Paracrine factors for neurodegenerative disorders: special emphasis on Parkinson’s disease
PERSPECTIVE
Paracrine factors for neurodegenerative disorders: special emphasis on Parkinson’s disease
The progressive loss of dopaminergic neurons in the ventral mesencephalon is the main pathological hallmark of Parkinson’s disease (PD). Drugs currently available only alleviate the principal symptomatic motor-related disturbances and their benefit is counteracted by side effects in the long time. While cell replacement strategies for approaching PD by means of intrastriatal implantation of dopaminergic neurons showed some encouraging results in a number of patients this therapeutical approach aims primarily to replenish the lack of dopamine but not halting disease progression. Hence, over the past decades various strategies have been exploited to protect the dopaminergic neurons in the ventral mesencephalon from dying. Of special importance are in this context neurotrophic factors, drugs striving against oxidative stress and bioenergetic supplements. Particularly, several neurotrophic factors have been described to specifically increase the survival and/or growth of dopaminergic neurons in vitro and in vivo, including neurotrophins and glial cell line-derived neurotrophic factor (GDNF) family members.
The use of stem cells for tissue regeneration elicited hope for the development of better treatment options for many neuropathological conditions. Indeed, in the last decade a considerable number of studies have been conducted to explore the potential of progenitor and stem cells. Importantly to note, in experimental stroke models significant improvement of symptoms was observed, however, histological analyzes revealed that only a small portion of transplanted cells differentiated into mature neurons. Hence, the concept that these cells exert therapeutic actions by replacing defective cells by differentiating into multiple cell types has been gradually revised. It is now believed that the restorative effects observed in cell transplantation settings mainly rely on autocrine/paracrine activities (Andres et al., 2011). In line with this notion, improved tissue functionality is typically associated with low levels of cell engraftment and cell trans-differentiation (Drago et al., 2013). There is a general consensus that the trophic and immunomodulatory paracrine activities are not simple bystander but rather main players of tissue regeneration supported by progenitor/stem cells. Hence, in the present perspective we communicate on the composition and mechanisms of action of the factors secreted by stem/progenitor cells commonly defined as secretome (Liang et al., 2014).
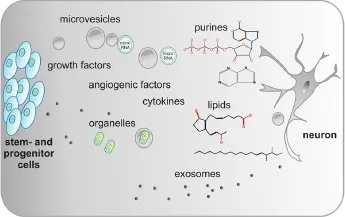
Figure 1 Schematic drawing of the major components in the secretome produced and released by various stem and progenitor cells.
The big variety of active elements released by cells can be tentatively classified in soluble factors and particulates. Classical growth factors and cytokines quantitatively predominate in the group of soluble factors that includes also lipids, extracellular matrix (ECM) com-ponents and nucleotides. Importantly to note, ECM components play an important role in juxtacrine signaling, however, in the present study we focus on its role in the secretome of stem/progenitor cells. The category of particulate factors is fundamentally composed of vesicular bodies, which are further distinguished in ectosomes and exosomes. Thereby, ectosomes range from 100 to 500 nm in diameter. Microvesicles arise from budding of the plasma membrane of a cell and their size range between 100 nm and 1,000 nm. Conversely, exosomes originate from an exocytosis process and are 30 nm to 100 nm sized (Anthony and Shiels, 2013). Microvesicles are important signaling mediators functioning as cargoes of variety of bioactive materials including genetic material (mRNA, microRNA, rRNA, and tRNA) and lipids (Choi et al., 2013). Interstingly, they can also shuttle mitochondria from one cell to the other (Spees et al., 2006). The importance of these particulates is highlighted by the fact that the portion of secreted proteins actually present both in microvesicles and exosomes may reach up 40% (Zullo et al., 2015) (Figure 1). Hence, it is important to note that a big variety of soluble factors and particulates are present in the secretome of stem/ progenitor cells.
Most of the current knowledge on the importance of soluble factors for preventing degeneration and promoting recovery has been gathered in studies using mesenchymal stem cells (MSC).
