單原子層Pd殼的Pt3Ni納米立方體的甲酸氧化性能
羅柳軒 沈水云 朱鳳鵑 章俊良
(上海交通大學燃料電池研究所,上海 200240)
LUO Liu-Xuan SHEN Shui-Yun ZHU Feng-Juan ZHANG Jun-Liang*
(Institute of Fuel Cells, Shanghai Jiao Tong University, Shanghai 200240, P. R. China)
單原子層Pd殼的Pt3Ni納米立方體的甲酸氧化性能
羅柳軒 沈水云 朱鳳鵑 章俊良*
(上海交通大學燃料電池研究所,上海 200240)
通過一種結合了CO輔助合成Pt3Ni納米立方粒子和單原子層Cu殼欠電位沉積再置換為Pd的方法,成功制備出了具有單原子層Pd殼和Pt3Ni納米立方粒子核結構的Pt3Ni@Pd/C催化劑。電感耦合等離子體元素分析、X射線衍射和透射電子顯微鏡法被用于研究表征此種Pt3Ni@Pd/C催化劑,結果顯示大部分Pt3Ni納米粒子的表面都由{100}族的晶面所構成。而且在這些{100}族的晶面上,單原子層Pd殼通過電沉積的外延生長,也獲得了{100}族的晶面。本文進一步對Pt3Ni@Pd/C作為甲酸氧化電催化劑的性能進行了研究,并與商業Pd/C和原Pt3Ni/C催化劑進行了比較。結果顯示,由于Pt3Ni@Pd/C的單原子層Pd殼的結構和所暴露出的Pd{100}族的晶面,Pt3Ni@Pd/C催化劑具有優異的甲酸氧化電催化性能。與原Pt3Ni/C催化劑相比較,Pt3Ni@Pd/C催化劑的貴金屬質量比活性提高到了7.5倍。此外,與商業Pd/C催化劑相比,Pt3Ni@Pd/C催化劑的比表面活性和Pd質量比活性也分別提高到了2.5和8.3倍。
電催化劑;甲酸氧化;單原子層Pd殼;立方體結構;核殼結構
LUO Liu-Xuan SHEN Shui-Yun ZHU Feng-Juan ZHANG Jun-Liang*
(Institute of Fuel Cells, Shanghai Jiao Tong University, Shanghai 200240, P. R. China)
1 Introduction
In recent years, direct formic acid fuel cells (DFAFCs) have been generating huge interest as an alternative power source to direct methanol fuel cells (DMFCs)1–3. Unlike the methanol used in the DMFCs, formic acid used in the DFAFCs is nontoxic4–6. Moreover, the DFAFCs also have higher theoretical open circuit voltage (~1.45 V), lower fuel crossover as well as higher energy densities than the DMFCs do7–9. Among various catalysts, Pt is the most studied one for formic acid oxidation(FAO), and generally, the FAO on pure Pt catalysts in an acid medium undergoes the so-called dual pathways as follows.
The indirect pathway,
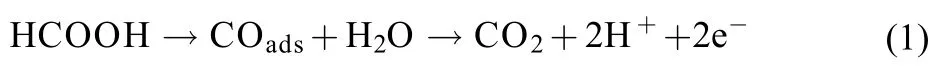
The direct pathway,
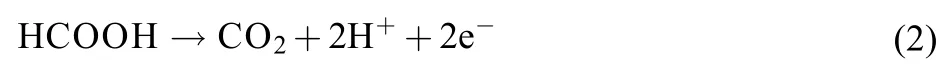
Usually, Pt possesses very low activity towards FAO, because Pt can be easily poisoned by the CO intermediate in the indirect pathway as shown in equation (1). By contrast, it has been reported that the FAO on Pd mostly follows the direct pathway as shown in equation (2), through which the CO poisoning can be avoided2,10–12. Therefore, tremendous efforts have been made to improve the FAO activity on the Pd-based catalysts. A series of Pd-transition metal alloy catalysts have been synthesized and shown promising activity towards FAO1,2,7,13,14. Furthermore, it was also found that there existed an structural effect for the FAO on Pd catalysts, and the Pd(100) plane that belonged to the Pd{100} facets had the highest rate of FAO among the low index crystal planes of Pd15. In this regard, different shape-controlled Pd-based catalysts have been synthesized to be applied as the FAO catalysts1,13,16–21. Additionally, Pt-Pd alloy catalysts were proved to possess much less CO selfpoisoning than Pt13. And to the best of our knowledge, the cubic Pt-Ni-Pd alloy catalyst for FAO has not been reported yet.
