二苯乙烯苷對淀粉樣前體蛋白/早老蛋白-1雙轉基因小鼠腦組織半胱氨酸天冬氨酸蛋白酶3和淀粉樣前體蛋白表達的影響
劉 寧,廖艷花,賴 術,黃 健,黎 昀,黃忠仕
533000廣西百色市,右江民族醫學院
·論著·
二苯乙烯苷對淀粉樣前體蛋白/早老蛋白-1雙轉基因小鼠腦組織半胱氨酸天冬氨酸蛋白酶3和淀粉樣前體蛋白表達的影響
劉 寧,廖艷花,賴 術,黃 健,黎 昀,黃忠仕*
533000廣西百色市,右江民族醫學院
目的 觀察二苯乙烯苷(TSG)對淀粉樣前體蛋白(APP)/早老蛋白-1(PS1)雙轉基因小鼠腦組織半胱氨酸天冬氨酸蛋白酶3(caspase-3)、APP表達水平的影響,探討其防治AD的可能機制。方法 2014年10月,將50只SPF級、雄性、APP/PS1雙轉基因小鼠按照隨機數字表法分為模型組、石杉堿甲組、TSG低劑量組、TSG中劑量組、TSG高劑量組,每組10只。并選取10只SPF級、雄性、C5B7L/6J小鼠為正常對照組。石杉堿甲組小鼠灌胃給予石杉堿甲0.020 mg/kg,TSG低劑量組、TSG中劑量組、TSG高劑量組小鼠分別灌胃給予TSG 0.033、0.100、0.300 g/kg,模型組、正常對照組小鼠灌胃給予0.9%氯化鈉溶液10.000 ml/kg;1次/d,連續給藥60 d。采用免疫組化法檢測各組小鼠腦皮質和海馬區caspase-3陽性細胞數,Western blotting法檢測各組小鼠腦組織APP表達水平。結果 正常對照組、石杉堿甲組、TSG低劑量組、TSG中劑量組、TSG高劑量組小鼠腦皮質、海馬區caspase-3陽性細胞數少于模型組(P<0.05);TSG低劑量組、TSG中劑量組、TSG高劑量組小鼠腦皮質、海馬區caspase-3陽性細胞數與石杉堿甲組比較,差異無統計學意義(P>0.05)。正常對照組、石杉堿甲組、TSG低劑量組、TSG中劑量組、TSG高劑量組小鼠腦組織APP表達水平均低于模型組(P<0.05);TSG低劑量組、TSG中劑量組、TSG高劑量組小鼠腦組織APP表達水平與石杉堿甲組比較,差異無統計學意義(P>0.05)。結論 TSG對APP/PS1雙轉基因小鼠有明顯的治療作用,其防治AD的機制可能與抑制caspase-3和APP表達、減少β淀粉樣蛋白產生、減緩神經元凋亡等機制有關。
阿爾茨海默病;二苯乙烯苷;半胱氨酸天冬氨酸蛋白酶3;淀粉樣前體蛋白分泌酶類
阿爾茨海默病(AD)是一種以記憶障礙為主要臨床表現、病程呈進行性發展的中樞神經變性病。目前中國55歲以上人群中,AD患病率已超過2.2%[1]。臨床上治療AD的藥物種類較多,但均僅能改善患者的臨床癥狀,而不能控制病情進展,因此,尋求療效確切且能控制病情發展的藥物成為當今研究的熱點。二苯乙烯苷(TSG)是何首烏中主要的生物活性成分,屬多羥基芪類化合物,具有保護神經、提高學習記憶力、抗衰老、防治AD、舒張血管、保護肝臟、降血脂、抗炎、抗腫瘤等多種生物活性[2-3]。石杉堿甲是從中草藥石杉中提取分離到的一種高活性的倍半萜類生物堿,相對分子量小,脂溶性好,容易透過血-腦脊液屏障,口服生物利用度高,口服吸收后在腦內主要分布于海馬區、額葉和顳葉等部位。多項研究表明,石杉堿甲對輕、中度AD有確切的臨床療效[4-5]。石杉堿甲治療AD的作用機制除了與抑制膽堿酯酶活性有關外,還與其抑制神經元凋亡有關[6-7]。
本課題組前期研究也發現,TSG能提高SAMP8鼠的學習記憶能力[8],抑制AD模型鼠腦組織中淀粉樣前體蛋白(APP)mRNA和早老蛋白-1(PS1)mRNA的表達[9-10]。本課題組前期研究初步表明TSG對癡呆動物模型有明顯的防治療效,但其對半胱氨酸天冬氨酸蛋白酶(caspase)-3表達水平是否有影響,尚未見相關報道。本研究擬在前期研究的基礎上以APP/PS1雙轉基因小鼠為模型,觀察TSG對其腦組織caspase-3、APP表達水平的影響,分析TSG防治AD的可能機制,為TSG的臨床應用提供實驗依據。
1 材料與方法
1.1 實驗材料
