First-principles study of helium clustering at initial stage in ThO2?
Kuan Shao(邵寬),Han Han(韓晗),Wei Zhang(張偉),Chang-Ying Wang(王昌英), Yong-Liang Guo(郭永亮),Cui-Lan Ren(任翠蘭),and Ping Huai(懷平),?
1 Shanghai Institute of Applied Physics,Chinese Academy of Sciences,Shanghai 201800,China
2 Key Laboratory of Interfacial Physics and Technology,Chinese Academy of Sciences,Shanghai 201800,China
3 University of Chinese Academy of Sciences,Beijing 100049,China
First-principles study of helium clustering at initial stage in ThO2?
Kuan Shao(邵寬)1,3,Han Han(韓晗)1,?,Wei Zhang(張偉)1,2,Chang-Ying Wang(王昌英)1, Yong-Liang Guo(郭永亮)1,Cui-Lan Ren(任翠蘭)1,2,and Ping Huai(懷平)1,2,?
1 Shanghai Institute of Applied Physics,Chinese Academy of Sciences,Shanghai 201800,China
2 Key Laboratory of Interfacial Physics and Technology,Chinese Academy of Sciences,Shanghai 201800,China
3 University of Chinese Academy of Sciences,Beijing 100049,China
The clustering behavior of helium atoms in thorium dioxide has been investigated by first-principles calculations.The results show that He atoms tend to form a cluster around an octahedral interstitial site(OIS).As the concentration of He atoms in ThO2increases,the strain induced by the He atoms increases and the octahedral interstitial site is not large enough to accommodate a large cluster,such as a He hexamer.We considered three different Schottky defect(SD)configurations (SD1,SD2,and SD3).When He atoms are located in the SD sites,the strain induced by the He atoms is released and the incorporation and binding energies decrease.The He trimer is the most stable cluster in SD1.Large He clusters,such as a He hexamer,are also stable in the SDs.
first-principles study,thorium dioxide,helium cluster,defective properties
1.Introduction
Thorium dioxide is a robust nuclear fuel candidate for generation IV reactors because of its low generation of minor actinides,excellent radiation resistance and chemical stability.[1–4]During the reactoroperation,production of noble gases may affect the mechanical and thermal properties of nuclear fuels.[5,6]One of these noble gases is helium,and most of the helium is generated by alpha decay during fuel burnup.Because of its high diffusivity,helium tends to cluster and form bubbles,resulting in swelling of nuclear fuels.[7–9]Therefore,it is necessary to investigate the clustering behavior of helium in nuclear fuels.
Considering the difficulties in nuclear fuel experiments, effective theoretical calculations should be performed.Density functional theory(DFT)is a reliable method to evaluate the point defect energies by atomic-scale calculation.[10–13]In recent years,a number of computational works concerned with the bulk and defect properties of nuclear fuels have been performed.[14–21]Zhang et al.[14,15]investigated the mechanical and thermal properties of ThO2by first-principles calculations.Thompson et al.[16]reported that the stability of noble gas atoms is related to the strain they caused in trap sites.Ma et al.[17]studied the swelling of UO2induced by noble gases based on hybrid DFT.Brillant et al.[18]studied the stability and solubility of fission products,including helium,using spin-polarized generalized gradient approximation with on-site Coulomb correction techniques.Yun et al.[19]investigated the clustering behavior of He in UO2,and found that He clusters affect the local mechanical properties of UO2. Dabrowski et al.[20]reported that diffusion of helium between two octahedral sites in UO2is along a polyline rather than a straight line.
In the present work,we investigate the clustering behavior of helium by choosing an octahedral interstitial site(OIS)and the Schottky defect(SD)as the trap sites.The volume change of ThO2and incorporation and solution energies are then calculated with increasing concentration of He atoms.Finally, the stability of He clusters is discussed from the perspective of their calculated binding energies.