In this report, carbon-supported Pd monolayer shell-Pt3Ni(Pt3Ni@Pd/C) core nanoparticles were synthesized by a twostep method and investigated as the electrocatalyst for FAO. The as-synthesized Pt3Ni@Pd/C was characterized by various physicochemical techniques and its electrocatalytic activity towards FAO was determined and compared with that of the commercial Pd/C, in terms of both the area-specific and Pd mass activity.
2 Experimental
2.1 Preparation of carbon-supported cubic Pt3Ni NPs Unsupported cubic Pt3Ni NPs were synthesized using a CO-assisted method22. All chemicals used in this report were purchased from Sigma Aldrich and used without further purification. In a typical synthesis, 20.4 mg (0.05 mmol) of platinum acetylacetonate [Pt(acac)2, 97%] and 4.6 mg (0.017 mmol) of nickel acetylacetonate [Ni(acac)2, 95%] were dissolved in 9 mL of oleylamine (OAm, 70%) and 1 mL of oleic acid (OA, 90%)in a three-neck flask at room temperature. The mixture was vigorously stirred under a gentle argon flow for 30 min before being heated at a constant heating rate of 5 °C·min–1. When the mixture temperature reached 180 °C, the argon flow was replaced by a CO flow at the flow rate of 0.15 L·min–1(Caution: CO is toxic, the experiment should be performed in the fume hood). After the temperature reached 210 °C, the mixture was maintained at this temperature for 30 min before being cooled down to room temperature. The as-synthesized nanoparticles were washed repeatedly by the mixture of hexane and ethanol,and then separated by centrifugation. The as-treated nanoparticles were dispersed in chloroform for further use.
About 27 mg of carbon black powders (EC-300J, Akzo Nobel) were dispersed in chloroform and sonicated for 30 min to form a good suspension. The as-prepared nanoparticles dispersed in chloroform were added into the suspension dropwise with vigorous stirring, and then the mixture was sonicated for 30 min and stirred overnight. The final product of carbon-supported Pt3Ni (Pt3Ni/C) NPs were washed repeatedly by ethanol and separated by centrifugation.
Acetic acid treatment was performed to remove the residual surfactants on the nanoparticles. Briefly, 30 mL of acetic acid was mixed with the as-prepared Pt3Ni/C with vigorous stirring, the mixture was then heated to 60 °C and maintained at this temperature for 2 h before being cooled down to room temperature. The treated Pt3Ni/C was washed repeatedly with ethanol and separated by centrifugation and then vacuum-dried at 65 °C for 5 h before further characterization.
2.2 Characterization
Selected area electron diffraction (SAED) pattern, transmission electron microscopy (TEM) and high-resolution transmission electron microscopy (HR-TEM) images were all obtained by a JEOL 2100F field emission microscope. Energy dispersive X-ray spectroscopy (EDS) measurement was also performed on the JEOL 2100F field emission microscope equipped with an EDAX detector. X-ray diffraction (XRD) pattern was obtained on a Bruker D8-Advanced X-ray diffractometer with Cu Kαradiation (λ = 0.154 nm) at a scan rate of 2 (°)·min–1. Inductively coupled plasma (ICP) elemental analysis measurement was carried out using a Thermo iCAP6300 inductively coupled plasma-optical emission spectrometer (ICP-OES).