1.1.1 實驗動物 50只APP/PS1雙轉基因小鼠和10只C5B7L/6J小鼠,均為雄性,SPF級,平均體質量(28±2)g,3月齡,均由北京華阜康生物科技股份有限公司提供〔許可證號:SCXK(京)2014-0004〕。
1.1.2 主要試劑 TSG(成都克洛瑪生物科技有限公司,批號:14112),純度≥70%;石杉堿甲(河南太龍藥業股份有限公司,批號:H10940156);二步法免疫組化檢測試劑盒(北京中杉金橋生物技術有限公司,批號:20150223);RIPA裂解液(產品編號P0013B)、十二烷基硫酸鈉-聚丙烯酰胺凝膠電泳(SDS-PAGE)凝膠配制試劑盒(產品編號P0012A)、免疫染色洗滌液(產品編號P0106)、特超敏ECL化學發光試劑盒(產品編號P0018A)及顯影定影試劑盒(產品編號P0020)均購自上海碧云天生物技術有限公司;PageRuler Plus Prestained Protein Ladder(Thermo Scientific公司,#26619);0.45 μm聚偏氟乙烯(PVDF)膜(Millipore公司,產品編號IPVH00010);caspase-3兔抗小鼠單克隆抗體(#9579)、APP多克隆抗體(#2452)、β-actin兔單克隆抗體(#8457)及HRP標記抗兔IgG(#7074)均購自美國Cell Signaling Technology公司。
1.1.3 主要儀器 HMIAS-2000W 高清彩色圖文分析系統(LO-GENE-I型)由右江民族醫學院附屬醫院病理科提供。BIO-RAD的電泳/轉膜儀(型號:041BR60422)、Mini型垂直電泳槽(型號:552BR068856)、濕法轉膜槽(型號:153BR57570)均由右江民族醫學院科學實驗中心提供。
1.2 實驗方法
1.2.1 動物分組與給藥 2014年10月,將50只APP/PS1雙轉基因小鼠按照隨機數字表法分為模型組、石杉堿甲組、TSG低劑量組、TSG中劑量組、TSG高劑量組,每組10只。并選取10只C5B7L/6J小鼠為正常對照組。石杉堿甲組灌胃給予石杉堿甲0.020 mg/kg,TSG低劑量組、TSG中劑量組、TSG高劑量組分別灌胃給予TSG 0.033、0.100、0.300 g/kg,模型組和正常對照組灌胃給予0.9%氯化鈉溶液10.000 ml/kg;1次/d,連續給藥60 d。
1.2.2 標本采集 治療2個月后,采用頸椎脫臼處死小鼠,斷頭,冰上迅速取出腦組織,每組隨機抽取4只小鼠的腦組織放入10%甲醛溶液中固定,剩余的小鼠腦組織裝入凍存管,用液氮罐轉移至-80 ℃冰箱保存用于后續實驗。
1.2.3 免疫組化法檢測caspase-3陽性細胞 10%甲醛溶液固定1周后的腦組織進行石蠟包埋、海馬區冠狀切片。切片經二甲苯脫蠟,梯度乙醇水化,0.01 mol/L枸櫞酸緩沖液于壓力鍋中加熱至沸騰2 min修復抗原,磷酸鹽緩沖液(PBS)沖洗3次,2 min/次;免疫染色封閉液封閉15 min(甩干);滴加caspase-3兔抗小鼠單克隆抗體(免疫染色一抗稀釋液1∶250稀釋),4 ℃孵育過夜,免疫染色洗滌液沖洗3次,2 min/次;HRP標記抗兔IgG孵育15 min,免疫染色洗滌液沖洗6次,2 min/次;DAB顯色5~8 min、蘇木素復染、從低到高濃度乙醇脫水、二甲苯透明、封固、觀察。DAB顯色后caspase-3陽性細胞為棕黃色,免疫染色一抗稀釋液代替一抗作為陰性對照。caspase-3陽性細胞測定方法:每組隨機抽取4張切片,每張切片在海馬區和腦皮質各采集連續且不重疊的5個視野(×400)的圖片,計算每個視野內的caspase-3陽性細胞數,取5個視野的平均值作為該組織caspase-3陽性細胞數。
