萘咪唑類稀土配合物的晶體結構和熒光性質
郝文輝 鄒曉艷*,,2 費博文 閆鵬飛 董艷萍 李光明*,
(1黑龍江大學化學化工與材料學院,功能無機材料化學教育部重點實驗室,哈爾濱150080)
(2《黑龍江大學工程學報》編輯部,哈爾濱150080)
萘咪唑類稀土配合物的晶體結構和熒光性質
郝文輝1鄒曉艷*,1,2費博文1閆鵬飛1董艷萍1李光明*,1
(1黑龍江大學化學化工與材料學院,功能無機材料化學教育部重點實驗室,哈爾濱150080)
(2《黑龍江大學工程學報》編輯部,哈爾濱150080)
通過2-(2′-羥基-3′-甲氧基)萘咪唑(HL)和Ln(NO)3·6H2O反應,合成了4種單核稀土配合物[Ln(HL)2(NO3)3]·CH2Cl2(Ln=Sm (1),Eu(2),Tb(3))和[Ln(HL)2(NO3)2(CH3OH)]NO3·CH3OH(Ln=Yb(4))。X射線單晶衍射分析表明配合物1~4均通過配體萘環間的π-π作用呈現蝴蝶狀結構。熒光性質表明僅有配合物4顯示Yb3+稀土離子的特征發光,固態和在乙腈溶液中的熒光壽命分別為8.27 μs和4.40 μs。
合成;結構;鑭系配合物;近紅外發光
0 Introduction
Owing to their unusual magnetic and luminescent behaviors,lanthanide complexes with various structures have currently attracted much attention from inorganic material community[1-4].In one respect,attentions have been drawn on the efficient luminescence of lanthanide complexes in the near-infrared(NIR) region such as Nd3+and Yb3+,in respective to their biological-friendly application with narrow and strongemission[5-6].More efforts for lanthanide complexes have focused mainly on the construction and preparation of versatile coordination structures,as well as the structure -property relationships.The large ionic radius of lanthanide ions affords high and variable coordination numbers,providing more difficulty in controlling the synthetic reaction than transition-metal ones[7-9].Among the strategies,the rational selection of organic ligands or co-ligands according to their length,rigidity and functional groups is important for the assembly of structurally controllable lanthanide complexes with NIR luminescent behaviors.From benzimidazole base ligands(HL1,HL1=2-(1H-benzoimidazol-2-yl)-4-bromo -6-methoxy-phenol,Scheme 1a),the luminescent lanthanide complexes with various structures were prepared by Jones,Yang and coworkers[10-11].Planar architecture of benzimidazole based ligands can engage in π-π stacking interactions and have also shown ligand-to-metal energy transfer(LMET)properties in lanthanide complexes[12].We envision that replacement of phenyl with larger conjugated aromatic system, such as naphthyl group,would increase the efficiency of luminescence and enlargement of the fused ring structure might provide different coordination mode. Herein,we synthesized 2-(2′-hydroxyphenyl-3′-methoxyl)naphthoimidazole(HL,Scheme 1b)and four mononuclear lanthanide complexes[Ln(HL)2(NO3)3]· CH2Cl2(Ln=Sm(1),Eu(2),Tb(3))and[Ln(HL)2(NO3)2)have been isolated with crystal structures determined by X-ray crystallographic analysis.The NIR luminescent of Yb3+complex has been investigated which has scarcely been reported.

Scheme 1 Structures of HL1(a)and HL(b)
1 Experimental
1.1Materials and general methods
2-(2′-hydroxyphenyl-3′-methoxyl)naphthoimidazole(HL)have been prepared according to the reported method[10].Ln(NO)3·6H2O were prepared by the reactions of Ln2O3with nitric acid in the aqueous solution. All the other chemicals were obtained from commercial sources and used without further purification. Fourier transform infrared(FT-IR)data were recorded on a PerkinElmer 100 spectrophotometer in the range of 4 000~500 cm-1using KBr disks.UV-Vis spectra (in CH3CN)were recorded on a PerkinElmer Lambda 35 spectrometer.Elemental(C,N,and H)analysis were carried out on a Perkin Elmer 2400 analyzer. Thermal analyses were carried out on a Perkin Elmer STA 6000 in the temperature range of 30~800℃with a heating rate of 10℃·min-1under atmosphere.The Powder X-ray diffraction(PXRD)patterns were recorded on a Rigaku D/Max-3B X-ray diffractometer with Cu Kα radiation as the radiation source(λ=0.154 06 nm,40 mA,200 kV)with 2θ range of 5°~40°.Luminescence spectra and luminescence lifetimes were conducted with an Edinburgh FLS 920 fluorescence spectrophotometer and a single photon counting spectrometer from Edinburgh Instruments(FLS 920) with a microsecond pulse lamp as the excitation, respectively.The detailed information of samples is shown in Fig.S1~S7.