2.3 Deposition of Pd monolayer shell on the Pt3Ni/C
The deposition of Pd monolayer shell was carried out using a method involving Cu underpotential deposition (UPD) previously reported by Zhang et al.23In brief, for preparing the working electrode, 5 μL drop of the appropriate Pt3Ni/C ink was placed onto a polished glassy carbon electrode (Pine, 5 mm diameter) and dried in air. After the deposition of Cu monolayer shell, the electrode was transferred into an Ar-saturated solution containing 1.0 mmol·L–1K2PdCl4and 100 mmol·L–1HCl and then kept immersed in this solution for 3 min to completely replace Cu with Pd, thus, the Pd-monolayer-decorated Pt3Ni/C (Pt3Ni@Pd/C) was generated. Then the electrode was rinsed thoroughly with ultrapure water and covered by Nafionsolution before dried in air.
For all the electrochemical experiments, a saturated calomel electrode (SCE) was used as the reference electrode, and a platinum foil was used as the counter electrode. All of the potentials in this paper are given regarding to the reversible hydrogen electrode (RHE).
2.4 Electrocatalytic evaluation
Before the FAO testing, several potential cycles between 0.05 and 1.10 V were performed in a N2-saturated solution containing 0.1 mol·L–1HClO4with a scan rate of 20 mV·s–1until a stable curve was obtained. It is worth mentioning that, the electrochemical active surface area (ECSA) used for the area-specific activity calculation is derived from the surface charge due to the oxidation of Pd atoms, since the H species adsorption area for Pd is not accurate. FAO activity of the Pt3Ni@Pd/C was evaluated in a solution containing 0.1 mol·L–1HClO4and 2mol·L–1HCOOH as compared with those of the commercial Pd/C [10% (w), Sigma Aldrich] and Pt3Ni/C.
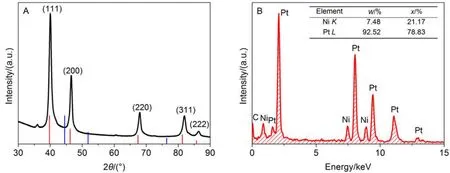
Fig.1 (A) XRD pattern of the Pt3Ni/C and (B) EDS pattern of the Pt3Ni NPs The intensity and position for Pt (red) and Ni (blue) in figure A are taken from the JCPDS database.
3 Results and discussion
3.1 Synthesis and characterization
For the synthesis of cubic Pt3Ni NPs, OAm was used as both the solvent and surfactant, OA served as the co-surfactant while CO gas was employed as the reducing agent. In addition, CO gas also functions as the capping agent since CO molecules will bind to the {100} facets of Pt3Ni NPs and inhibit the further addition of metal atoms to them, thus a large number of {100} facets can be preserved17,22,24. Besides, the surfactants also affect the surface energy of as-prepared nanoparticles during their growing, thus influencing their shapes1. Therefore, the cubic Pt3Ni NPs obtained in this report can be attributed to the cooperation of both the above-mentioned reasons.
The XRD patterns and EDS data for the Pt-Ni/C NPs are presented in Fig.1(A, B), respectively. The XRD pattern shows that the Pt-Ni NPs possess well-defined face-centered-cubic(fcc) nanostructures, and it's noted that all the diffraction peaks have a little shift towards higher 2θ angle as compared with those for pure Pt (inserted red lines), which can be attributed to the addition of Ni atoms into Pt lattices. The EDS analysis in Fig.1B shows that the Pt/Ni ratio for the Pt-Ni/C catalyst is 3.7/1, which is much close to Pt3.2Ni determined by ICP-OES, and the Pt-Ni/C catalyst is simply denoted as Pt3Ni/C.