2.Methodology
Our calculations were performed using the density functional theory as implemented in the Vienna ab initio simu-lation package(VASP).[22,23]The projected augmented wave method(PAW)[24]and the generalized gradient approximation(GGA)[25]were used.The exchange and correlation energies were calculated using the Perdew–Burke–Ernzerh of (PBE)functional.[26]The wave functions were expanded in a plane-wave basis set with an energy cutoff of 500 eV. Since ThO2is a diamagnetic material,[14]the spin polarization was not considered in the calculation.The results were also checked with spin polarized calculations,which showed no obvious differences.Due to no inclusion of occupied 5f states,the strong correlation effect of ThO2was negligible.Ithas been reported[15,27]that the GGA approximation can give nearly correct energy information for ThO2,and therefore the GGA+U method[28,29]was not adopted in this work.The lattice constants and internal freedom of the unit cell were fully optimized until the Hellman–Feynman forces on the atoms were less than 0.01 eV/?A.The effective charge for each atom was calculated using Bader charge analysis.[30]
In order to simulate the helium clusters incorporated in ThO2,a 2×2×2 supercell containing 96 atoms was used in the calculation.The previous results[27,28]have proven that a supercell of this size can make the energies sufficiently converged.Depending on the unit cell size and shape,a 2×2×2 Monkhorst–Pack sampling mesh[31]of k-points was used.We implemented a k-mesh test for the He–ThO2system.The incorporation energy of He(discussed in the next section)calculated by 2×2×2 and 3×3×3 k-meshes has a little difference within the range of meV.This indicated that a 2×2×2 k-mesh is sufficient to avoid significant numerical errors in our calculations.All these calculations were checked using larger energy cutoffs and k-meshes;the results of total energy and Hellmann–Feynman forces were converged within 0.01 eV and 0.01 eV/?A,respectively.According to the previous work,[32–34]the zero point energy(ZPE)of helium in oxides is small,which does not affect the numerical results. This can be seen in some previous studies of similar material systems.For instance,when helium interacts with O atoms in Al2O3,ZPE corrections are in a range of 10?2–10?3eV.[32]Therefore,calculations without ZPE correction were employed in this work.

Table 1.Incorporation energy of He in octahedral interstitial site for different k-meshes.
3.Results and discussion
ThO2crystallizes in a cubic fluorite structure(space group:F m3m).Our calculated lattice constant is 5.617?A, which agrees with the theoretical result(5.619?A)reported by Zhang et al.[14]and the experimental value(5.597?A)reported by Olsen et al.[35]To investigate the clustering behavior of helium in ThO2,the following trap sites are considered:the octahedral interstitial site(OIS)and the Schottky defect(SD) as shown in Fig.1.The SD clusters can be made by removing one thorium atom and two neighboring oxygen atoms from the lattice.[36]Thus three configurations of the Schottky defect (SD1,SD2,SD3)can be considered,differing by the distance between the two oxygen vacancies.
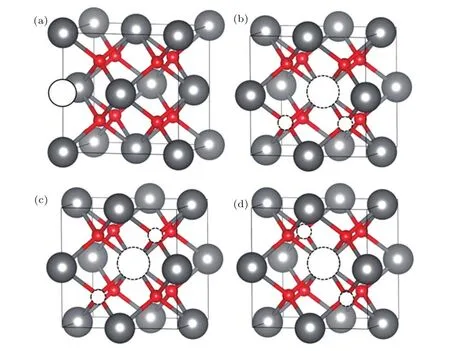
Fig.1.(color online)(a)The cubic fluorite structure of ThO2.The gray and red spheres represent the thorium and oxygen atoms,respectively. The solid spheres represent the OIS.(b)Three configurations of the Schottky defect:(b)SD1,(c)SD2,and(d)SD3.The dashed spheres represent the vacancies.
It is known that helium atoms prefer to reside in octahedral interstitial sites in the perfect fuel matrix.[37]He atoms spontaneously move to OISs after atomic relaxation from their initial positions in other interstitial sites.The diffusion barrier plays an important role in the clustering process of He.We calculated the migration energy of He by the nudged elastic band(NEB)method.[38]The migration energy of He between two OISs is 3.80 eV,which is in agreement with the results reported by Da?rowski et al.[39]Considering the environment with a high temperature in nuclear fuels,we suggest that He tends to be mobile in ThO2.In this work,we considered the clustering behavior of He atoms around an OIS with increasing concentration of He atoms.Firstly,we positioned two He atoms at the center of the edge between two oxygen atoms (Fig.2),which is the midpoint between two interstitial sites. After relaxation,they both move to the center of an OIS and form a He dimer,as shown in Fig.2(a).