1.2Syntheses of complexes 1~4
Complexes 1~4 were prepared by the similar procedures.In a typical synthesis of complex 1,Sm (NO)3·6H2O(0.1 mmol,0.045 7 g)and 1,8-naphthalenediamine(0.2 mmol,0.085 2 g)were dissolved in a mixture solution of CH3OH/CH2Cl2(20 mL,1∶1,V/V). The mixture was stirred at room temperature for 4 h. Then hexane(60 mL)was allowed to diffuse slowly into the filtrate.Crystals suitable for single crystal X-ray analysis were obtained in 7 days.
[Sm(HL)2(NO3)3]·CH2Cl2(1)
Yield:68%.Anal.Calcd.for C37H30SmCl2N7O13(%): C,44.35;H,3.02;N,9.79.Found(%):C,44.55 H, 2.96;N,9.85..IR(KBr,cm-1):3 056,2 939,2 841, 1 624,1 508,1 383,1 220,1 065,1 028,819.UV-Vis(MeOH,nm):206,232,267,279,338.
[Eu(HL)2(NO3)3]·CH2Cl2(2)
Yield:75%.Anal.Calcd.for C37H30EuCl2N7O13(%): C,44.28;H,3.01;N,9.77.Found(%):C,44.23 H, 2.97;N,9.82.IR(KBr,cm-1):3 054,2 940,2 842, 1 624,1 507,1 384,1 221,1 066,1 026,820.UVVis(MeOH,nm):203,229,270,279,342.
[Tb(HL)2(NO3)3]·CH2Cl2(3)
Yield:78%.Anal.Calcd.for C37H30TbCl2N7O13(%): C,43.98;H,2.99;N,9.70.Found(%):C,43.69 H, 2.92;N,9.68.IR(KBr,cm-1):3 060,2 941,2 845, 1 624,1 508,1 384,1 286,1 065,819,737.UV-Vis (MeOH,nm):203,230,265,280,335.
[Yb(HL)2(NO3)2(CH3OH)]NO3·CH3OH(4)
Yield:74%.Anal.Calcd.for C38H36YbN7O15(%): C,45.47;H,3.61;N,9.77.Found(%):C,45.42;H, 3.59;N,9.75.IR(KBr,cm-1):3 059,2 941,2 845, 1 622,1 507,1 384,1 286,1 065,819,737.UV-Vis (MeOH,nm):203,230,270,279,342.
1.3Determination of the crystal structures
Single crystals of complexes 1~4 were selected for X-ray diffraction analysis on an Oxford XcaliburGemini Ultra diffractometer using graphite-monochromated Mo Kα radiation(λ=0.071 073 nm)at room temperature.Empirical absorption corrections based on equivalent reflections were applied.The structures of complexes 1~4 were solved by direct methods,and all non-hydrogen atoms were anisotropically refined by full-matrix least squares methods on F2using SHELXS -97 crystallographic software package[13].It should be noticed that the SQUEEZE/PLATON tools were employed in the refinement for the crystal structure, and largest residual density peaks(0.113 nm)has been found taking a very close distance 0.171 9 and 0.19 nm with carbon atom(C61 and C60)in complex 1,0.196 2 and 1.881 nm with carbon atom(C38 and C41)in complex 2,0.193 7 and 0.193 7 nm with carbon atom(C25 and C36)in complex 3 respectively, However,the positions of the residual density peaks are unreasonable to name as any atoms.The crystal data and structure refinement details were summarized in Table 1.