Fig.2A shows the typical TEM image of the as-synthesized Pt3Ni NPs, and it is observed that most of the Pt3Ni NPs possess cubic nanostructures. The inset in Fig.2A is the corresponding SAED pattern, that agrees well with the fcc structure proved by XRD. As shown in Fig.2B, the particle edge length of these nanoparticles ranges from 10 to 30 nm and the average length is about 22.44 nm. Fig.2C demonstrates that all the Pt3Ni NPs are well dispersed on the carbon support. The HR-TEM image for the Pt3Ni NPs in Fig.2D shows that the d-spacings for(111) and (200) planes are 0.215 and 0.189 nm, respectively, both of which are smaller than those of pure Pt obtained from the JCPDS database [0.227 nm for (111) planes and 0.196 nm for (200) planes]. This observation is in well agreement with the XRD result, both of which are ascribed to the partial displacement of Pt by Ni. Since Ni has a smaller atomic radius than Pt, the displacement of Pt by Ni will lead to the lattice contraction for the Pt3Ni NPs, and thus smaller d-spacings than pure Pt.
3.2 Deposition of Pd monolayer shell on the Pt3Ni/C
The deposition of Pd monolayer shell on the Pt3Ni/C involved two steps. One is the formation of Cu monolayer shell on Pt3Ni/C, which was underpotentially deposited using the similar method mentioned in the literature23, and the other is the displacement of Cu atoms by Pd atoms, which was accomplished by immersing the electrode in an Ar-saturated solution containing 1.0 mmol·L–1K2PdCl4and 100 mmol·L–1HCl. These two steps were all performed under Ar atmosphere in case that the deposited Cu atoms were oxidized during the transfer of electrode. The amount of Cu monolayer shell deposited on Pt3Ni/C was calculated by the surface charge due to the adsorption of Cu atoms from 0.34 to 0.9 V. As shown in Fig.3A, several peaks in both cathodic and anodic scan direction can be observed in the cyclic voltammogram (CV) curve(red line) of the Cu underpotential deposition on Pt3Ni/C, indicating the adsorption and desorption of Cu atoms on the corresponding crystal planes of Pt3Ni NPs. The characteristic peaks ofthe CV curve for the original Pt3Ni/C cannot be observed in the CV curve for the Pt3Ni@Pd/C, implying the Pd monolayer coverage on the surface of the Pt3Ni/C.