1.2.4 Western blotting法檢測APP表達水平 于-80 ℃冰箱中取出腦組織,冰上剪碎,加入預冷的RIPA裂解液,勻漿器勻漿至充分裂解;4 ℃14 000×g離心15 min,取上清液,測定并計算樣本總蛋白;每個樣本取30 μg進行SDS-PAGE,300 mA恒流濕式電印跡法轉至PVDF膜,雙蒸水洗膜3 min,封閉液室溫封閉1 h后剪膜,分別加入APP兔多克隆抗體和β-actin兔單克隆抗體(1∶1 000稀釋),4 ℃冰箱搖床孵育過夜;TBST漂洗5次,10 min/次,加入HRP標記的二抗(1∶1 000~1∶3 000稀釋)孵育1~2 h;TBST洗膜3次,20 min/次,暗室曝光,壓片。采用Image J軟件分析,目的蛋白條帶與β-actin蛋白條帶灰度值的比值表示APP相對表達水平。為去除曝光時間差異對結果的影響,本實驗再將同一張X片上各實驗組目的條帶灰度相對值與正常對照組目的條帶灰度相對值相比,用其最終比值代表各目的蛋白的相對表達水平,納入統計數據。
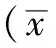
2 結果
2.1 各組小鼠腦皮質、海馬區caspase-3陽性細胞數比較 免疫組化法結果顯示,正常對照組小鼠腦皮質和海馬區caspase-3陽性細胞數較少,細胞內反應物染色較淺;模型組小鼠腦皮質和海馬區caspase-3陽性細胞數較多,細胞內反應物染色較深;石杉堿甲組與模型組相比,小鼠腦皮質和海馬區caspase-3陽性細胞數明顯減少,且細胞內反應物染色也較淺;TSG低劑量組、TSG中劑量組、TSG高劑量組與模型組相比,小鼠腦皮質和海馬區caspase-3陽性細胞數明顯減少,且以TSG高劑量組減少最明顯(詳見圖1~2,本文圖1~2彩圖見本刊官網www.chinagp.net電子期刊相應文章)。
6組小鼠腦皮質、海馬區caspase-3陽性細胞數比較,差異有統計學意義(P<0.05);正常對照組、石杉堿甲組、TSG低劑量組、TSG中劑量組、TSG高劑量組小鼠腦皮質、海馬區caspase-3陽性細胞數少于模型組,差異有統計學意義(P<0.05);TSG低劑量組、TSG中劑量組、TSG高劑量組小鼠腦皮質、海馬區caspase-3陽性細胞數與石杉堿甲組比較,差異無統計學意義(P>0.05,見表1)。
2.2 各組小鼠腦組織APP表達水平比較 6組小鼠腦組織APP表達水平比較,差異有統計學意義(P<0.05);正常對照組、石杉堿甲組、TSG低劑量組、TSG中劑量組、TSG高劑量組小鼠腦組織APP表達水平均低于模型組,差異有統計學意義(P<0.05);TSG低劑量組、TSG中劑量組、TSG高劑量組小鼠腦組織APP表達水平與石杉堿甲組比較,差異無統計學意義(P>0.05,見表2、圖3)。

注:a為正常對照組,b為模型組,c為石杉堿甲組,d為二苯乙烯苷(TSG)低劑量組,e為TSG中劑量組,f為TSG高劑量組
圖1 各組小鼠腦皮質caspase-3陽性細胞表達情況(免疫組化染色,×400)
Figure 1 Expressions of caspase-3 positive cells in the cerebral cortex stained by immunohistochemistry of six groups of mice

注:a為正常對照組,b為模型組,c為石杉堿甲組,d為TSG低劑量組,e為TSG中劑量組,f為TSG高劑量組
圖2 各組小鼠海馬區caspase-3陽性細胞表達情況(免疫組化染色,×400)
Figure 2 Expressions of caspase-3 positive cells in the hippocampus stained by immunohistochemistry of six groups of mice

注:APP=淀粉樣前體蛋白;a為正常對照組,b為模型組,c為石杉堿甲組,d為TSG低劑量組,e為TSG中劑量組,f為TSG高劑量組
圖3 TSG對各組小鼠腦組織中APP表達水平的影響
Figure 3 Effect of TSG on the expression level of APP in the brain tissues of six groups of mice

Table1Numberofcaspase-3positivecellsinthecerebralcortexandhippocampusofsixgroupsofmice