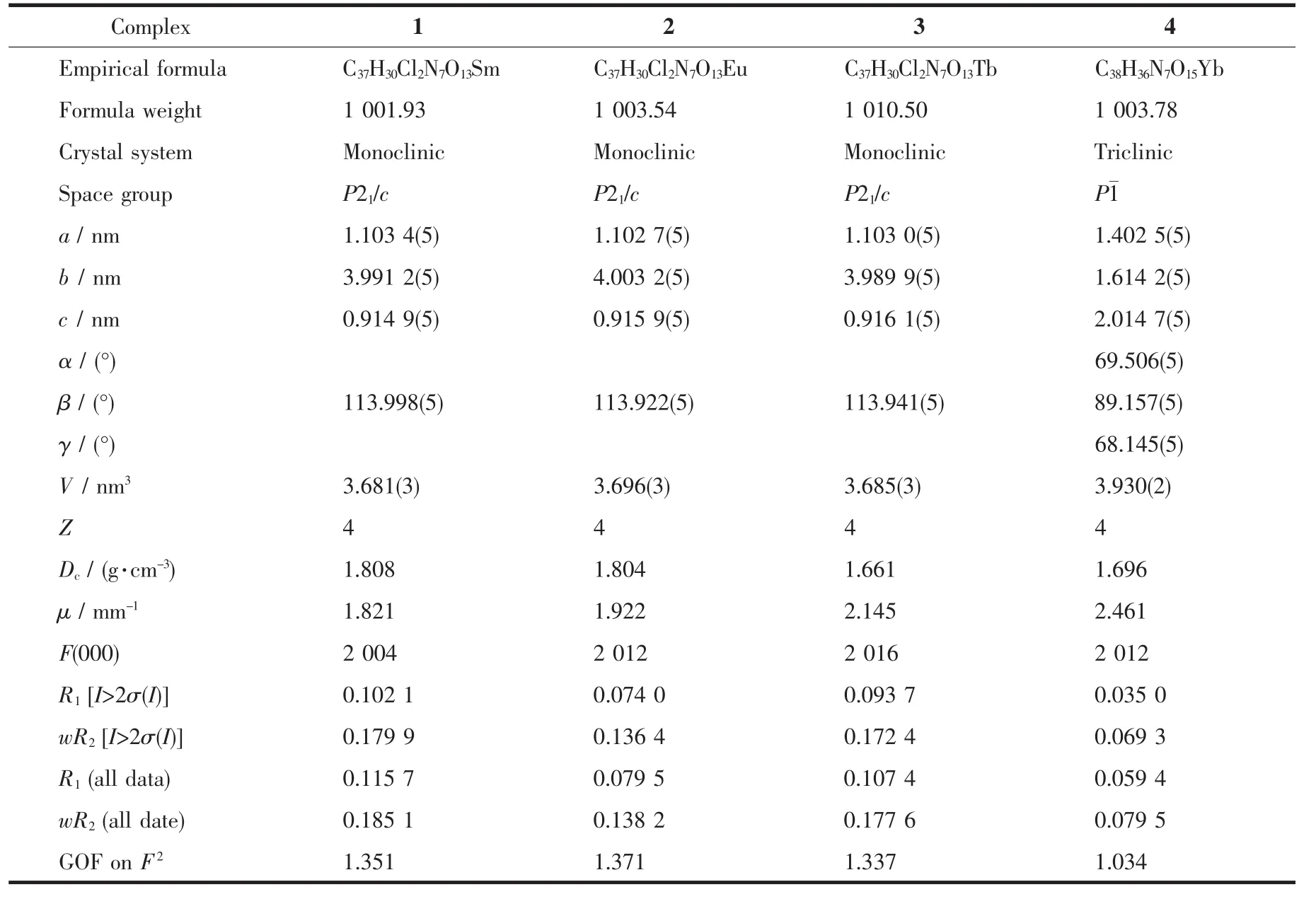
Table1 Crystal data and structures refinement for complexes 1~4
CCDC:1441075,1;1441076,2;1441077,3; 1441080,4.
