can be obtained, which are shown in Fig. 3 and Fig. 4. The prominent feature at all temperatures in XRD patterns was the (002) diffraction peak at ~25.6, which was identified as from turbostratic carbon[26]. The (002) diffraction peaks of the samples catalyzed below 673 K were broad and obscure, and that of the C-673 sample was more obvious. The (002) peaks tended to become sharp gradually (Fig. 4) with carbonization temperature, which could be explained by the fact that the disordered degree of carbon decreases with temperature. Table 1 lists thed002and crystallite sizeLcof the C series samples. The interlayer spacingd002increased with carbonization temperature below 1 173 K, possibly due to the gas released that pushed open carbon layers[27]. Thed002decreased with a further increase in carbonization temperature. The crystallite size increased from ~1.2 to 5.0 nm when samples were polymerized from 633 to 673 K. However, when the sample was carbonized at 1 173 and 1 623 K, its crystallite size decreased to 3.2 and 4.2 nm, respectively. This could be ascribed to the impurities between the graphitic layers, which reacted with carbon atoms and caused a decrease of the crystallite size at these temperatures[27]. At 1 873 K, the crystallite size in the sample increased since the impurities were volatilized below 1 623 K and the crystallite size began to increase with temperature.

Fig. 3 XRD patterns of as-prepared samples catalyzed by AlCl3.
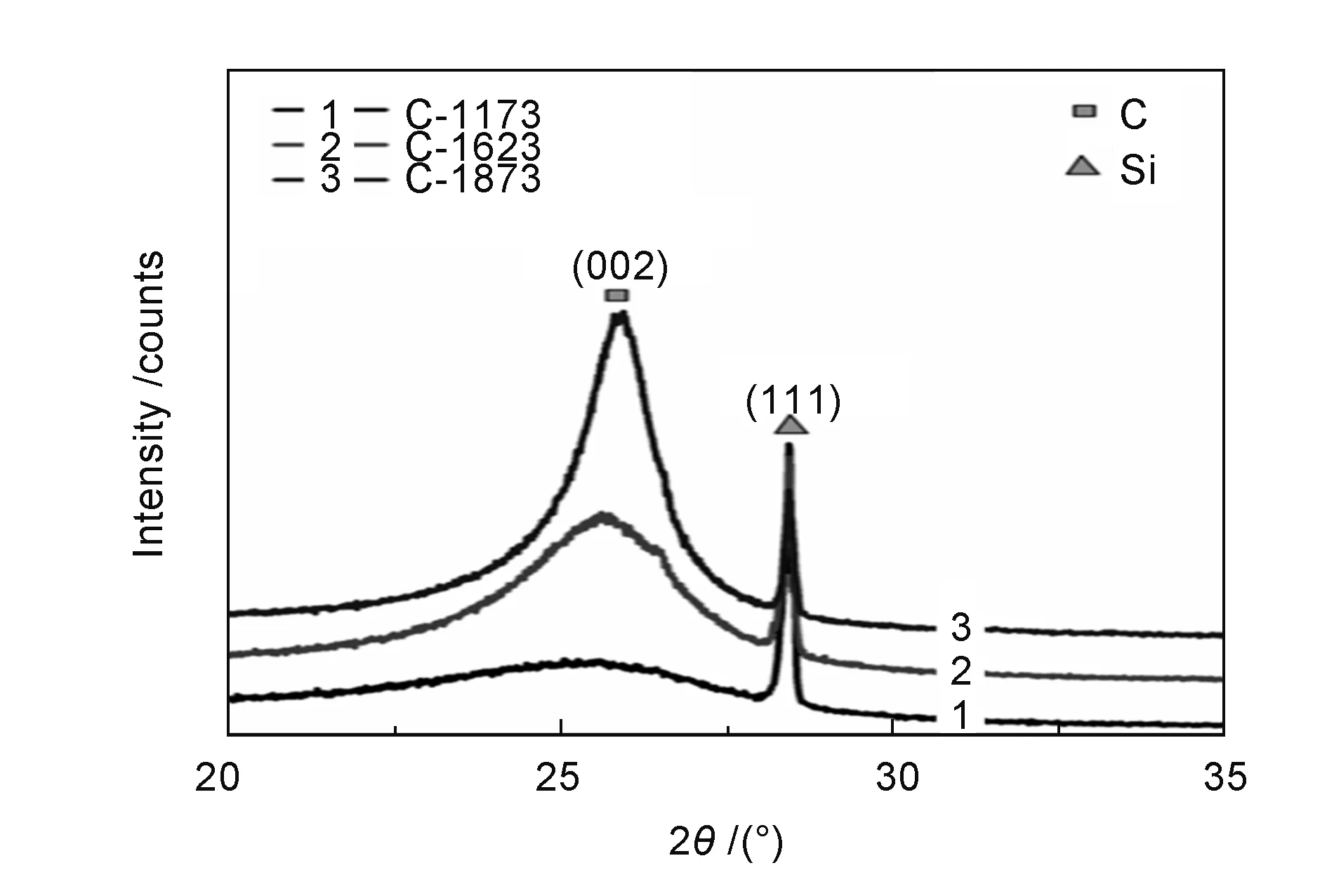
Fig. 4 XRD patterns of C-673 samples obtained by carbonization at high temperature.