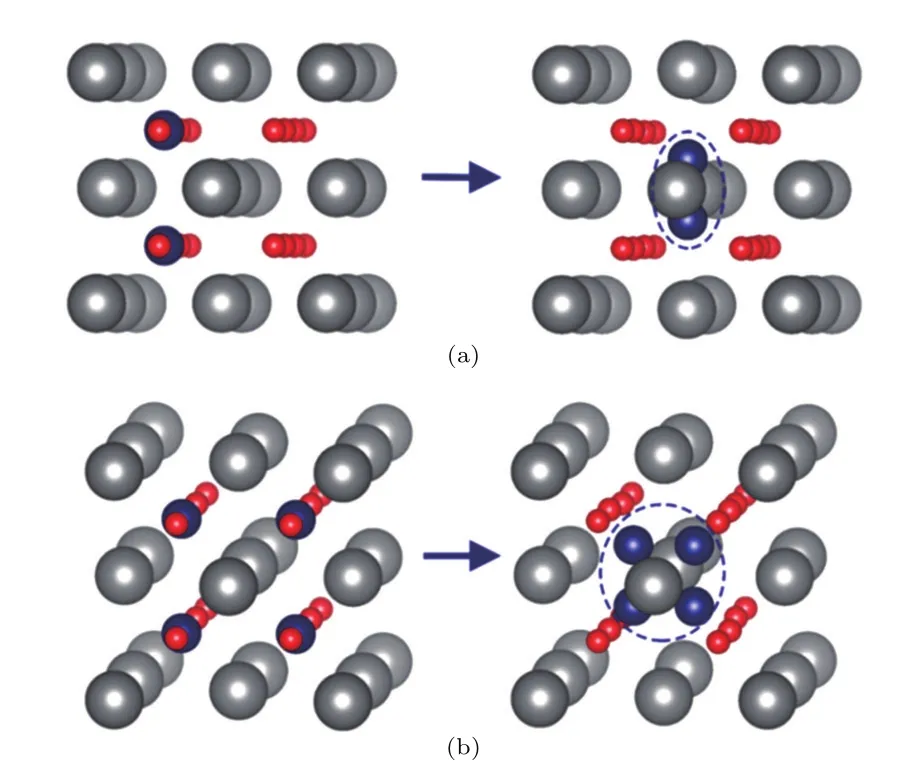
Fig.2.(color online)The initial configurations of(a)two and(b)four He atoms and their configurations after atomic relaxation.The gray, red,and blue spheres represent the thorium,oxygen,and helium atoms, respectively.
The clustering behavior was investigated by increasing the concentration of He atoms in ThO2.For four He atoms, a tetra mer forms in an OIS,as shown in Fig.2(b).For six He atoms,the He atoms do not form a hex-amer,which we might except,but they form three dimers trapped in different OISs (see Fig.3).This indicates that the OIS in ThO2does not have sufficient space to accommodate a large cluster,such as a hex-amer.This result is different from the case in UO2,where six He atoms tend to form a hex-amer in the OIS and greatly displace the surrounding atoms.[19]The largest displacement of the surrounding oxygen atoms in our work is 0.23?A(see Table 1),while the displacement of oxygen atoms can be as large as 0.65?A in UO2.We suggest that the oxygen and thorium atoms in ThO2are less mobile than the uranium and oxygen atoms in UO2.
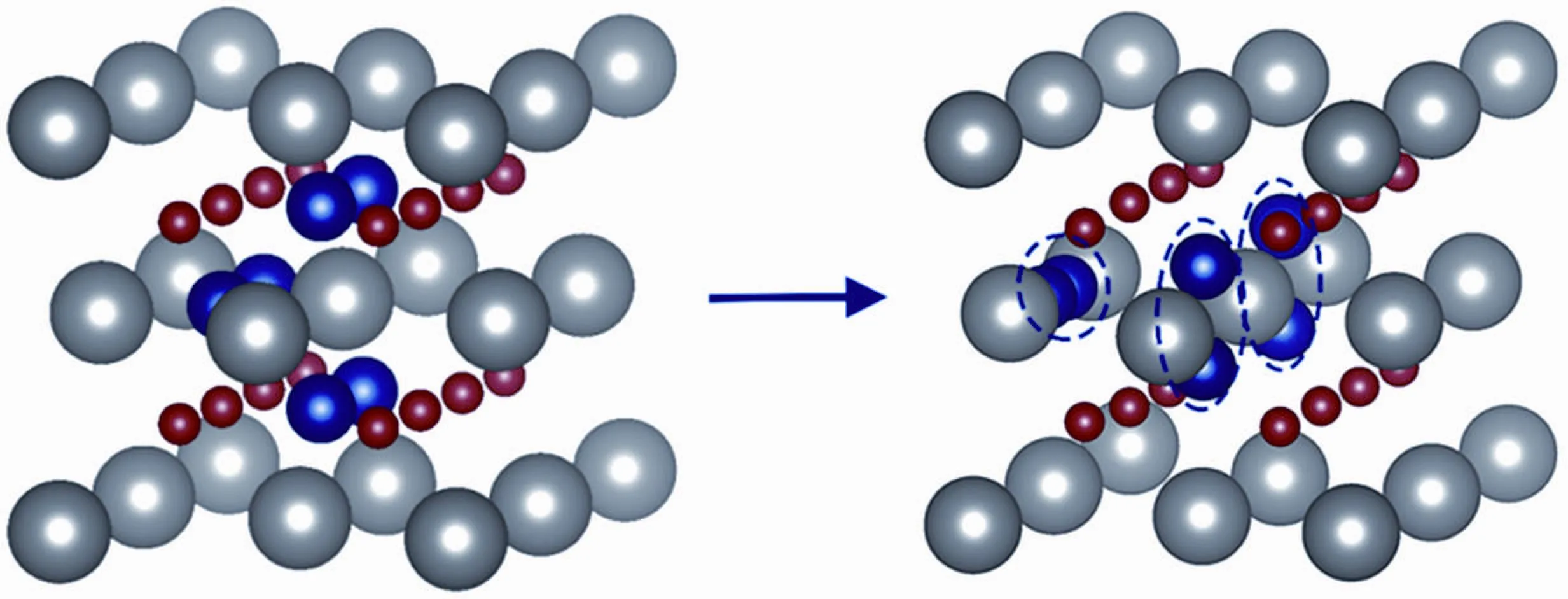
Fig.3.(color online)The initial configurations of six He atoms and the configuration after atomic relaxation.Three dimers form in different OISs.
When He atoms are introduced into the supercell structure,the volume of the structure changes according to the doping site and the number of He atoms.This volume change is given by