2 Results and discussion
2.1Description of crystal structures
Structural analysis shows that complexes 1~3 are isostructural and crystallize in the monoclinic P21/c space group,while complex 4 crystallized in the triclinic P1 space group.In a typical structure of complex 1(Fig.1a),the Sm3+ion is ten-coordinated to four phenolate O atoms from two ligands and six O atoms from three bidentate nitrate groups adopting sphenocorona geometry(Fig.1b).The Sm-O bond lengths are in the range of 0.227 3(90)~0.269 0(107) nm in accordance with the reported values[14].The N atoms of the ligands remain exclusively uncoordinated.Notably,the two adjacent ligands attached to the Sm3+ions pack in an offset head-to-head fashion. The distinct strands comprised of Sm3+ions interact with each other through π-π interactions of the conjugated rings of adjacent ligands as shown in Fig. 1c.The naphthalene rings of two ligands are found to form π-π interactions in the range of 0.358 9~0.360 4 nm.Interestingly,complex 1 features with“butterflylike”structure attributed to π-π interactions of the naphthalene rings of two ligands(Fig.1d).The coordination mode of Ln3+ion in complex 4 is distinguished from complexes 1~3,more specifically,the Yb3+ion is nine-coordinated and accomplishes by nine O atoms,four phenolate O atoms from two ligands,five O atoms from two bidentate nitrate groups and one methanol molecule adopting distorted muffin geometry (Fig.2).It should be noticed that the coordination numbers reducing from ten-coordinated Ln3+ions(Sm, Eu and Tb)to nine-coordinated the Yb3+ion are attributed to the lanthanide contract,which is consistent with literatures[15].
2.2Luminescence properties
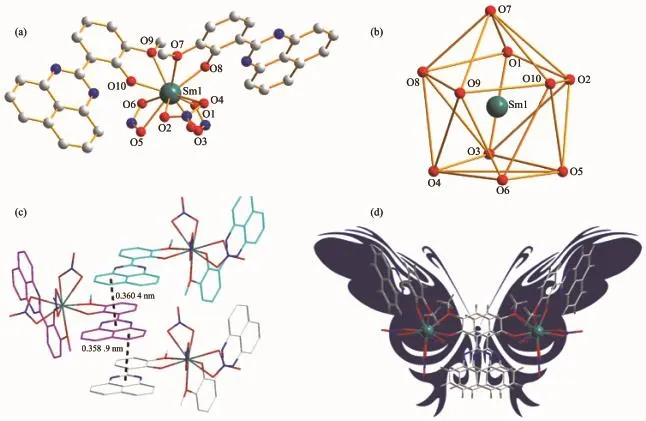
Fig.1(a)Molecular structures of complex 1;(b)Polyhedron view of the coordination geometry;(c)π-π interactions between the naphthalene rings of complex 1;(d)“Butterfly-like”structure of complex 1
NIR photoluminescence spectra of complex 4 was recorded both in solid and in CH3CN(1×10-5mol·L-1) under the excited wavelength of 380 nm at room temperature(Fig.3and Fig.S3).The emission spectra exhibit a wide band of characteristic luminescence of Yb3+ion in the range of 950~1 050 nm,which isassigned to the2F5/2→2F7/2transition.Remarkably,NIR luminescence intensity in solid is stronger than that in CH3CN.The resulting distinction between solid state and solution is mainly attributed to the solvent effect[16]. In addition to the steady-state emission,the timeresolved measurement was also carried out in the NIR region by using the time-correlated single photon counting(TCSPC)technique.The lifetimes for complex 4 show a satisfactory fit to a monoexponential lifetime with 8.27 μs in solid and 4.40 μs in CH3CN,respectively(Fig.S4).It is higher than the reported 4.22 μs in solid for complex[H2L3][Yb(HL3)2(NO3)4]2·2CH3OH·2H2O(HL3=2-(2′-hydroxyphenyl) benzimidazole)[12].The quantum yields of Φ=0.41%in solid and Φ=0.22%in CH3CN for Yb3+ion were estimated by ΦLn=τobs/τ0,where τobsis the observed emission lifetime and τ0is the“nature lifetime”,i.e. 2.0 ms for the Yb3+ion[17].However,the characteristic emissions corresponding to Sm3+,Eu3+and Tb3+ions were not observed in both solid and in CH3CN(1×10-5mol·L-1)at room temperature(Fig.S5).It suggests that the ligand is not able to sensitive the Sm3+,Eu3+and Tb3+ions.