Samplesd002(nm)g'(%)Lc(nm)C?6330.3468?~1.2C?6430.3462?~0.98C?6530.3481?~0.98C?6630.3448?4.3C?6730.3464?5.0C?11730.3518?3.2C?16230.3474?4.2C?18730.34364.76.3
Fig. 5 shows Raman spectra of the polymerized samples catalyzed by AlCl3at different temperatures. In general, the first-order Raman spectrum of a carbon consists of two bands around 1 360 cm-1for the D band and 1 580 cm-1for theGband. TheDband contains four components at ~1 240, 1 290 and 1 430 cm-1except 1 360 cm-1, this feature is in common with disordered carbon materials such as pitches and hard carbon[26, 28, 29]. It was noted that the three shoulder bands became weak with polymerization temperature. As given in Fig. 6, the shoulder bands of the samples carbonized at high temperatures disappeared andDband became sharp with carbonization temperature, which can be explained by the improvement of graphitization degree. In addition, the peak ofGband shifted from 1 600 to 1 582 cm-1with carbonization temperature, indicating an increasing proportion of a graphitized carbon structure[30]. On the other hand, the intensity ratio (ID/IG) ofDband andGband is in inversely proportional to the graphitization degree[31-33]. Table 2 lists the variations ofID/IGwith polymerization and carbonization temperatures. The values ofID/IGof C-633, C-643, C-653, C-663 samples all ranged from 2.8 to 3.2 as a result of the presence of multiple disordered carbons. The values ofID/IGincreased and then decreased with carbonization temperature, which could be closely related to the evolution of crystallite structure with carbonization temperature, the same as the variation ofd002.
To confirm the effect of AlCl3on microstructures of the formed coke, the coke in different distances from AlCl3obtained from C-673 sample was analyzed by Raman spectroscopy. The three sharp bands of AlCl3at 1 453, 1 516 and 2 427 cm-1are shown in Fig. 7a. Five main bands are observed in the spectra of the coke located at AlCl3(Fig. 7b), theDband at 1 368 cm-1, theGband at 1 603 cm-1and other three bands of AlCl3at 1 452, 1 515 and 2 426 cm-1. In comparison, the Raman spectra of the coke nearby and far away from AlCl3showed that the bands of AlCl3became weak gradually and finally disappeared(Fig. 7c, d) with the distance. TheDband of the coke far away from AlCl3became asymmetric, indicating the existence of multiple disordered carbon due to a low graphitization degree. Moreover, the value ofID/IGincreased with the distance from AlCl3.

