Enantioseparation of 2-(substituted phenyl)propanoic acids with hydroxypropyl-β-cyclodextrin as a chiral additive:investigation of substituent influence on enantiorecognition
WANG Xiaoping, LU Mengxia, BU Zhisi, Lü Liqiong, TONG Shengqiang
(College of Pharmaceutical Science, Zhejiang University of Technology, Hangzhou 310032, China)
Article
Enantioseparation of 2-(substituted phenyl)propanoic acids with hydroxypropyl-β-cyclodextrin as a chiral additive:investigation of substituent influence on enantiorecognition
WANG Xiaoping, LU Mengxia, BU Zhisi, Lü Liqiong, TONG Shengqiang*
(CollegeofPharmaceuticalScience,ZhejiangUniversityofTechnology,Hangzhou310032,China)
2-(Substituted phenyl)propanoic acids were successfully enantioseparated by reversed-phase high-performance liquid chromatography with hydroxypropyl-β-cyclodextrin as a mobile phase additive. The effect of the mobile phase composition, including the aqueous buffer, the organic modifier, and the concentration of the additive, were investigated. The aqueous buffer pH, the type and percentage of the organic modifier, and the additive concentration had a great influence on the retention time and peak resolution. Enantioseparations were achieved on an YMC ODS-C18(150 mm×4.6 mm, 5 μm) column. The mobile phase was composed of acetonitrile and 0.10 mol/L of phosphate buffer at pH 3.3 containing 25 mmol/L of the hydroxypropyl-β-cyclodextrin additive. The binding constants of the inclusion complex between each of the 2-(substituted phenyl) propanoic acids and hydroxypropyl-β-cyclodextrin were determined, and the formation of the inclusion complex was investigated. The results showed that the stoichiometries for all of the inclusion complexes between hydroxypropyl-β-cyclodextrin and enantiomers were 1∶1. It was found that the electron-donating group of the enantiomer was advantageous for enantiorecognition by hydroxypropyl-β-cyclodextrin. The results provide a useful reference for the influence of the enantiorecognitionfactors produced by hydroxypropyl-β-cyclodextrin.
enantioseparation; high-performance liquid chromatography (HPLC); 2-(substituted phenyl)propanoic acids; hydroxypropyl-β-cyclodextrin
2-(Substituted phenyl)propanoic acids are important intermediates for synthesis of non-steroidal phenyl propanoic acid antipyretic analgesic and anti-inflammatory drugs. Different pharmacological activities, mechanisms, and toxicities exist between theR- andS-enantiomers. Accordingly, the enantioseparation of 2-(substituted phenyl)propanoic acids has become an important issue [1]. An increasing number of studies regarding enantioseparation of 2-(substituted phenyl)propanoic acids by liquid chromatography have become available, such as enantioseparation by chiral stationary phases [2-7] and chiral mobile phase additives [8,9]. As chiral stationary phases are generally expensive, the application of a chiral mobile phase additive is an alternative method for enantioseparation owing to its relatively low cost. In the current work, a method is presented for enantioseparation of four racemic 2-(substituted phenyl)propanoic acids via high-performance liquid chromatography (HPLC) with the chiral mobile phase additive hydroxypropyl-β-cyclodextrin, including 2-(4-nitrophenyl)propanoic acid, 2-(4-methylphenyl)propanoic acid, 2-(4-hydroxyphenyl)propanoic acid, and 2-(4-chlorophenyl)propanoic acid.