Different studies have provided indirect evidence that MSC mediate enhanced survival of nigral dopaminergic neurons and functional recovery in a parkinsonian model of rats by means of secreted factors (Wang et al., 2010). Additionally, the use of conditioned medium (CM) has allowed investigating more precisely the potential of paracrine secretions for tissue repair/protection. In this respect, it has been demonstrated that CM derived from bone marrow MSC supports the viability of rat primary dopaminergic neurons while preconditioning with CM enhances the survival of transplanted dopaminergic neurons in an animal model of PD (Shintani et al., 2007). Moreover, MSC-derived CM has been described to exert neuroprotective effects in vitro on dopaminergic and serotoninergic cells against oxidative stress (Whone et al., 2012) with GDNF acting as a crucial mediator. Interestingly, the protein DJ-1 whose gene mutations are closely associated with the development of PD, is protective for neuronal cells as well as primary dopaminergic neurons challenged respectively by ischemia and neurotoxins (Mullett and Hinkle, 2009; Kaneko et al., 2014) and has been found to be secreted by MSC, astrocytes, and neuronal progenitor cells.
There is compelling evidence that the tissue regenerative properties of MSC do not rely solely on the trophic and anti-apoptotic functions of neurotrophic factors. It is in fact recognized that the chemotactic stromal cell-derived factor-1 α secreted by MSC promote endogenous repair through the activation of resident neuronal stem cells (NSC) (Wang et al., 2010). Moreover, stimulation of vascular growth by released soluble angiogenic factors is another relevant mechanism for the repair of ischemic tissues, but also for ameliorating the progression of tissue degeneration in Alzheimer’s disease (AD) and PD. Furthermore, the capacity to modulate the inflammation by paracrine factors is of outmost importance to steer neuronal tissues towards damage or repair for all neurodegenerative conditions. In this respect, MSC secretevarious pro- and anti-inflammatory molecules. These include not only cytokines but also lipids as sphingosine-1-phosphate (S1P) and prostaglandins and their metabolizing enzymes. Importantly, alterations in the S1P signalling have been associated to the pathogenesis of AD while S1P administration has demonstrated neuroprotective effects on experimental models of AD and PD (Pyszko and Strosznajder, 2014).
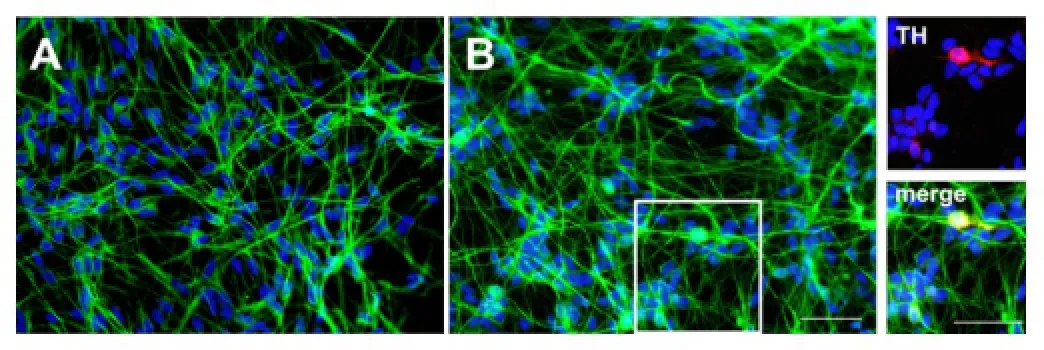
Figure 2 Photomicrographs of human midbrain cell cultures.
Nucleotides and nucleosides constitute an important group of paracrine messengers involved in cell growth/differentiation and immunomodulation in a number of physiological and pathological conditions (Cavaliere et al., 2015). Given that MSC and NSC release purines and express the purinergic receptors and ectonucleotide metabolizing machinery, it has been proposed that extracellular purines might be considered a valuable tool to promote neuronal tissue homeostasis and repair (Cavaliere et al., 2015). Furthermore, MSC and endothelial progenitor cells (EPC) secretomes are rich of ECM elements like heparan or chondroitin sulfate proteoglycans as well as metalloproteinases. In addition to the essential role in growth factor signaling and synaptic transmission, ECM directly modulates plasticity. So, a number of proteins, as for example SPARC and Cyr61 that bind to ECM, are important regulators of cellular functions (Estrada et al., 2009). These so called matricellular proteins are important constituents of the MSC secretome. It is thus not surprising that an altered ECM composition is involved in the development of many neurodegenerative disorders including AD, epilepsy and schizophrenia.