Fig. 5 Raman spectra of as-prepared samples polymerized at different temperatures with AlCl3 as catalyst.(Exposure time: 20 s; Laser power: 0.5%)
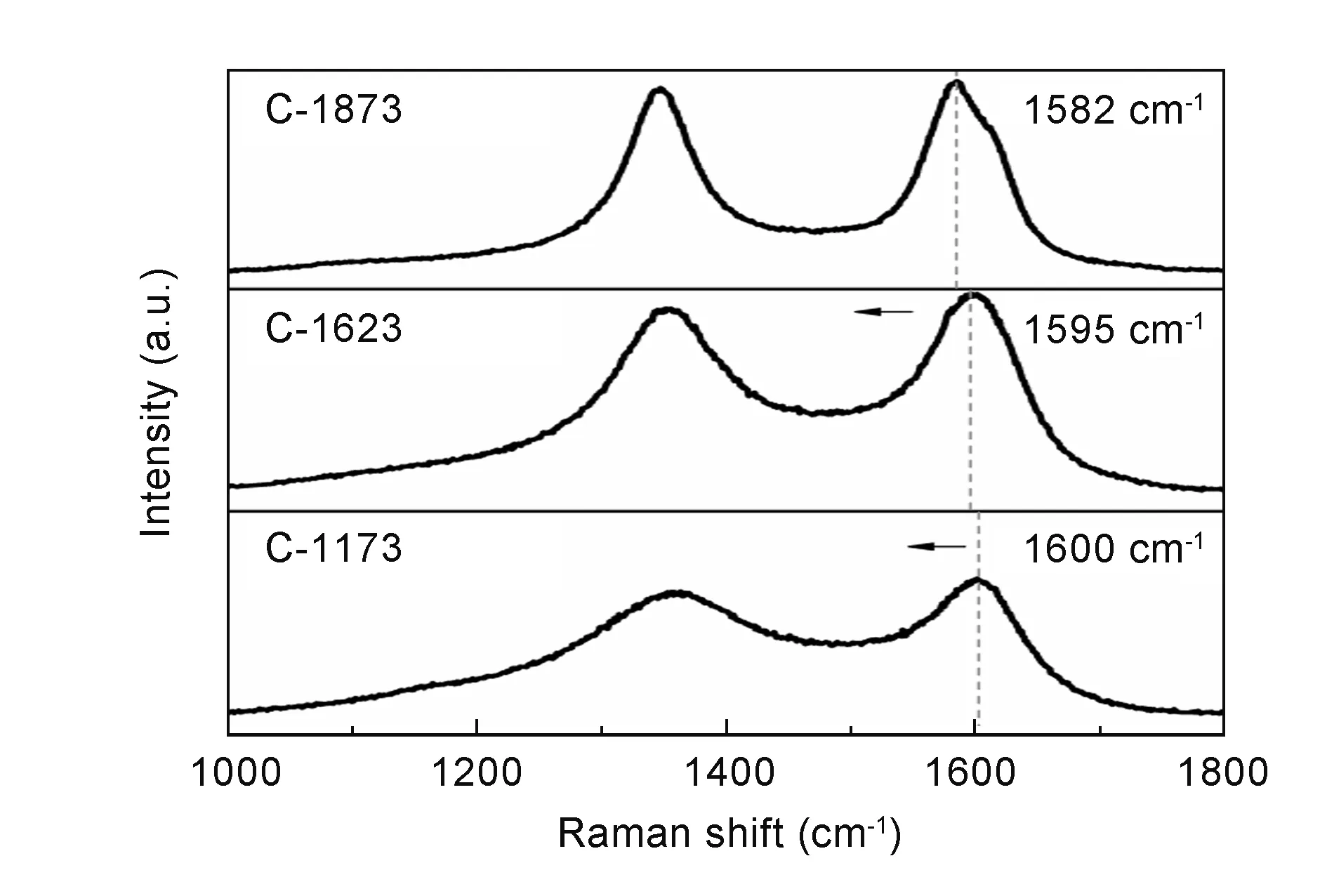
Fig. 6 Raman spectra of the C673 carbonized at different temperatures.(Exposure time: 30 s; Laser power: 5%)

SamplesC?633C?643C?653C?663C?673C?1173C?1623C?1873ID/IG2.8?3.22.42.81.61.0
3.3Optical texture
Mesophase formation generally occurs in liquid phase carbonization, however, its optical texture is sensitive to the reactivity of carbonaceous precursors and carbonization conditions[6, 34]. From the polarized light micrographs of the C-673 sample (Fig. 8a), it was found that a great number of fine anisotropic nucleus (<2 um) was observed. The fine anisotropic nucleus in the coke accounted for ~63%, domain texture ~5% and isotropic texture ~32% (Fig. 8b), as determined by the measurement of 50 randomly samples.