組別只數腦皮質海馬區正常對照組46955±1557a5185±1020a模型組411670±371710330±2462石杉堿甲組47410±1717a6495±1179aTSG低劑量組48520±1434a7400±1235aTSG中劑量組48070±1266a6930±1315aTSG高劑量組47140±1364a5820±2631aF值29924162P值00390010
注:TSG=二苯乙烯苷;與模型組比較,aP<0.05

表2 各組小鼠腦組織APP表達水平比較±s)
注:APP=淀粉樣前體蛋白;與模型組比較,aP<0.05
3 討論
本實驗選用的APP/PS1雙轉基因小鼠,其腦內斑塊形成較早,可以較好模擬AD腦組織神經病理特征[11],得到國際的認可[12-14],目前該模型已被廣泛用于AD的相關研究[15-16]。
神經元纖維纏結、腦內老年斑和神經元丟失是AD的主要病理特征[17]。AD患者腦組織切片中單位面積的凋亡細胞數增加[18],提示凋亡參與了AD神經元丟失的過程。目前AD公認的發病機制為腦內積累具有神經毒性的β淀粉樣蛋白(Aβ),其通過引發一系列反應,導致大量的神經元凋亡、丟失[19]。有研究表明,AD患者的神經元凋亡主要是通過caspase激活途徑[20-21]。caspase-3是caspase家族中的重要成員,是細胞凋亡的主要執行者[22]。王述菊等[23]研究結果顯示,艾灸治療AD的作用機制可能與減少caspase-3的釋放有關。AD主要受累部位為腦皮質和海馬區[24]。本研究中免疫組化法結果顯示,治療2個月后,石杉堿甲組及TSG各劑量組小鼠腦皮質和海馬區caspase-3陽性細胞數均減少,TSG各劑量組腦皮質和海馬區caspase-3陽性細胞數與石杉堿甲組相比無差異,表明TSG可以有效抑制小鼠腦皮質和海馬區caspase-3表達,減少小鼠腦神經元的凋亡和丟失。
APP與AD的發生密切相關,老年斑的核心成分Aβ是由APP裂解產生的。APP是一種跨膜糖蛋白,在體內各組織均有表達,而在腦中以APP695為主。大量生成的APP可激活caspase-3,加速神經元凋亡[25]。此外,病理狀態下,過量表達的APP除了依次被β-分泌酶和γ-分泌酶裂解產生有神經毒性的Aβ外,其胞質部分還能直接被激活的caspase-3裂解,加大Aβ的生成量,加快老年斑的形成,加速神經元的凋亡[26-28]。因此,過量表達的APP既是caspase-3的激活劑,又是caspase-3的底物,APP和caspase-3之間相互作用,放大凋亡信號,形成毒性網絡,加速神經元凋亡。本課題組前期研究發現,TSG治療AD的機制可能和下調AD模型小鼠腦組織中APP表達水平有關[29]。本研究Western blotting法結果顯示,治療2個月后,石杉堿甲組及TSG各劑量組小鼠腦組織APP表達水平均低于模型組,且TSG各劑量組小鼠腦組織APP表達水平與石杉堿甲組相比無差異,表明TSG可以有效降低小鼠腦組織APP表達水平,減少Aβ產生,減緩AD進程。
綜上所述,TSG防治AD的機制除了與抑制caspase-3表達、直接抑制神經元凋亡有關外,還可能與減少APP作為caspase-3底物的裂解途徑、抑制Aβ的產生及其神經毒性有關,也可能與TSG作用于APP和caspase-3產生協同作用有關,鑒于中藥作用的多靶點、多途徑的特點,其具體作用機制有待進一步探究。
作者貢獻:劉寧負責查閱資料,購買小鼠、實驗試劑和耗材,進行實驗操作、文章寫作;廖艷花負責輔助實驗操作;賴術、黃健、黎昀負責查閱資料及小鼠飼養及給藥;黃忠仕負責課題設計、實驗指導、文章審核。
本文無利益沖突。
本研究不足與展望:
(1)淀粉樣前體蛋白(APP)/早老蛋白-1(PS1)雙轉基因小鼠的價格限制了本次實驗的樣本量,這在一定程度上可能影響實驗結果的準確性,后續研究中若能增加樣本量,將為二苯乙烯苷(TSG)的臨床應用和研究提供更可靠的實驗數據。(2)本課題組關于TSG的研究目前尚處于動物實驗研究階段,關于其防治阿爾茨海默病的臨床療效和具體作用機制還有待進一步驗證。