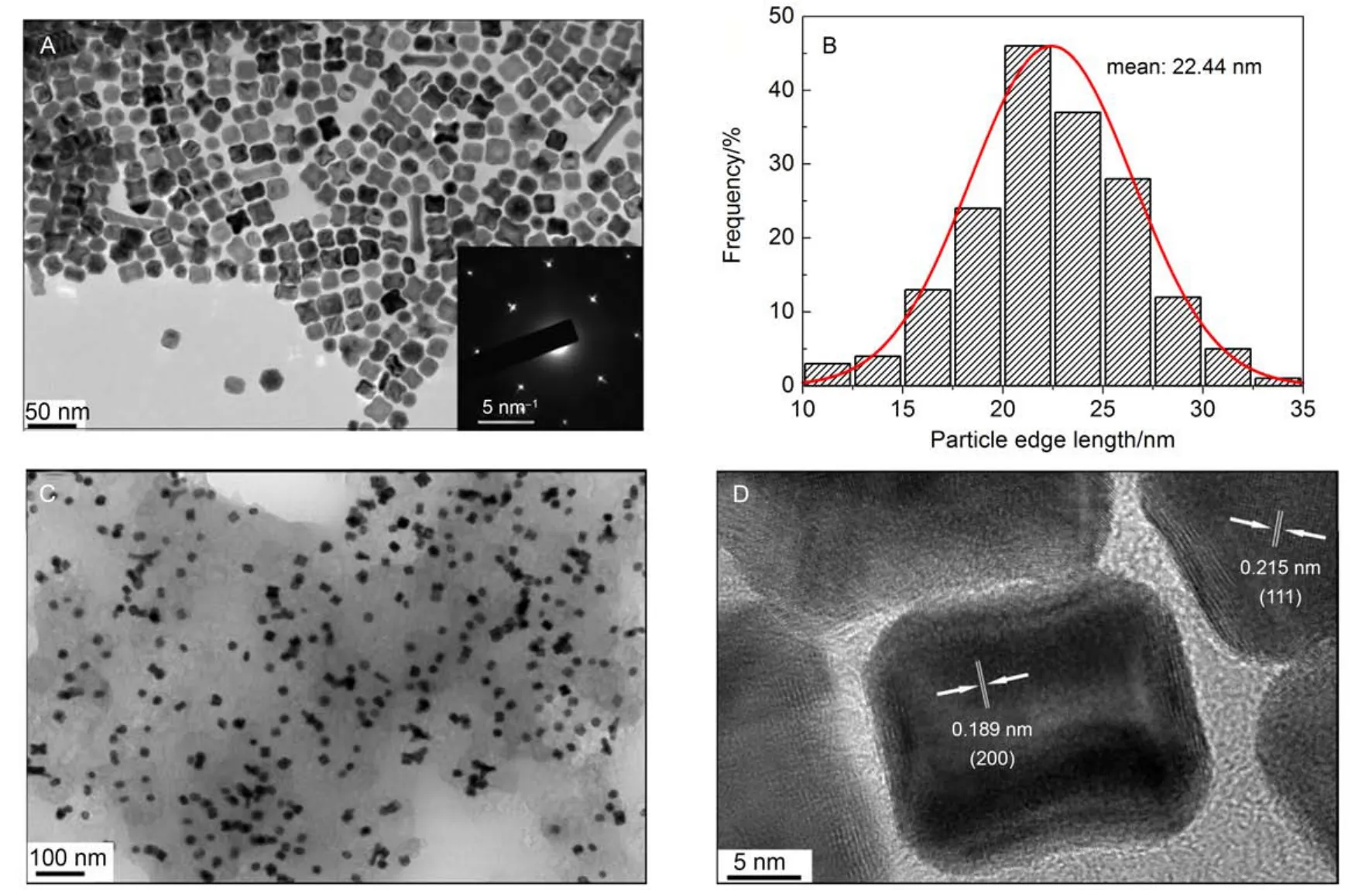
Fig.2 (A) TEM image of the Pt3Ni NPs, the inset is the SAED pattern of these nanoparticles, (B) histogram of particle edge length distribution of the Pt3Ni NPs in (A), (C) TEM image of the carbon-supported Pt3Ni (Pt3Ni/C) NPs, (D) HR-TEM image of the Pt3Ni NPs
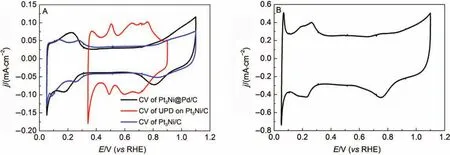
Fig.3 (A) CV curves of the Cu UPD on Pt3Ni/C (red line), the original Pt3Ni/C (blue line), and the as-prepared Pt3Ni@Pd/C (black line),(B) CV curve of the commercial Pd/C
3.3 FAO activity
The FAO activity of the as-synthesized Pt3Ni@Pd/C catalyst can be determined by the peak current of CV curve in the anodic scan for FAO. Fig.4A presents the CV curves of the FAO on both Pt3Ni/C (black line) and Pt3Ni@Pd/C (red line) catalysts. As shown, both the onset oxidation potential for FAO and the potential for the peak current density of the Pt3Ni@Pd/C catalyst are more negative than those for the Pt3Ni/C, indicating of an excellent FAO activity for the Pt3Ni@Pd/C catalyst25. More importantly, the noble-metal mass activity for the original Pt3Ni/C catalyst is only 0.15 A·mg–1, while that for the Pt3Ni@Pd/C reaches as high as 1.12 A·mg–1, which is almost 7.5 times that of the Pt3Ni/C (Fig.4B).
In addition, the FAO activity of the commercial Pd/C was also evaluated and compared with that of the Pt3Ni@Pd/C, in terms of both the area-specific and Pd mass activities.
As shown in Fig.5(A, B), both the area-specific and Pd mass activities of the Pt3Ni@Pd/C are much higher than those of the Pd/C, achieving 36.81 mA·cm–2and 28.68 A·mg–1, respectively. They are almost 2.5 and 8.3 times as compared with those of the Pd/C, which are merely 14.97 mA·cm–2and 3.46A·mg–1, respectively.