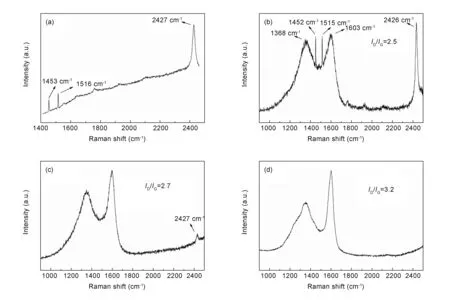
Fig. 7 Raman spectra of (a) AlCl3, (b) coke located at AlCl3, (c) coke nearby AlCl3 and (d) coke far away from AlCl3.
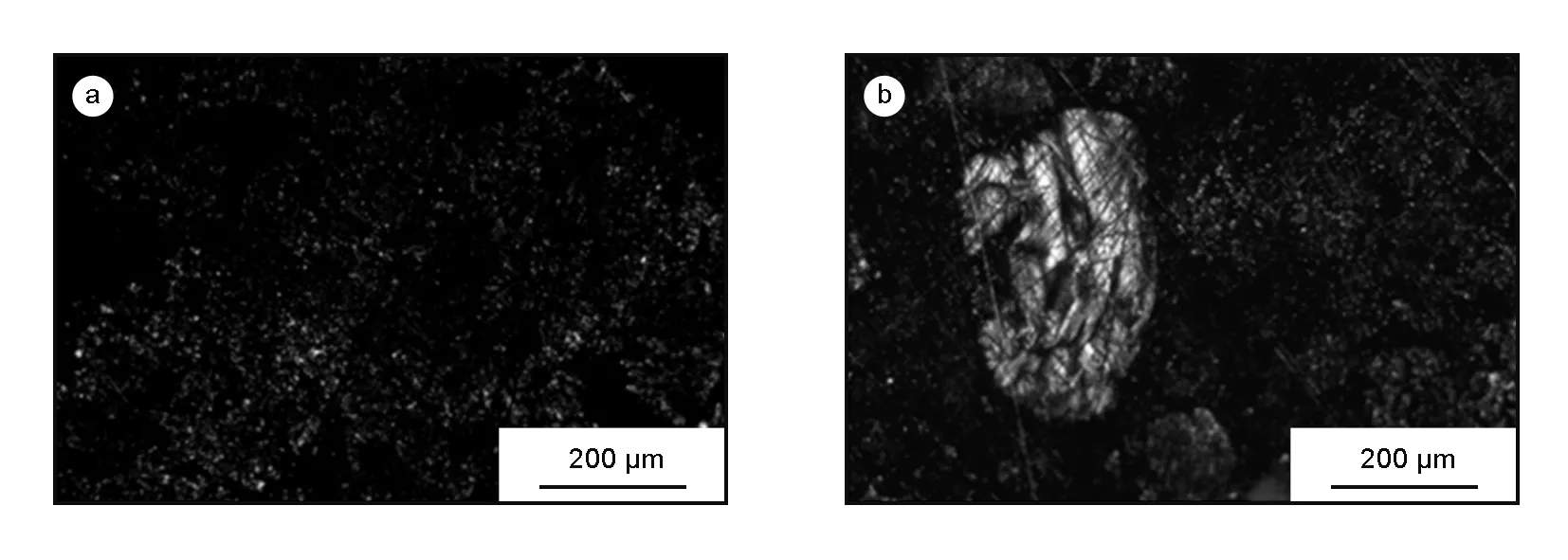
Fig. 8 Polarized light micrographs of the C-673 sample.
4 Conclusions
The role of AlCl3in the polymerization and carbonization of coal tar was demonstrated, which effectively accelerated dehydrogenation of aromatic hydrocarbons in polymerization, and increased weight of carbonaceous residues in carbonization. The AlCl3apparently affected the microstructures of the formed coke. In the presence of AlCl3, most coke seemed to be isotropy, which was different from the coke prepared by traditional liquid phase carbonization. Therefore, this method provided a cost-effective and rapid routine to produce isotropic graphite and its related carbon materials.
[1]Inagaki M, Kang F Y. Carbon Materials Science and Engineering—From Fundamentals to Applications[M]. Beijing: Tsinghua University Press, 2011: 3-6.
[2]Walker P L. Carbon: An old but new material revisited[J]. Carbon, 1990, 28(2-3): 261-279.