It is now well established that exosomes can exert cytoprotective effects. This phenomenon has been observed also for dopaminergic cells incubated with exosomes derived from MSC (Jarmalaviciute et al., 2015). A growing amount of observations have shown that the genetic transfer through mRNAs and/or miRNAs carried by microvesicles and exosomes is a crucial element in cell-to-cell communication and instructs both degenerative and reparative processes. The list of miRNA shuttled by the vesicles according to the cells of origin and the disease states is continuously updated and represents an extraordinary biological tool to harness tissue repair (Smith et al., 2015).
It is clear that the effects exerted by secretomes are likely the result of a interplay of multiple factors with a different biochemical nature including proteins, lipids, and nucleic acids, activating different downstream events at the cellular level. Due to this heterogeneous mode of action and assorted composition, predictions of targeted cell populations are challenging. Nevertheless, given that many of the factors present in the secretomes are finally dependent on their specific receptors to be operative multireceptor mapping may offer a novel tool for the identification of such targets.
Studies from our laboratory have shown that the secretome derived from EPC promotes angiogenesis, resistance against oxidative stress in endothelial cells but also the dif-ferentiation of neuronal stem/progenitor cells. Importantly to note in the context of PD, is the potential of EPC-derived CM to induce a dopaminergic phenotype in neurons of midbrain cultures (Figure 2). These effects which are mediated by proteinaceous and lipid factors are paradigmatic of pleiotropic actions of the secretome (Di Santo et al., 2016).
The use of secretome originated from different cell types is emerging in the field of tissue repair/regeneration due in part to advantages over cell transplantation. Notably, secretome-based therapies pose few concerns with regards to immunogenic reactions and oncogenicity. These features offer the advantage of an allogenic and off the shelf use. Thus, in consideration of the high scalability and the possible, countless modifications, secretomes can be considered an ideal interface between cellbased therapies and conventional drugs. It is, however, important to note that secretome-based approaches have also limitations and drawbacks. In addition to beneficial effects, the variety of active factors that can be found in secretomes can promote tissue fibrosis or even elicit inflammatory responses. Moreover, it has been reported that secretomes derived from senescent cells can in turn propagate senescence in neighbor cells; a phenomenon termed senescence associated secretory phenotype (Zullo et al., 2015).
In sum, the detailed characterization of the different secretomes including mechanisms of action will be an important topic for the full exploitation of their tissue regenerative potentials. Thus, new studies are needed to overcome the current limitations of secretome-based treatments and to achieve alternative and/or complementary therapeutic tools for neurodegenerative disorders.
This research was supported by the HANELA Foundation and the Swiss National Science Foundation, No. 31003A_135565 and 406340_128124.
Stefano Di Santo, Hans Rudolf Widmer*
Department of Neurosurgery, Neurocenter and Regenerative Neuroscience Cluster, University Hospital Bern, Switzerland University of Bern, Inselspital, CH-3010 Berne, Switzerland
*Correspondence to: Hans Rudolf Widmer, Ph.D., hanswi@insel.ch.
Accepted: 2016-03-10
orcid: 0000-0002-4032-999X (Hans Rudolf Widmer)
Andres RH, Horie N, Slikker W, Keren-Gill H, Zhan K, Sun G, Manley NC, Pereira MP, Sheikh LA, McMillan EL, Schaar BT, Svendsen CN, Bliss TM, Steinberg GK (2011) Human neural stem cells enhance structural plasticity and axonal transport in the ischaemic brain. Brain 134:1777-1789.
Anthony DF, Shiels PG (2013) Exploiting paracrine mechanisms of tissue regeneration to repair damaged organs. Transplant Res 2:10.
Cavaliere F, Donno C, D’Ambrosi N (2015) Purinergic signaling: a common pathway for neural and mesenchymal stem cell maintenance and differentiation. Front Cell Neurosci 9:211.
Choi DS, Kim DK, Kim YK, Gho YS (2013) Proteomics, transcriptomics and lipidomics of exosomes and ectosomes. Proteomics 13:1554-1571.