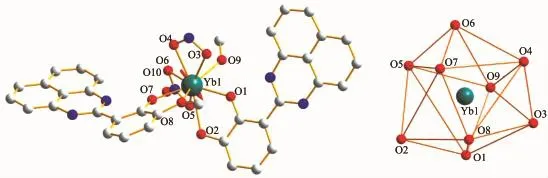
Fig.2 Molecular structure of complex 4 and polyhedron view of the coordination geometry
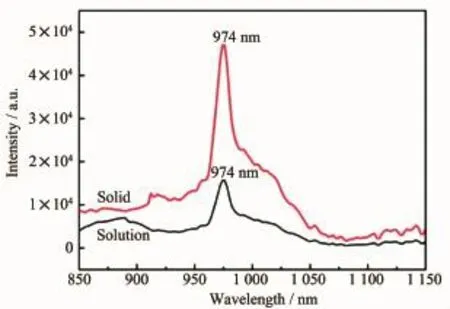
Fig.3 NIR emission spectra of complex 4 in solid and in CH3CN(1×10-5mol·L-1)excited at 380 nm
2.3Intramolecular energy transfer between ligand and Ln3+ions
According to the Dexter theory,the energy-level match between the triple state energy of the ligand and the resonance emission energy level of lanthanide ion is the most significant factor on determining the luminescence of the lanthanide complexes[18].The single(1ππ*)state energy level of H2L(32 258 cm-1, 310 nm)is estimated by referencing its absorbance edge(Fig.S3).The triplet(3ππ*)energy level of H2L (22 124 cm-1,452 nm),is calculated by referring to the lower wavelength emission peak of the corresponding phosphorescence spectra of Gd(HL)2(NO3)3·CH2Cl2(Fig.S6).According to Reinhoudt′s empirical rule[19], the intersystem crossing(ISC)process will be effective when the ΔE(1ππ*-3ππ*)is at least greater than 5 000 cm-1.The energy gap between the1ππ*and3ππ* states of H2L is 10 134 cm-1.Therefore,the ISC process is effective in this ligand,as shown in Fig.4.The energy differences between the triplet state of H2L (22 124 cm-1,452 nm)and the resonance energy level of Sm3+ion(4G5/2,17 910 cm-1),Eu3+ion(5D0,17 300 cm-1)and Tb3+ion(5D4,20 500 cm-1)are 1 294,4 824 and 1 624 cm-1,which are out of the optimized range of 2 500~3 200 cm-1,2 500~4 500 cm-1and 2 500~5 000 cm-1[20],respectively.Therefore,the characteristic luminescence of Sm3+ion,Eu3+ion and Tb3+ion in photoluminescence spectra were not detected for complexes 1,2 and 3 both in solid and CH3CN at room temperature(Table 2).Notably,the energy differences of 11 124 cm-1between the triplet state ofH2L(22 124 cm-1,452 nm)and the resonance energy level of Yb3+ion(2F5/2,11 000 cm-1)enable the characteristic luminescence of Yb3+ion to be observed both in solid and in CH3CN.

Table2 Energy differences between the triplet state of H2L and the resonance energy level of Ln3+(Sm3+,Eu3+and Tb3+)ions for complexes 1,2 and 3

Fig.4 Schematic energy level diagram and energy transfer processes for complex 4
3 Conclusions
Isolation of a series of four HL-lanthanide complexes 1~4 demonstrates that 2-(2′-hydroxyphenyl-3′-methoxyl)naphthoimidazole(HL)is able to coordinate to the lanthanide ions affording mononuclear lanthanide complexes.Photo-luminescent analysis reveals that HL is able to sensitive the characterize NIR luminescence of Yb3+ions in complex 4 but not Sm3+,Eu3+and Tb3+ions.
Acknowledgements:This work is financially supported by the National Natural Science Foundation of China(Grants No.51402092,51272069 and 21601132).