[1]秦俊法.微量元素與阿爾茨海默病(9)[J].廣東微量元素科學,2016,23(1):1-8. QIN J F.Trace elements and Alzheimer′s disease(9)[J].Guangdong Trace Elements Science,2016,23(1):1-8.
[2]黃世瓊,張毅,楊軍宣.何首烏主要成分二苯乙烯苷的研究進展[J].海峽藥學,2016,28(6):37-39.DOI:10.3969/j.issn.1006-3765.2016.06.016. HUANG S Q,ZHANG Y,YANG J X.Recent advances on stilbene glucoside of the main component of polygonum multiflorum Thunb[J].Strait Pharmaceutical Journal,2016,28(6):37-39.DOI:10.3969/j.issn.1006-3765.2016.06.016.
[3]陳冰冰,姜愛玲,張巖.何首烏有效成分二苯乙烯苷的藥理活性研究進展[J].中國臨床藥理學與治療學,2016,21(6):710-715. CHEN B B,JIANG A L,ZHANG Y.Research progress on pharmacological activities of stilbene glucoside,active component from Polygonum multiflorum Thunb[J].Chinese Journal of Clinical Pharmacology and Therapeutics,2016,21(6):710-715.
[4] 史寶和,李玉鋒,李成洋,等.石杉堿甲治療輕中度阿爾茨海默病的臨床研究[J].實用藥物與臨床,2013,16(1):37-38.DOI:10.3969/j.issn.1673-0070.2013.01.015. SHI B H,LI Y F,LI C Y,et al.Clinical study of huperzine A on patients with mild and moderate Alzheimer disease[J].Practical Pharmacy and Clinical Remedies,2013,16(1):37-38.DOI:10.3969/j.issn.1673-0070.2013.01.015.
[5]唐建良.石杉堿甲與多奈哌齊對阿爾茨海默病患者認知功能損害的對照研究[C]//中國心理衛生協會.第七屆全國心理衛生學術大會論文匯編.中國心理衛生協會,2014:2. TANG J L.A comparative study of huperzine A and donepezil on cognitive impairment in patients with Alzheimer′s disease[C]//Chinese Association for Mental Health.Paper compilation of the Seventh National Conference on mental Hygiene.Chinese Association for Mental Health,2014:2.
[6]XIAO X Q,ZHANG H Y,TANG X C.Huperzine A attenuates amyloid beta-peptide fragment 25-35-induced apoptosis in rat cortical neurons via inhibiting reactive oxygen species formation and caspase-3 activation[J].J Neurosci Res,2002,67(1):30-36.