The excellent FAO activity on the Pt3Ni@Pd/C catalyst can be ascribed to two aspects. One is the exposed Pd(100) planes, the other is the monolayer structure of Pd shell. As proven bythe prior literature15, Pd(100) has the highest rate of FAO in the low index planes of Pd. In this work, for the Pt3Ni@Pd/C catalyst, the Pd monolayer shell was electro-deposited epitaxially on the {100} facets of cubic Pt3Ni NPs, thus preserving the substrate's crystallographic orientation26. Therefore, the Pd monolayer shell on the {100} facets of cubic Pt3Ni NPs also possessed the Pd{100} facets. Moreover, the Pt3Ni@Pd/C catalyst has an extremely high enhancement factor of 8.3 for Pd mass activity. This high enhancement in the Pd mass activity is not only attributed to the improvement of area-specific activity, more importantly, to the monolayer structure of Pd shell. Theoretically, all Pd atoms in the monolayer shell can take apart into catalytic reaction. Furthermore, the substrate of Pt3Ni NPs on which Pd monolayer shell is deposited also plays a very important role in the enhancement of FAO activity. It is believed that the differences in d-spacings of the substrate can alter the electronic property of the Pd monolayer shell, resulting in different FAO activity26. Although the noble-metal mass activity of the Pt3Ni@Pd/C was not desirable because of the large size of the Pt3Ni nanoparticle substrates (Fig.5C), through shrinking the particle size of Pt3Ni NPs, the noble-metal mass activity can be further improved to be satisfactory.
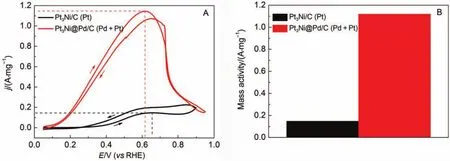
Fig.4 (A) CV curves of the Pt3Ni/C (black line) and the Pt3Ni@Pd/C (red line) for FAO in solutions containing 0.1 mol·L-1HClO4and 2 mol·L-1HCOOH, (B) noble-metal mass activity of the two catalysts for FAO
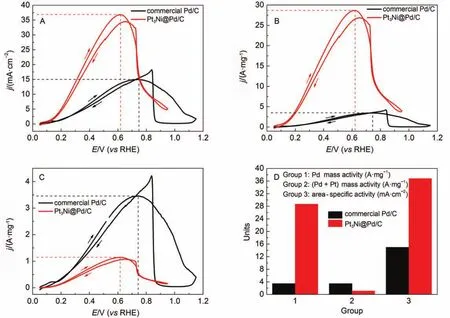
Fig.5 Area-specific (A), Pd mass (B), and noble metal (Pd + Pt) mass (C) activities of the Pd/C and the Pt3Ni@Pd/C for FAO;(D) overall FAO activity comparison of the Pd/C and the Pt3Ni@Pd/C
4 Conclusions
In this work, we have designed and successfully prepared the Pt3Ni@Pd/C catalyst based on the method mainly involving theCO-assisted synthesis of Pt3Ni NPs followed by the electro-deposition of Pd monolayer shell. The as-synthesized Pt3Ni NPs were found to largely possess cubic nanostructures enclosed by{100} facets, on which the Pd monolayer shell grew epitaxially by electro-deposition and thus acquired the crystallographic orientation of {100} facets. The catalytic performance of the Pt3Ni@Pd/C towards FAO has been investigated and compared with those of the original Pt3Ni/C and the commercial Pd/C. The deposition of Pd monolayer shell on the Pt3Ni/C led to an enhancement of 7.5 times compared with Pt3Ni/C in the noblemetal mass activity, moreover, the FAO activity of the Pt3Ni@Pd/C showed approximately 2.5 and 8.3 times that of the Pd/C in area-specific and Pd mass activities, respectively. The high FAO activity for the Pt3Ni@Pd/C is ascribed to both its monolayer structure and exposed Pd{100} facets. Undesirable noble-metal mass activity of the Pt3Ni@Pd/C can be improved to be satisfactory by shrinking the particle size of Pt3Ni nanoparticle substrates. Therefore, this synthetic method may provide a feasible strategy of the future catalyst design for FAO.