[3]Lewis I C. Chemistry of carbonization[J]. Carbon, 1982, 20(6): 519-529.
[4]QIAN Shu-an. A Brief exposition on the formation and progress of carbon science IV. History and modern state of researches on the gas-phase carbonization mechanisms[J]. Carbon (Chinese), 1996, (1): 4-12.
(錢樹安. 略論炭素科學的形成和進展IV. 氣相炭化機理研究的歷史和現狀[J]. 炭素, 1996, (1): 4-12.)
[5]QIAN Shu-an. A brief exposition on the formation and progress of carbon science V. History and modern state of the researches on liquid-phase carbonization mechanisms[J]. Carbon (Chinese), 1996, (2): 6-17.
(錢樹安. 略論炭素科學的形成和進展V. 液相炭化機理研究的歷史和現狀[J]. 炭素, 1996, (2): 6-17.)
[6]Marsh H, Menendez R. Carbons from pyrolysis of pitches, coals, and their blends[J]. Fuel Processing Technology, 1988, 20: 269-296.
[7]Taylor G H. Development of optical properties of coke during carbonization[J]. Fuel, 1961, 40: 465-472.
[8]Brooks J D, Taylor G H. The formation of graphitizing from the liquid phase[J]. Carbon, 1965, 3(2): 185-193.
[9]Chang Y C, Sohn H J, Ku C H, et al. Anodic performances of mesocarbon microbeads (MCMB) prepared from synthetic naphthalene isotropic pitch[J]. Carbon, 1999, 37(8): 1285-1297.
[10]Mochida I, Maeda K, Takeshita K. Structure of anisotropic spheres obtained in the course of needle coke formation[J]. Carbon, 1977, 15(1): 17-23.
[11]Mochida I, Korai Y, Ku C, et al. Chemistry of synthesis, structure, preparation and application of aromatic-derived mesophase pitch[J]. Carbon, 2000, 38(2): 305-328.
[12]Otani S, Oya A. Effect of AlCl3on graphitizability of coal tar pitch[J]. The Journal of the Society of Chemical Industry, 1970, 73(6): 1110-1116.
[13]Otani S, Oya A. Graphitizability of pitch carbon[J]. The Journal of the Society of Chemical Industry, 1970, 73(3): 493-498.
[14]Otani S, Oya A, Nagasima T. On the procedure and the properties of the carbon product prepared from chlorinated pitch materials[J]. The Journal of the Society of Chemical Industry Japan, 1970, 73(10): 2095-2100.
[15]Yamada S. Carbonization and graphitization of molded and extruded mixtures of carbons and binder pitch added with nitro compound[J]. The Journal of the Society of Chemical Industry, 1959, 62(10): 1524-1532.
[16]Mochida I, Nakamura E, Maeda K, et al. Carbonization of aromatic hydrocarbons—III: Carbonization catalyzed by alkali metals[J]. Carbon, 1975, 13(6): 489-493.
[17]Mochida I, Inoue S, Maeda K, et al. Carbonization of aromatic hydrocarbons—VI: Carbonization of heterocyclic compounds catalyzed by aluminum chloride[J]. Carbon, 1977, 15(1): 9-16.
[18]Mochida I, Nakamura E, Maeda K, et al. Carbonization of aromatic hydrocarbons—IV: Reaction path of carbonization catalyzed by alkali metals[J]. Carbon, 1976, 14(2): 123-129.
[19]Rey Boero J F, Wargon J A. Study of the AlCl3catalytic activity on aromatic hydrocarbons-II: Mesophase formation[J]. Carbon, 1981, 19(5): 341-346.
[20]LU Chun-xiang, LING Li-cheng, LU Wei-ming, et al. The reaction properties of naphthalene catalyzed and the structure of the oligomer and mesophase pitch[J]. New Carbon Materials, 1999, 14(3): 19-25.
(呂春祥, 凌立成, 呂維明, 等. 催化改性萘的反應特性及產物分析[J]. 新型炭材料, 1999, 14(3): 19-25.)
[22]Cullity B D. Elements of X-ray Diffraction [M]. New York: Addison Wesley, 1978: 84-85.
[23]Short M A, Walker P L. Measurement of interlayer spacings and crystal sizes in turbostratic graphite[J]. Carbon, 1963, 1(1): 3-9.