A method for enantioseparation of eight nonsteroidal anti-inflammatory drugs by reversed-phase HPLC has been established [9]. Hydroxypropyl-β-cyclodextrin was added as a chiral mobile phase additive, and stereoselective skin permeation of different enantiomers were investigated. All of the evaluated racemic drugs were 2-(substituted phenyl)propanoic acids. The major difference in their chemical structures was the substituent on the benzene ring of 2-phenylpropionic acid. Additionally, major variance in enantiorecognition for each racemate was found when investigating enantioseparation of these racemates by HPLC with hydroxypropyl-β-cyclodextrin as a chiral additive. Meanwhile, in our recent studies [10-12], high-speed countercurrent chromatography was used for preparative enantioseparation of some 2-(substituted phenyl)propanoic acids. We determined the distribution ratio and enantioseparation factor for all the racemates by enantioselective liquid-liquid extraction. It was found that the racemates with an electron-donating group on the benzene ring presented a higher enantiorecognition produced by the same chiral selector hydroxypropyl-β-cyclodextrin than that of racemates with an electron-withdrawing group on the benzene ring [10]. In the present work, in order to further verify the abovementioned results, enantioseparation of the four racemic 2-(substituted phenyl)propanoic acids along with four racemic nonsteroidal anti-inflammatory drugs (suprofen, ibuprofen, ketoprofen, and naproxen)were investigated. It was carried out by reversed-phase HPLC with hydroxypropyl-β-cyclodextrin as a chiral mobile phase additive. Enantiorecognition between the enantiomers and chiral additive was evaluated by determining binding constants for the different inclusion complexes. The separation mechanism of HPLC is different than that of high-speed countercurrent chromatography; yet, enantiorecognition between the enantiomers and hydroxypropyl-β-cyclodextrin could be well evaluated by the determination of binding constant ratios for the inclusion complexes from enantioseparation by liquid chromatography. The binding constant ratios of the inclusion complexes could be compared with enantioseparation factors obtained from countercurrent chromatography. Fig. 1 shows the chemical structures of the eight 2-(substituted phenyl)propanoic acids.
1 Experimental
1.1 Chemicals and reagents
2-(4-Nitrophenyl)propanoic acid, 2-(4-methylphenyl)propanoic acid, 2-(4-hydroxyphenyl)propanoic acid, 2-(4-chlorophenyl)propanoic acid, suprofen, ibuprofen, ketoprofen, and naproxen were purchased from J&K Scientific Ltd., Shanghai, China. Hydroxypropyl-β-cyclodextrin was purchased from Qianhui Fine Chemical Co. Inc., Shandong, China. Analytical grade sodium dihydrogen phosphate, phosphoric acid, acetic acid, and sodium acetate were purchased from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China. Chromatographic grade acetonitrile and methanol used for HPLC were purchased from Sigma-Aldrich, Shanghai, China. Water used for HPLC was redistilled by the distillation unit purchased from Shanghai Huxi Analysis Instrument Factory Co., Ltd., Shanghai, China.

Fig. 1 Chemical structures of the eight 2-(substituted phenyl)propanoic acids
1.2 Apparatus
The chromatographic studies were performed on a Shimadzu HPLC LC-solution system. The instrument was composed of a Shimadzu LC-10Avp UV detector, a SCL-10Avp controller, a Shimadzu LC-10ATvp multisolvent delivery system, and a dual Shimadzu LC pump. The pH value was determined with a Delta 320-s pH meter, Shanghai, China.
1.3 Chromatographic conditions
The chiral separations of all the analytes were performed on an YMC ODS C18column (150 mm×4.6 mm i. d., 5 μm, Shanghai, China). The mobile phase was composed of acetonitrile and 0.10 mol/L phosphate buffer (pH 3.3) containing 25 mmol/L of hydroxypropyl-β-cyclodextrin. It was filtered through a 0.45-μm filter and degassed for 20 min prior to use. All of the analytes were detected at 212 nm. The temperature of the column was 35 ℃. The sample injection volume was 20 μL.
1.4 Preparation of stock solution
Each racemate of the 2-(substituted phenyl)propanoic acids was accurately weighed, transferred to volumetric flasks, dissolved in methanol, and stored at 5 ℃. The concentration of each solution was approximately 0.5 mg/mL.
2 Results and discussion
2.1 Chromatographic conditions for method development
Enantioseparation of four 2-(substituted phenyl)propanoic acids, including 2-(4-methylphenyl)propanoic acid, 2-(4-hydroxyphenyl)propanoic acid, 2-(4-chlorophenyl)propanoic acid, and 2-(4-nitrophenyl)propanoic acid, by HPLC using hydroxypropyl-β-cyclodextrin as a chiral mobile phase additive was investigated. The main influence factors, organic modifier and concentration of the chiral additive, were investigated with regard to peak resolution.