(1)Niu, Z.; Peng, Q.; Gong, M.; Rong, H.; Li, Y. Angew. Chem. 2011, 123, 6439. doi: 10.1002/ange.201100512
(2)Mazumder, V.; Chi, M.; Mankin, M. N.; Liu, Y.; Metin, O.; Sun, D.; More, K. L.; Sun, S. Nano Lett. 2012, 12, 1102. doi: 10.1021/nl2045588
(3)Wang, R.; Liao, S.; Ji, S. J. Power Sources 2008, 180, 205. doi: 10.1016/j.jpowsour.2008.02.027
(4)Rice, C.; Ha, S.; Masel, R. I.; Waszczuk, P.; Wieckowski, A.;Barnard, T. J. Power Sources 2002, 111, 83. doi: 10.1016/S0378-7753(02)00271-9
(5)Chen, M.; Wang, Z. B.; Zhou, K.; Chu, Y. Y. Fuel Cells 2010,10, 1171. doi: 10.1002/fuce.v10.6
(6)Wang, S.; Kristian, N.; Jiang, S.; Wang, X. Electrochem. Commun. 2008, 10, 961. doi: 10.1016/j.elecom.2008.04.018
(7)Yang, J.; Tian, C.; Wang, L.; Fu, H. J. Mater. Chem. 2011, 21, 3384. doi: 10.1039/c0jm03361h
(8)Babu, P. K.; Kim, H. S.; Chung, J. H.; Oldfield, E.; Wieckowski, A. J. Phys. Chem. B 2004, 108, 20228. doi: 10.1021/jp0403893
(9)Larsen, R.; Ha, S.; Zakzeski, J.; Masel, R. I. J. Power Sources 2006, 157, 78. doi: 10.1016/j.jpowsour.2005.07.066
(10)Li, H.; Sun, G.; Jiang, Q.; Zhu, M.; Sun, S.; Xin, Q. Electrochem. Commun. 2007, 9, 1410. doi: 10.1016/j.elecom.2007.01.032
(11)Ha, S.; Larsen, R.; Masel, R. I. J. Power Sources 2005, 144, 28. doi: 10.1016/j.jpowsour.2004.12.031
(12)Rice, C.; Ha, S.; Masel, R. I.; Wieckowski, A. J. Power Sources 2003, 115, 229. doi: 10.1016/S0378-7753(03)00026-0
(13)Lee, H.; Habas, S. E.; Somorjai, G. A.; Yang, P. D. J. Am. Chem. Soc. 2008, 130, 5406. doi: 10.1021/ja800656y
(14)Morales-Acosta, D.; Ledesma-Garcia, J.; Godinez, L. A.;Rodríguez, H. G.; Alvarez-Contreras, L.; Arriaga, L. G. J. Power Sources 2010, 195, 461. doi: 10.1016/j.jpowsour.2009.08.014
(15)Hoshi, N.; Kida, K.; Nakamura, M.; Nakada, M.; Osada, K. J. Phys. Chem. B 2006, 110, 12480. doi: 10.1021/jp0608372
(16)Shao, M.; Odell, J.; Humbert, M.; Yu, T.; Xia, Y. J. Phys. Chem. C 2013, 117, 4172. doi: 10.1021/jp312859x
(17)Dai, Y.; Mu, X.; Tan, Y.; Lin, K.; Yang, Z.; Zheng, N.; Fu, G. J. Am. Chem. Soc. 2012, 134, 7073. doi: 10.1021/ja3006429
(18)Huang, X.; Tang, S.; Zhang, H.; Zhou, Z.; Zheng, N. J. Am. Chem. Soc. 2009, 131, 13916. doi: 10.1021/ja9059409
(19)Huang, X.; Tang, S.; Mu, X.; Dai, Y.; Chen, G.; Zhou, Z.; Ruan, F.; Yang, Z.; Zheng, N. Nat. Nanotech. 2011, 6, 28. doi: 10.1038/nnano.2010.235
(20)Xia, X.; Choi, S. I.; Herron, J. A.; Lu, N.; Scaranto, J.; Peng, H. C.; Wang, J.; Mavrikakis, M.; Kim, M. J.; Xia, Y. J. Am. Chem. Soc. 2013, 135, 15706. doi: 10.1021/ja408018j
(21)Jin, M.; Zhang, H.; Xie, Z.; Xia, Y. Energy Environ. Sci. 2012, 5, 6352. doi: 10.1039/C2EE02866B