[7]ZHANG H Y,TANG X C.Huperzine A attenuates the neurotoxic effect ofstaurosporine in primary rat cortical neurons[J].Neurosci Lett,2003,340(2):91-94.
[8]劉玲,賴術,黎昀,等.二苯乙烯苷對SAMP8小鼠學習記憶能力的改善作用[J].中國現代中藥,2012,14(10):12-15.DOI:10.3969/j.issn.1673-4890.2012.10.003. LIU L,LAI S,LI Y,et al.TSG on learning and memory abilities in senescence accelerated SAMP8 mouse[J].Modern Chinese Medicine,2012,14(10):12-15.DOI:10.3969/j.issn.1673-4890.2012.10.003.
[9]劉玲,朱曉瑩,黎昀,等.二苯乙烯苷對SAMP8小鼠APP mRNA和PS1 mRNA表達的影響[J].中國實驗方劑學雜志,2013,19(1):240-243. LIU L,ZHU X Y,LI Y,et al.Effects of TSG on expression of APP mRNA and PS1 mRNA in SAMP8 mice[J].Chinese Journal of Experimental Traditional Medical Formulae,2013,19(1):240-243.
[10]楊曉穎,劉寧,黃岑漢,等.二苯乙烯苷對APP/PS1雙轉基因小鼠APP、PS1基因表達的影響[J].中國老年學雜志,2016,36(7):1573-1575.DOI:10.3969/j.issn.1005-9202.2016.07.016. YANG X Y,LIU N,HUANG C H,et al.Effect of TSG on gene expression of APP and PS1 in APP/PS1 double transgenic mice[J].Chinese Journal of Gerontology,2016,36(7):1573-1575.DOI:10.3969/j.issn.1005-9202.2016.07.016.
[11]LEE J E,HAN P L.An update of animal models of Alzheimer disease with areevaluation of plaque depositions[J].Exp Neurobiol,2013,22(2):84-95.
[12]YAO Z G,ZHANG L,LIANG L,et al.The effect of PN-1,a traditional chineseprescription,on the learning and memory ina transgenic mouse model of Alzheimer′s disease[J].Evid Based Complement Alternat Med,2013,2013:518421.DOI:10.1155/2013/518421.
[13]NEUMEISTER K L,RIEPE M W.Bupropion and citalopram in the APP23 mouse model of Alzheimer′s disease:a study in a dry-land maze[J].Int J Alzheimers Dis,2012,2012:673584.
[14]LUO H,WU Q,YE X,et al.Genome-wide analysis of miRNA signature in the APPswe/PS1ΔE9 mouse model of Alzheimer′s disease[J].PLoS One,2014,9(8):e101725.DOI:10.1371/journal.pone.0101725.
[15]趙曉燕,王丹丹,單燁,等.APP/PS1轉基因鼠腦內小泛素化修飾物-1上調可能參與阿爾茨海默病老年斑形成和神經突起變性的調節[J].生理學報,2013,65(3):253-262. ZHAO X Y,WANG D D,SHAN Y,et al.Potential involvement of abnormal increased SUMO-1 in modulation of the formation of Alzheimer′s disease senile plaques and neuritic dystrophy in APP/PS1 transgenic mice[J].Acta Physiologica Sinica,2013,65(3):253-262.
[16]邱昕,陳國華,汪弢,等.黃連解毒湯對APP/PS1雙轉基因阿爾茨海默病小鼠自由基代謝及海馬區病理形態學影響[J].中國中西醫結合雜志,2011,31(10):1379-1382. QIU X,CHEN G H,WANG T,et al.Effects of Huanglian Jiedu Decoction on free radicals metabolism and pathomorphism of the hippocampus in APP/PS1 double transgenic mice[J].Chinese Journal of Integrated Traditional and Western Medicine,2011,31(10):1379-1382.
[17]肖穩,丁萍,楊飛.阿爾茨海默病早期生物標記物及其檢測方法的研究進展[J].中國預防醫學雜志,2016,17(3):225-228.DOI:10.16506/j.1009-6639.2016.03.016. XIAO W,DING P,YANG F.Research progress of biomarkers and detection methods of Alzheimer′s disease[J].Chinese Preventive Medicine,2016,17(3):225-228.DOI:10.16506/j.1009-6639.2016.03.016.