Supporting information is available at http://www.wjhxxb.cn
References:
[1]Yan L,Tan C,Zhang G,et al.J.Am.Chem.Soc.,2015,137: 8550-8556
[2]Xu B,Chen Q,Hu H,et al.Cryst.Growth Des.,2015,15: 2318-2329
[3]Bala S,Bishwas M S,Pramanik B,et al.Inorg.Chem.,2015, 54:8197-8201
[4]ZOU Xiao-Yan(鄒曉艷),MA Hui-Yuan(馬慧媛),PANG Hai -Jun(龐海軍),et al.Chinese J.Inorg.Chem.(無機化學學報),2016,32(9):16470-1652
[5]Dong H,Sun L,Yan C.Chem.Soc.Rev.,2015,44:16084-16089
[6]Li J,Chen L,Hao Z,et al.Inorg.Chem.,2015,54:4806-4812
[7]Herm Z R,Swisher J A,Smit B,et al.J.Am.Chem.Soc., 2011,133:5664-5671
[8]Li X J,Jiang F L,Wu M Y,et al.Inorg.Chem.,2012,51: 4116-4121
[9]Feng X,Ling X L,Liu L,et al.Dalton Trans.,2013,42: 10292-10299
[10]Yang X P,Jones R A,Oye M M,et al.New J.Chem.,2011, 35:310-319
[11]Yang X P,Jones R A,Wiester M J,et al.Cryst.Growth Des.,2010,10:970-977
[12]Chen P,Li Q,Chen S,et al.Inorg.Chem.Commun.,2012, 17:17-20
[13]Sheldrick G M.SHELXL-97,A Crystallographic Program for Structure Refinement,University of G?ttingen,Germany, 1997.
[14]Ma Y,Xu G F,Yang X,et al.Chem.Commun.,2010,46: 8264-8269
[15]Sun O,Gao T,Sun J W,et al.CrystEngComm,2014,16: 10460-10466
[16]Zhang H,Fan R,Chen W,et al.J.Lumin.,2013,143:611-615
[17]He H,Zhu X,Hou A,et al.Dalton Trans.,2004:4064-4068
[18]Ahmed Z,Iftikhar K.J.Phys.Chem.A,2013,117:11183-11187
[19]Steemers F J,Verboom W,Reinhoudt D N,et al.J.Am. Chem.Soc.,1995,117:9408-9415
[20]Latva M,Takalo H,Mukkala V M,et al.J.Lumin.,1997, 75:149-160
Crystal Structures and Near-IR Luminescence of Naphthoimidazole Lanthanide Complexes
HAO Wen-Hui1ZOU Xiao-Yan*,1,2FEI Bo-Wen1YAN Peng-Fei1DONG Yan-Ping1LI Guang-Ming*,1
(1Key Laboratory of Functional Inorganic Material Chemistry(MOE),School of Chemistry and Materials Science,Heilongjiang University,Harbin 150080,China)
(2Editorial Board of Journal of Engineering of Heilongjiang University,Harbi n 150080,China)
Self-assembly reactions of 2-(2′-hydroxyphenyl-3′-methoxyl)naphthoimidazole(HL)and Ln(NO)3·6H2O afford four mononuclear lanthanide complexes[Ln(HL)2(NO3)3]·CH2Cl2(Ln=Sm(1),Eu(2),Tb(3))and[Ln(HL)2(NO3)2(CH3OH)]NO3·CH3OH(Ln=Yb(4)).X-ray crystallographic analysis reveals that complexes 1~4 feature“butterfly-like”structure attributed to π-π interactions of the naphthalene rings of two ligands.Luminescent analysis reveals that only complex 4 exhibit essential NIR luminescence of Yb3+ion with lifetime of 8.27 μs in solid and 4.40 μs in CH3CN(c=1×10-5mol·L-1).CCDC:1441075,1;1441076,2;1441077,3;1441080,4.
synthesis;structure;lanthanide complexes;NIR luminescence
O614.33+7;O614.33+8;O614.341;O614.346
A
1001-4861(2016)11-2063-06
10.11862/CJIC.2016.260
2016-08-05。收修改稿日期:2016-10-11。
國家自然科學基金(No.51402092,51272069,21601132)資助項目。*通信聯系人。E-mail:zxy_18889@126.com,gmli_2000@163.com