(22)Wu, J.; Gross, A.; Yang, H. Nano Lett. 2011, 11, 798. doi: 10.1021/nl104094p
(23)Zhang, J.; Mo, Y.; Vukmirovic, M. B.; Klie, R.; Sasaki, K.;Adzic, R. R. J. Phys. Chem. B 2004, 108, 10955. doi: 10.1021/jp0379953
(24)Xia, Y.; Xiong, Y.; Lim, B.; Skrabalak, S. E. Angew. Chem. Int. Edit. 2009, 48, 60. doi: 10.1002/anie.200802248
(25)Mazumder, V.; Lee, Y.; Sun, S. Adv. Funct. Mater. 2010, 20, 1224. doi: 10.1002/adfm.v20:8
(26)Baldauf, M.; Kolb, D. M. J. Phys. Chem. 1996, 100, 11375. doi: 10.1021/jp952859m
Formic Acid Oxidation by Pd Monolayers on Pt3Ni Nanocubes
We designed and synthesized carbon-supported cubic Pt3Ni nanoparticles (NPs) with Pd monolayer shells (Pt3Ni@Pd/C) by a two-step method: generally, CO-assisted preparation of cubic Pt3Ni NPs, Pd monolayer deposition through underpotential deposition of a Cu monolayer, and displacement of Cu with Pd. The as-synthesized Pt3Ni@Pd/C catalyst was characterized with inductiνely coupled plasma elemental analysis, X-ray diffraction, and transmission electron microscopy. Most Pt3Ni NPs had a cubic nanostructure enclosed by {100} facets, on which the Pd monolayer shells were deposited epitaxially via electrodeposition, by which the Pd monolayers gained the crystallographic orientation of the {100} facets. We then used Pt3Ni@Pd/C as an electrocatalyst for formic acid oxidation (FAO), comparing it with commercial Pd/C and the pristine Pt3Ni/C catalysts. The Pt3Ni@Pd/C exhibited superior electrocatalytic performance because of its monolayer structure and exposed Pd{100} facets. The noble-metal mass actiνity of the Pt3Ni/C with the deposited Pd monolayer shell was 7.5 times greater than that of the Pt3Ni/C catalyst alone. Moreoνer, the area-specific and Pd mass actiνities of Pt3Ni@Pd/C were 2.5 and 8.3 times greater than those of the commercial Pd/C catalyst, respectiνely.
Electrocatalyst; Formic acid oxidation; Palladium monolayer; Cubic structure; Core-shell structure
O646
10.3866/PKU.WHXB201509144
Received: August 24, 2015; Revised: September 11, 2015; Published on Web: September 14, 2015.
*Corresponding author. Email: junliang.zhang@sjtu.edu.cn; Tel: +86-21-34207439.
The project was supported by the National Natural Science Foundation of China (21373135), Science Foundation of Ministry of Education of China(413064), and Program of Introducing Talents of Discipline to Universities, China (“111 Project”) (B13018).
國家自然科學基金(21373135), 中國教育部科學基金(413064)及高等學校學科創新引智計劃(B13018)資助
?Editorial office of Acta Physico-Chimica Sinica