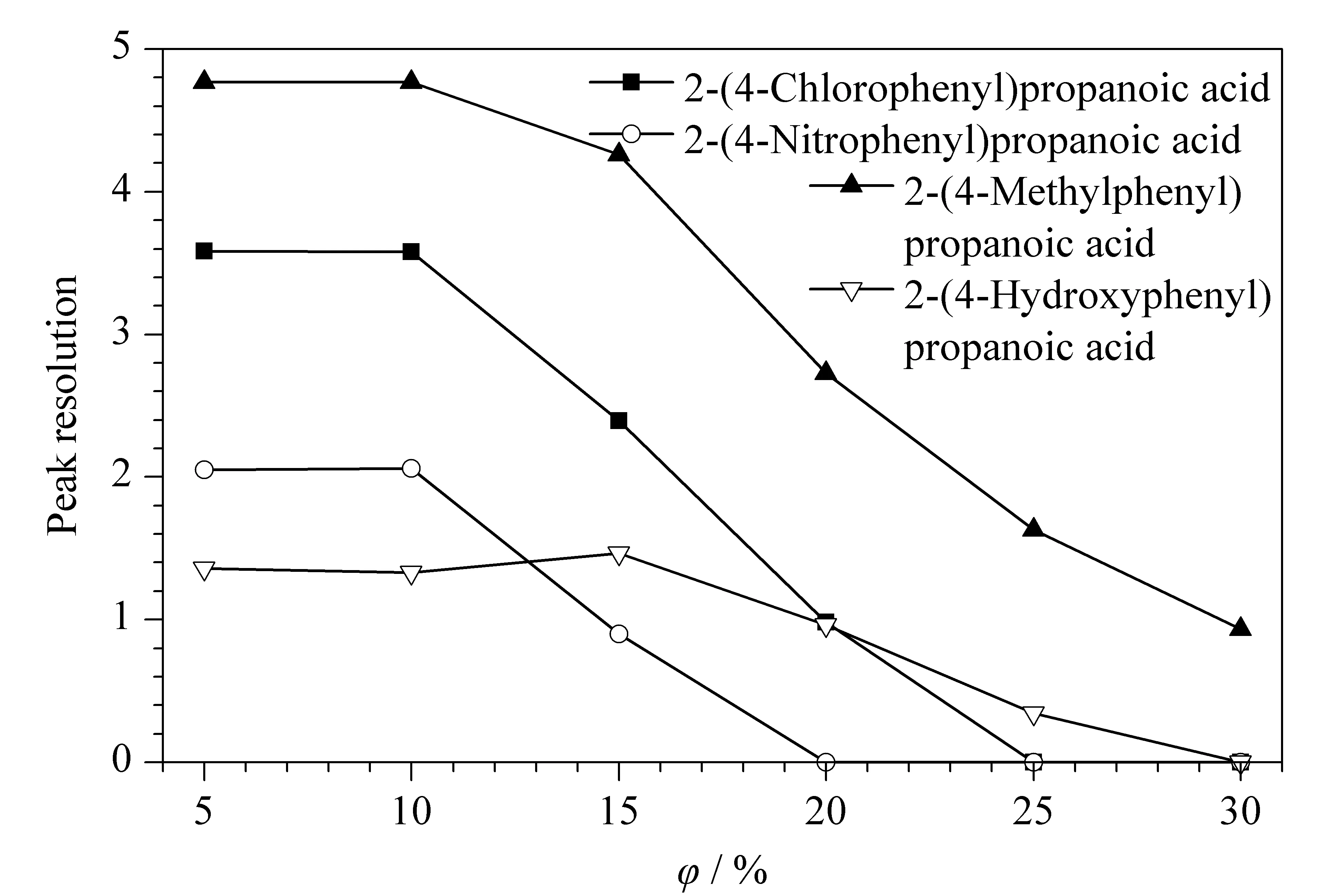
Fig. 2 Effects of the volume percentage of acetonitrile on peak resolutionAqueous phase: 0.10 mol/L of phosphate buffer (pH 3.3) containing 25 mmol/L of hydroxypropyl-β-cyclodextrin; flow rate: 0.8 mL/min; wavelength: 212 nm; column temperature: 35 ℃.