[18]孟艷,王蓉,劉夢霞,等.阿爾茨海默病患者腦神經元凋亡機制中相關因素的研究[J].中華老年醫學雜志,2006,25(4):245-247. MENG Y,WANG R,LIU M X,et al.Study on the factors related with mechanism of neuron apoptosis in Alzheimer disease[J].Chinese Journal of Geriatrics,2006,25(4):245-247.
[19]余云舟,徐青.靶向β-淀粉樣蛋白的阿爾茨海默病免疫治療研究進展[J].國際藥學研究雜志,2016,43(2):216-223.DOI:10.13220/j.cnki.jipr.2016.02.005. YU Y Z,XU Q.Immunotherapy targeting β-amyloid for Alzheimer′s disease:research advances[J].Journal of International Pharmaceutical Research,2016,43(2):216-223.DOI:10.13220/j.cnki.jipr.2016.02.005.[20]YAMAZAKI Y,TSURUGA M,ZHOU D,et al.Cytoskeletal disruption accelerates caspase-3 activation and alters theintracellular membrane reorganization in DNA damage-induced apoptosis[J].Exp Cell Res,2000,259(1):64-78.[21]ROHN T T,RISSMAN R A,DAVIS M C,et al.Caspase-9 activationand caspase cleavage of tau in the Alzheimer′s disease brain[J].Neurobiol Dis,2002,11(2):341-354.
[22]陳雪,孫婧霞,蔣常文.Bcl-2、Caspase-3與阿爾茨海默病關系的研究進展[J].臨床醫學工程,2013,20(9):1177-1179. CHEN X,SUN J X,JIANG C W.Research progress on Bcl-2,Caspase-3 and Alzheimer′s disease[J].Clinical Medical & Engineering,2013,20(9):1177-1179.
[23]王述菊,孫國杰,馬駿,等.艾灸對Alzheimer病模型大鼠行為學及海馬區Bcl-2、Bax、Caspase-3影響的研究[J].世界科學技術-中醫藥現代化,2015,17(6):1243-1248.DOI:10.11842/wst.2015.06.022. WANG S J,SUN G J,MA J,et al.Effects of moxibustion on behaviors and Bcl-2,Bax,Caspase-3 in hippocampus of Alzheimer′s disease model rats[J].World Science and Technology-Modernization of Traditional Chinese Medicine,2015,17(6):1243-1248.DOI:10.11842/wst.2015.06.022.
[24]ZHOU Z D,CHAN C H,MA Q H,et al.The roles of amyloidprecursor protein(APP) in neurogenesis:implications to pathogenesis and therapy of Alzheimer disease[J].Cell Adh Migr,2011,5(4):280-292.
[25]許志強,周華東,蔣曉江.淀粉樣β蛋白沉淀及其神經毒性與阿爾茨海默病[J].中國臨床康復,2006,10(14):138-140.DOI:10.3321/j.issn:1673-8225.2006.14.058. XU Z Q,ZHOU H D,JIANG X J.Amyloid beta protein deposit and its neurotoxicity associated with Alzheimer disease[J].Chinese Journal of Clinical Rehabilitation,2006,10(14):138-140.DOI:10.3321/j.issn:1673-8225.2006.14.058.
[26]GERVAIS F G,XU D,ROBERTSON G S,et al.Involvement ofcaspases in proteolytic cleavage of Alzheimer′s amyloid-beta precursor proteinand amyloidogenic A beta peptide formation[J].Cell,1999,97(3):395-406.
[27]LEBLANC A,LIU H,GOODYER C,et al.Caspase-6 role inapoptosis of human neurons,amyloidogenesis,and Alzheimer′s disease[J].J Biol Chem,1999,274(33):23426-23436.
[28]BARNES N Y,LI L,YOSHIKAWA K,et al.Increased production of amyloid precursor protein provides a substrate for caspase-3 in dying motoneurons[J].J Neurosci,1998,18(15):5869-5880.