[24]Dasgupta K, Sathiyamoorthy D. Disordered carbon-its preparation, structure, and characterization[J]. Materials Science and Technology, 2003, 19(8): 995-1002.
[25]Fernandez J J, Figueiras A, Granda M, et al. Modification of coal-tar pitch by air-blowing--I. Variation of pitch composition and properties[J]. Carbon, 1995, 33(3): 295-307.
[26]Dumont M, Chollon G, Dourges M A, et al. Chemical, microstructural and thermal analyses of a naphthalene-derived mesophase pitch[J]. Carbon, 2002, 40(9): 1457-1486.
[27]Haridoss P, Uribe F A, Garzon F H, et al. Structural modifications of diordered mesocarbon microbeads with lower temperatures of heat treatment[J]. Journal of Materials Research, 1998, 13(7): 2015-2022.
[28]Kudryavtsev A B, Schopf J W, Agresti D G, et al. In situ laser-Raman imagery of precambrian microscopic fossils[J]. Proceedings of the National Academy of the Sciences of the United States of America, 2001, 98(3): 823-826.
[29]Nestler K, Dietrich D, Witke K, et al. Thermogravimetric and Raman spectroscopic investigations on different coals in comparison to dispersed anthracite found in permineralized tree fern psaronius sp[J]. Journal of Molecular Structure, 2003, 661-662: 357-362.
[30]Rodríguez-Mirasol J, Cordero T, Rodríguez J J. High-temperature carbons from kraft lignin[J]. Carbon, 1996, 34(1): 43-52.
[31]Zou L H, Huang B Y, Huang Y, et al. An investigation of heterogeneity of the degree of graphitization in carbon-carbon composites[J]. Materials Chemistry and Physics, 2003, 82(3): 654-662.
[32]Yen B K, Ishihara T. An investigation of friction and wear mechanisms of carbon-carbon composites in nitrogen and air at elevated temperatures[J]. Carbon, 1996, 34(4): 489-498.
[33]Tuinstra F, Koenig J L. Raman spectrum of graphite[J]. The Journal of Chemical Physics, 1970, 53: 1126.
[34]Marsh H, Latham C S. The chemistry of mesophase formation[J]. American Chemical Society Division of Petroleum Chemistry , 1984, 29(2): 354-381.
The catalytic role of aluminium trichloride in the polymerization and carbonization of coal tar
DUAN Miao1,TIAN Yong-ming2,LI Si-zhong1
(1.CollegeofMaterialScienceandEngineering,HuaqiaoUniversity,Xiamen361021,China;2.MaterialsDepartment,NewMexicoTech, 801LeroyPl,Socorro,NM87801,USA)
The polymerization and carbonization of coal tar was carried out at different temperatures from 633 to 1 873 K using aluminium trichloride as catalyst. The polymerization degree, microstructures and optical texture of samples were analyzed by thermogravimetry, X-ray diffraction, Raman spectroscopy and polarized light microscopy. Results indicated that aluminium trichloride accelerated dehydrogenation during the polymerization of coal tar, and increased the toluene-insoluble fraction and carbonization yield. The presence of aluminium trichloride in the polymerized coal tar affected the microstructure of the coke by the formation of a quasi-isotropic structure.
Coal tar; Aluminium trichloride; Catalytic carbonization; Microstructure; Optical texture
date: 2015-12-10;Reviseddate: 2016-01-10
Special Foundation for Young Scientists of Fujian Province (2011J05131); Scientific Research Staring Foundation of Huaqiao University (11BS214); Cultivate Project of National Natural Science Foundation of Huaqiao University (JB-ZR1214).
LI Si-zhong, Ph. D., Lecturer. E-mail: lisi202001@tom.com
introduction: DUAN Miao, Master Student. E-mail: duan@hqu.edu.cn
1007-8827(2016)01-0062-06
TQ127.1+1
A
福建省自然科學基金(2011J05131);華僑大學青年教師啟動基金(11BS214);華僑大學自然基金培育基金(JB-ZR1214).
李四中,博士,講師. E-mail: lisi202001@tom.com
段淼,碩士研究生. E-mail: duan@hqu.edu.cn
10.1016/S1872-5805(16)60005-7
English edition available online ScienceDirect ( http:www.sciencedirect.comsciencejournal18725805 ).