First, the type and percentage of the organic modifier were investigated. Methanol and acetonitrile are two frequently used organic modifiers. A shorter retention time, higher theoretical plates, better peak shape, and a slightly higher peak resolution was obtained with acetonitrile as the modifier compared to methanol. Moreover, the percentage of acetonitrile in the mobile phase had a great effect on the retention time and peak resolution. The results showed that a longer retention time and higher peak resolution were obtained with a low volume percentage of acetonitrile. For example, the retention time for enantiomers of 2-(4-chlorophenyl)propanoic acid was 75.81 min and 94.08 min with 5% of acetonitrile compared with 61.46 min and 67.16 min with 20% of acetonitrile, while the peak resolution was approximately 3.58 with 5% of acetonitrile compared with 0.984 with 20% of acetonitrile. As shown in Fig. 2, the peak resolution decreased with increasing concentration of acetonitrile. Therefore, suitable acetonitrile volume percentages of 10%, 20%, 5%, and 15% were chosen for 2-(4-nitrophenyl)propanoic acid, 2-(4-methylphenyl)propanoic acid, 2-(4-hydroxyphenyl)propanoic acid, and 2-(4-chlorophenyl)propanoic acid, respectively.

Fig. 3 Effects of the concentration of hydroxypropyl-β-cyclodextrin on peak resolutionChromatographic conditions: 0.10 mol/L of phosphate buffer (pH 3.3) containing different concentrations of hydroxypropyl-β-cyclodextrin and acetonitrile, (90∶10, v/v) for 2-(4-nitrophenyl)propanoic acid, (80∶20, v/v) for 2-(4-methylphenyl)propanoic acid, (95∶5, v/v) for 2-(4-hydroxyphenyl)propanoic acid, and (85∶15, v/v) for 2-(4-chlorophenyl)propanoic acid; flow rate: 0.4 mL/min for 2-(4-hydroxyphenyl)propanoic acid and 0.8 mL/min for 2-(4-nitrophenyl)propanoic acid, 2-(4-methylphenyl)propanoic acid, and 2-(4-chlorophenyl)propanoic acid; detection wavelength: 212 nm; column temperature: 35 ℃.

Fig. 4 Effects of mobile phase pH on peak resolutionMobile phase: 0.5% (v/v) acetate buffer (pH 3.5-5.5) and 0.10 mol/L of phosphate buffer (pH 2.5-3.3) containing 25 mmol/L of hydroxypropyl-β-cyclodextrin and methanol (85∶15, v/v); flow rate: 0.8 mL/min; wavelength: 212 nm; column temperature: 35 ℃.
Then, the effects of the chiral additive concentration on the enantioseparation were investigated. Fig. 3 shows the effects of hydroxypropyl-β-cyclodextrin concentration on peak resolution. The results showed that the peak resolution increased with increasing concentration of hydroxypropyl-β-cyclodextrin (HP-β-CD) for 2-(4-nitrophenyl)propanoic acid, 2-(4-methylphenyl)propanoic acid, and 2-(4-chlorophenyl)propanoic acid. However, it was found that the peak resolution of 2-(4-hydroxyphenyl)propanoic acid decreased owing to its large polarity. A higher concentration of the additive generally led to higher column pressure. Hence, 25 mmol/L of hydroxypropyl-β-cyclodextrin was selected.
Lastly, the pH value and flow rate of the mobile phase were investigated. As all of the racemates possessed a carboxyl group, the peak resolution was strongly influenced by the pH value of the mobile phase. The results showed that the higher the pH of the buffer, the lower the peak resolution observed for all racemates, as shown in Fig. 4. Though this effect of pH on enantioseparation of 2-arylpropionic acid derivatives using cyclodextrins as chiral selectors has been known, different analytes were tested in our work. Therefore, the pH of the mobile phase was kept below 4.0. A flow rate of 0.8 mL/min was suitable for all racemates except for 2-(4-hydroxyphenyl)propanoic acid, which was eluted early owing to its chemical structure and large polarity resulting in poor peak resolution. However, when the flow rate was set at 0.4 mL/min, a satisfactory peak resolution (Rs=1.52) for 2-(4-hydroxyphenyl) propanoic acid was obtained.
Table 1 and Fig. 5 show the optimized chromatographic conditions and resultant chromatogram for enantioseparation of the four 2-(substituted phenyl)propanoic acids.