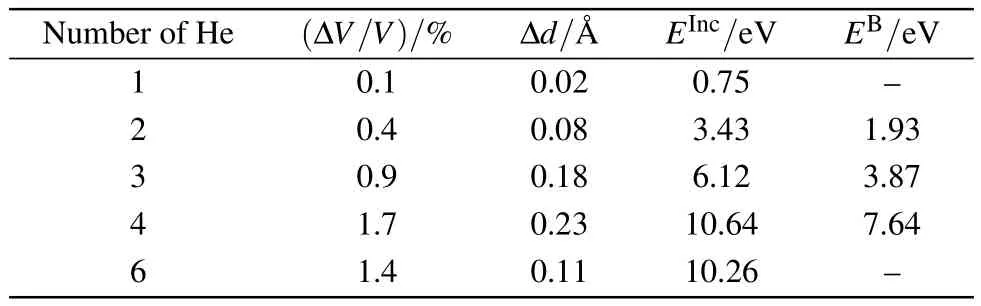
Table 2.Volume changes relative to the perfect supercell ΔV/V,displacement of the nearest-neighbouring atoms of He Δd,incorporation energies E Inc,and binding energies E B with the increasing number of He atoms.
To investigate the stability of the He clusters in ThO2,we calculated the incorporation energies of He interstitials.The incorporation energyis defined as the energy required to incorporate a He atom in a pre-existing defect site,
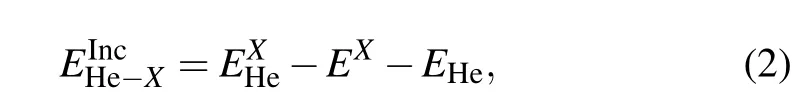
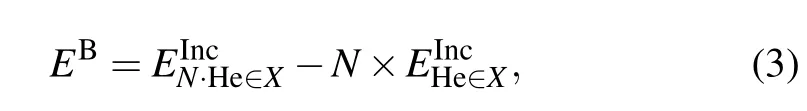
where N is the number of He atoms andis the incorporation energy of the He cluster in defect X.As shown in Table 2,all of the binding energies are positive,suggesting that binding of these He clusters in an OIS is an endothermic process.The binding energy increases with the size of the He cluster,and the tetramer has the largest binding energy of 7.64 eV.This indicates that a large energy barrier needs to be overcome when four He atoms migrate from isolated OISs to form a cluster in one OIS of ThO2.The He dimer is the most stable cluster in the OIS with a binding energy of 1.93 eV.
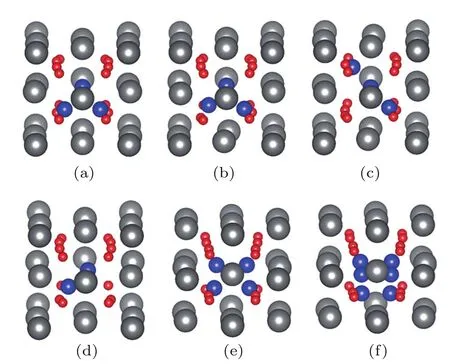
Fig.4.(color online)The configurations of He trimmers in(a)SD1, (b)SD2,(c)SD3;and(d)a He dimer,(e)a He tetramer,and(f)a He hexamer in SD1.