[29]楊曉穎,劉寧,黃岑漢,等.二苯乙烯苷對APP/PS1雙轉基因阿爾茨海默病小鼠腦內β淀粉樣前體蛋白及分揀蛋白相關受體1 mRNA表達的影響[J].中國老年學雜志,2016,36(3):536-539.DOI:10.3969/j.issn.1005-9202.2016.03.009. YANG X Y,LIU N,HUANG C H,et al.Effect of TSG on protein expression of APP in APP/PS1 double transgenic AD mice brain tissues[J].Chinese Journal of Gerontology,2016,36(3):536-539.DOI:10.3969/j.issn.1005-9202.2016.03.009.
(本文編輯:毛亞敏)
Effect of TSG on the Expressions of Caspase-3 and APP in Brain Tissues of APP/PS1 Double Transgenic Mouse Model of Alzheimer′s Disease
LIUNing,LIAOYan-hua,LAIShu,HUANGJian,LIYun,HUANGZhong-shi*
YoujiangMedicalCollegeyforNationalities,Baise533000,China
*Correspondingauthor:HUANGZhong-shi,Professor;E-mail:hzs1004@163.com
Objective To observe the effect of tetrahydroxy stilbene glucoside(TSG) on the expressions of caspase-3 and amyloid precursor protein(APP) in brain tissues of APP/presenilin-1(PS1) double transgenic mouse model of Alzheimer′s disease(AD),and to investigate the possible mechanisms of therapeutic effect about TSG on AD.Methods In October 2014,50 SPF rank male APP/PS1 double transgenic mice were randomly divided into model group,huperzine A group and low-dose TSG group,mid-dose TSG group,high-dose TSG group with 10 mice in each based on the random number table.Another 10 SPF rank male C5B7L/6J mice were assigned to the normal control group.Huperzine A group,low-,mid- and high-dose TSG groups,model group and normal control group were respectively given by gavage with huperzine A(0.020 mg/kg),TSG(0.033,0.100,0.300 g/kg),0.9% sodium chloride solution(10.000 ml/kg),0.9% sodium chloride solution(10.000 ml/kg),once a day,for 60 days.At the end of intervention,all the mice were sacrificed and the brain tissues were taken out for experiments.Immunohistochemistry was used to determine the number of caspase-3 positive cells in each group mice′s cerebral cortex and hippocampus,and Western blotting was adopted to detect the expression level of APP in each group mice′s brain tissues.Results The number of caspase-3 positive cells in the cerebral cortex and hippocampus of the mice in the normal control group,huperzine A group and low-,mid- and high-dose TSG groups were less than those in the model group(P<0.05).The number of caspase-3 positive cells in the cerebral cortex and hippocampus of mice in huperzine A group was not significantly different from that of the mice in low-,mid- and high-dose TSG groups(P>0.05).The mice in the model group had higher expression level of APP in brain tissues than those in the normal control group,huperzine A group and low-,mid- and high-dose TSG groups(P<0.05).The expression level of APP in brain tissues of mice in huperzine A group did not differ substantially from that of the mice in low-,mid- and high-dose TSG groups(P>0.05).Conclusion TSG has obvious therapeutic effect on APP/PS1 double transgenic mice,the mechanism might be related to the inhibition of the expression levels of caspase-3 and APP so as to reduce the production of amyloid-β protein,and delay the apoptosis of nerve cells.
Alzheimer disease;Tetrahydroxy stilbene glucoside;Caspase 3;Amyloid precursor protein secretases
國家自然科學基金資助項目(81260495)
R 745.7
A
10.3969/j.issn.1007-9572.2017.15.014
2017-01-02;
2017-03-01)
*通信作者:黃忠仕,教授;E-mail:hzs1004@163.com
劉寧,廖艷花,賴術,等.二苯乙烯苷對淀粉樣前體蛋白/早老蛋白-1雙轉基因小鼠腦組織半胱氨酸天冬氨酸蛋白酶3和淀粉樣前體蛋白表達的影響[J].中國全科醫學,2017,20(15):1854-1859.[www.chinagp.net]
LIU N,LIAO Y H,LAI S,et al.Effect of TSG on the expressions of caspase-3 and APP in brain tissues of APP/PS1 double transgenic mouse model of Alzheimer′s disease[J].Chinese General Practice,2017,20(15):1854-1859.