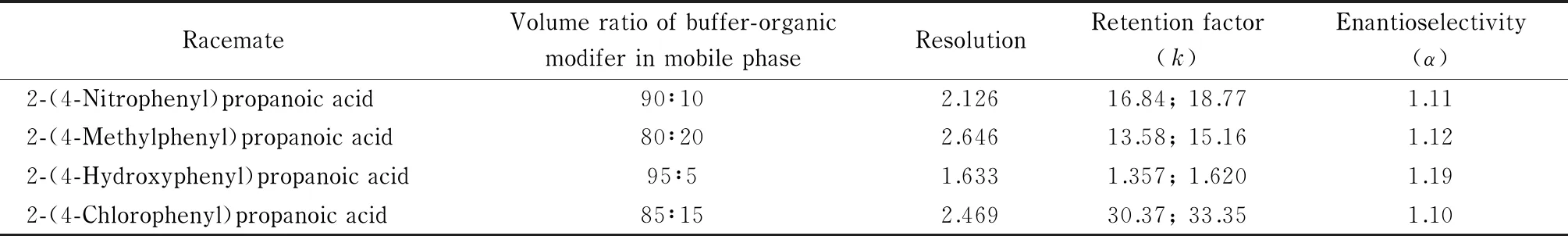
Table 1 Optimized chromatographic conditions for enantioseparation of four 2-(substituted phenyl)propanoic acids
Mobile phase: 0.10 mol/L of phosphate buffer (pH 3.3) containing 25 mmol/L hydroxypropyl-β-cyclodextrin and acetonitrile; flow rate: 0.8 mL/min for all racemates except 2-(4-hydroxyphenyl)propanoic acid (0.4 mL/min); wavelength: 212 nm; column temperature: 35 ℃.
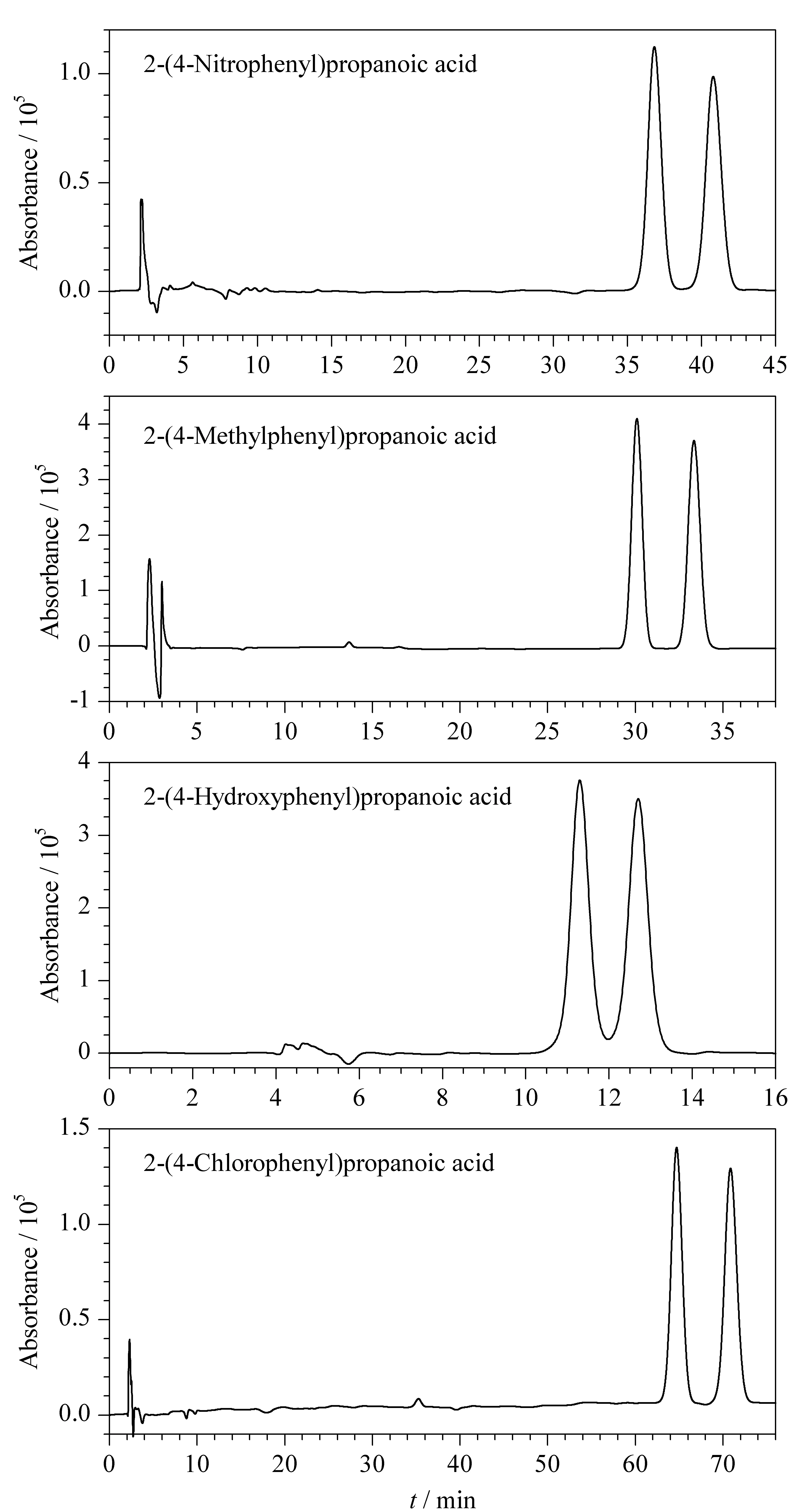
Fig. 5 Chromatograms of the enantioseparation of 2- (substituted phenyl)propanoic acids by reversed-phase HPLC with hydroxypropyl-β-cyclodextrin as a chiral mobile phase additive under optimized chromatographic conditions
2.2 Investigation of the influence of substituents on enantiorecognition
In enantioseparation by HPLC using a chiral mobile phase additive, enantiorecognition between hydroxypropyl-β-cyclodextrin and enantiomers may be greatly affected by the percentage of organic modifier. Generally, a higher enantiorecognition can be achieved with a lower concentration of organic modifier because the organic modifier in the mobile phase competitively forms an inclusion complex with hydroxypropyl-β-cyclodextrin. Therefore, a higher peak resolution is generally always observed for a racemate with a lower concentration of the organic modifier. As shown in Table 1, the highest peak resolution of 2.646 was obtained for 2-(4-methylphenyl)propanoic acid even with the highest percentage of organic modifier (20%) in the mobile phase among all of the racemates, which indicated that the best enantiorecognition occurred between enantiomers of 2-(4-methylphenyl)propanoic acid and hydroxypropyl-β-cyclodextrin among all the racemates examined. Interestingly, the racemate peak resolution of 2-(4-methylphenyl)propanoic acid, 2-(4-chlorophenyl)propanoic acid, and 2-(4-nitrophenyl)propanoic acid decreased successively, although the percentage of organic modifier used in the optimized conditions decreased (as shown in Table 1), which indicated that enantiorecognition between the enantiomers of each of the three racemates and hydroxypropyl-β-cyclodextrin decreased successively. Enantiorecognition between the enantiomers and hydroxypropyl-β-cyclodextrin