Di Santo S, Fuchs AL, Periasamy R, Seiler S, Widmer HR (2016) The cytoprotective effects of human endothelial progenitor cell-conditioned medium against an ischemic insult are VEGF and IL-8 dispensable. Cell Transplant doi:10.3727/096368916X690458.
Drago D, Cossetti C, Iraci N, Gaude E, Musco G, Bachi A, Pluchino S (2013) The stem cell secretome and its role in brain repair. Biochimie 95:2271-2285.
Estrada R, Li N, Sarojini H, An J, Lee MJ, Wang E (2009) Secretome from mesenchymal stem cells induces angiogenesis via Cyr61. J Cell Physiol 219:563-571.
Jarmalaviciute A, Tunaitis V, Pivoraite U, Venalis A, Pivoriunas A (2015) Exosomes from dental pulp stem cells rescue human dopaminergic neurons from 6-hydroxy-dopamine-induced apoptosis. Cytotherapy 17:932-939.
Kaneko Y, Shojo H, Burns J, Staples M, Tajiri N, Borlongan CV (2014) DJ-1 ameliorates ischemic cell death in vitro possibly via mitochondrial pathway. Neurobiol Dis 62:56-61.
Liang X, Ding Y, Zhang Y, Tse HF, Lian Q (2014) Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transplant 23:1045-1059.
Mullett SJ, Hinkle DA (2009) DJ-1 knock-down in astrocytes impairs astrocyte-mediated neuroprotection against rotenone. Neurobiol Dis 33:28-36.
Pyszko J, Strosznajder JB (2014) Sphingosine kinase 1 and sphingosine-1-phosphate in oxidative stress evoked by 1-methyl-4-phenylpyridinium (MPP+) in human dopaminergic neuronal cells. Mol Neurobiol 50:38-48.
Shintani A, Nakao N, Kakishita K, Itakura T (2007) Protection of dopamine neurons by bone marrow stromal cells. Brain Res 1186:48-55.
Smith JA, Leonardi T, Huang B, Iraci N, Vega B, Pluchino S (2015) Extracellular vesicles and their synthetic analogues in aging and age-associated brain diseases. Biogerontology 16:147-185.
Spees JL, Olson SD, Whitney MJ, Prockop DJ (2006) Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci U S A 103:1283-1288.
Wang F, Yasuhara T, Shingo T, Kameda M, Tajiri N, Yuan WJ, Kondo A, Kadota T, Baba T, Tayra JT, Kikuchi Y, Miyoshi Y, Date I (2010) Intravenous administration of mesenchymal stem cells exerts therapeutic effects on parkinsonian model of rats: focusing on neuroprotective effects of stromal cell-derived factor-1alpha. BMC Neurosci 11:52.
Whone AL, Kemp K, Sun M, Wilkins A, Scolding NJ (2012) Human bone marrow mesenchymal stem cells protect catecholaminergic and serotonergic neuronal perikarya and transporter function from oxidative stress by the secretion of glial-derived neurotrophic factor. Brain Res 1431:86-96.
Zullo J, Matsumoto K, Xavier S, Ratliff B, Goligorsky MS (2015) The cell secretome, a mediator of cell-to-cell communication. Prostaglandins Other Lipid Mediat 120:17-20.
10.4103/1673-5374.180739 http://www.nrronline.org/
How to cite this article: Di Santo S, Widmer HR (2016) Paracrine factors for neurodegenerative disorders: special emphasis on Parkinson’s disease. Neural Regen Res 11(4):570-571.
- 中國神經再生研究(英文版)的其它文章
- Gait deterioration due to neural degeneration of the corticoreticular pathway: a case report
- Complement components of nerve regeneration conditioned fluid influence the microenvironment of nerve regeneration
- Electrical stimulation of dog pudendal nerve regulates the excitatory pudendal-to-bladder reflex
- Supplementary motor area deactivation impacts the recovery of hand function from severe peripheral nerve injury
- Combined use of Y-tube conduits with human umbilical cord stem cells for repairing nerve bifurcation defects
- Senegenin inhibits neuronal apoptosis after spinal cord contusion injury