may be greatly affected by the size, polarity, and electronegativity of the differing benzene ring substituents. Since the chromatographic conditions were almost identical, the difference in enantiorecognition might be caused by the different benzene ring substituents of the analytes. It was found that methyl and chloro radicals were electron-donating groups but nitro was a typical electron-withdrawing group. The chloro radical may be thought of as an electron-donating group in this case because of the high electronegativity of carboxyl on the para-position. As for 2-(4-hydroxyphenyl)propanoic acid, though the hydroxyl was an electron-donating group, the peak resolution for this analyte was pretty low, which was mainly caused by early elution owing to its chemical structure and high polarity.
It could be proposed that high enantiorecognition may be obtained by 2-(substituted phenyl) propanoic acids with an electron-donating group on the benzene ring. To further testify the validity of this proposal, the four nonsteroidal anti-inflammatory drugs were investigated. In order to evaluate enantiorecognition ability between different enantiomers and hydroxypropyl-β-cyclodextrin, binding constants of the inclusion complexes between host and guest molecule were investigated. Binding constants could be well determined by the retention equation in HPLC [13]. Generally, the stoichiometry of the inclusion complex between enantiomers and cyclodextrin was 1∶1. The retention equation could be expressed as:
(1)
wherekis the chromatographic definition of retention factor,φis the phase ratio, [A] is the concentration of stationary phase adsorption site, [CD] is the concentration of cyclodextrin molecule, andK1is the equilibrium constant. Eq (1) gives the retention behavior of the analytes bound with the cyclodextrins, which shows the stoichiometry of the inclusion complexes to be 1∶1. The binding constants for the inclusion complexes could be determined using Eq (1).
Table 2 shows the regression equation of 1/kand the concentration of the cyclodextrin. All of the correlation coefficients of plots were greater than 0.99, indicating that the stoichiometry of all inclusion complexes was 1∶1.
The value of the binding constant ratio of each racemate listed in Table 2 indicated that the enantiorecognition ability between each enantiomer and hydroxypropyl-β-cyclodextrin was different. Moreover, the sequence of the binding constant ratios of the eight 2-(substituted phenyl)propanoic acids further indicated that high enantiorecognition between hydroxypropyl-β-cyclodextrin and 2-(substituted phenyl)propanoic acids would most likely be obtained when an electron-donating group is on the benzene ring of 2-(substituted phenyl)propanoic acids.
3 Conclusions
The enantioseparation of four 2-(substituted phenyl)propanoic acids by reversed-phase HPLC with hydroxypropyl-β-cyclodextrin as a chiral mobile phase additive was established. The ratios of binding constants of eight 2-(substituted phenyl)propanoic acids were determined, and the enantiorecognition between each enantiomer and chiral additive was evaluated. The results showed that an electron-donating group on the benzene ring is advantageous for enantiorecognition, which led to a relatively high ratio of binding constants. The results obtained in our current work were in agreement with the countercurrent chromatography results from our previous work.
[1] Evans A M. Eur J Clin Pharmacol, 1992, 42: 237
[2] Wang R Q, Ong T T, Tang W H, et al. Anal Chim Acta, 2012, 718: 121
[3] Zhong Q Q, He L F, Beesley T E, et al. J Chromatogr A, 2006, 1115: 19
[4] Wang Y, Ong T T, Li L S, et al. J Chromatogr A, 2009, 1216: 2388
[5] Overbeke A V, Baeyens W, Dewaele C. Anal Chim Acta, 1996, 321: 245
[6] Luo A, Wan Q, Fan H J, et al. Chinese Journal of Chromatography, 2014, 32(9): 1013
[7] Wang M. Chinese Journal of Chromatography, 2014, 32(2): 198
[8] Zheng Y, Yan J Z, Tong S Q, et al. Chinese Journal of Pharmaceutical Analysis, 2013, 33(4): 827
[9] Ye J C, Yu W Y, Chen G S, et al. Biomed Chromatogr, 2010, 24: 799
[10] Tong S Q, Wang X P, Lu M X, et al. J Sep Sci, 2016, 39: 1567
[11] Tong S Q, Guan Y X, Yan J Z, et al. J Chromatogr A, 2011, 1218: 5434
[12] Tong S Q, Zheng Y, Yan J Z. J Chromatogr A, 2013, 1281: 79
[13] Armstrong D W, Nome F, Spino L A, et al. J Am Chem Soc, 1986, 108: 1418
以羥丙基-β-環糊精為手性添加劑拆分2-取代芳基丙酸:取代基對手性識別的影響
王小平, 魯夢霞, 步知思, 呂力瓊, 童勝強*
(浙江工業大學藥學院, 浙江 杭州 310032)
以羥丙基-β-環糊精為手性添加劑,采用反相高效液相色譜法對2-取代芳基丙酸類物質進行了手性拆分。考察了流動相的組成,包括緩沖溶液、有機改性劑以及添加劑的濃度等。緩沖溶液的pH值、有機改性劑的種類與濃度,以及添加劑的濃度對色譜峰的保留時間和分離度均有較大的影響。以YMC ODS-C18(150 mm×4.6 mm, 5 μm)為色譜柱,乙腈-0.10 mol/L磷酸鹽緩沖液(pH 3.3,含25 mmol/L添加劑)為流動相,測定了各2-取代芳基丙酸與羥丙基-β-環糊精的包結常數,考察了羥丙基-β-環糊精對各物質的包結形式。實驗結果表明,羥丙基-β-環糊精與各對映體均以1∶1的形式包結,同時發現推電子取代基更有利于羥丙基-β-環糊精的包結行為,為羥丙基-β-環糊精對手性拆分的影響提供了一個有利的參考因素。
手性分離;高效液相色譜;2-取代芳基丙酸;羥丙基-β-環糊精
10.3724/SP.J.1123.2016.12020
Foundation item: Project of Department of Education of Zhejiang Province, China (No. pd2013031).
O658 Document code: A Article IC:1000-8713(2017)05-0544-07
* Received date: 2016-12-09
* Corresponding author. Tel: +86-571-88320984, Fax: +86-571-88320913, E-mail: sqtong@zjut.edu.cn.