The incorporation energy of He atoms is related to the strain.[16]Owing to the large strain induced by large clusters, an OIS does not seem to be the energetically favorable trap site for He atoms.Thus,we also considered the Schottky defect (SD)as the trap site,which can provide more empty space for strain release.As shown in Fig.4,three configurations of the Schottky defect(SD1,SD2,and SD3)were considered,which differ by the distance between the two oxygen vacancies.He atoms tend to aggregate in the SDs after atomic relaxation, and even a He hexamer can exist in ThO2(see Fig.4).The calculated ΔV/V and EIncof He clusters trapped in the SDs are listed in Table 3.As the concentration of He atoms in ThO2increases,both ΔV/V and EIncincrease.A He hexamer in SD1has the largest incorporation energy of 3.35 eV,which also results in the largest volume change of 1.31%.As shown in Fig.5,the incorporation energies of He atoms in the SDs are significantly smaller than those in OISs,suggesting that He clusters prefer to reside in SDs.The volume changes induced by He atoms in the SDs are also smaller than those in OISs.This indicates that these SDs are favorable for the release of the strain induced by incorporation of He clusters in ThO2,especially for large clusters.
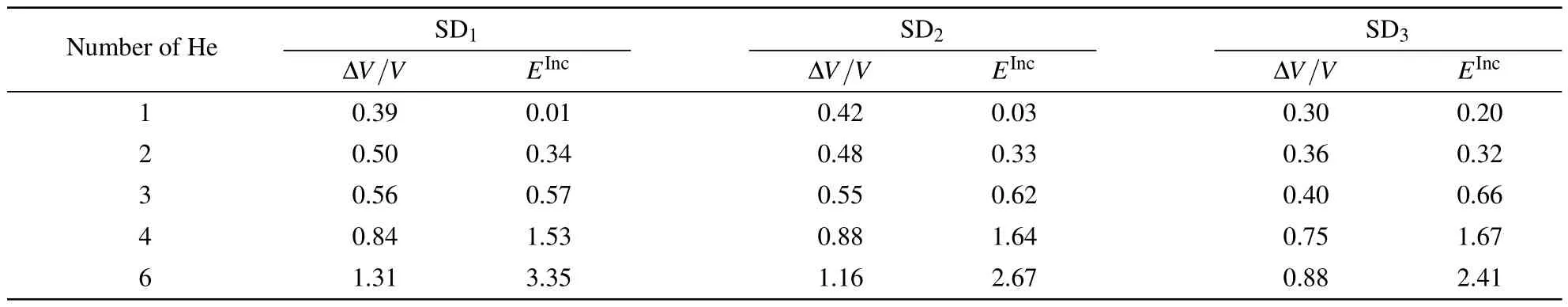
Table 3.Volume changes ΔV/V(in%)and incorporation energies E Inc(in eV)of He clusters trapped in SDs with the increasing number of He atoms.
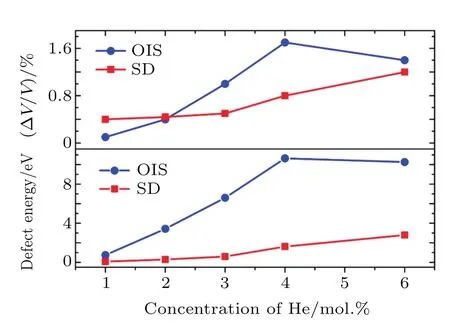
Fig.5.(color online)The average volume changes ΔV/V and incorporation energies E Inc of He clusters trapped in SDs,with comparison to those in an OIS.
Considering the energy cost for formation of the SD,we also calculated the solution energy
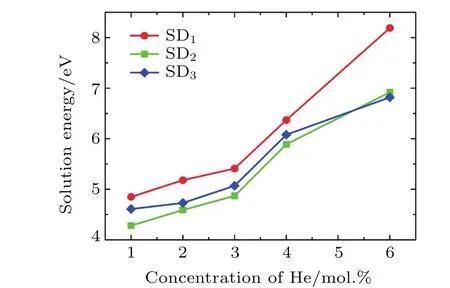
Fig.6.(color online)Solution energies of He clusters in SDs,with the increasing concentration of He atoms in ThO2.Three configurations of SD are considered.
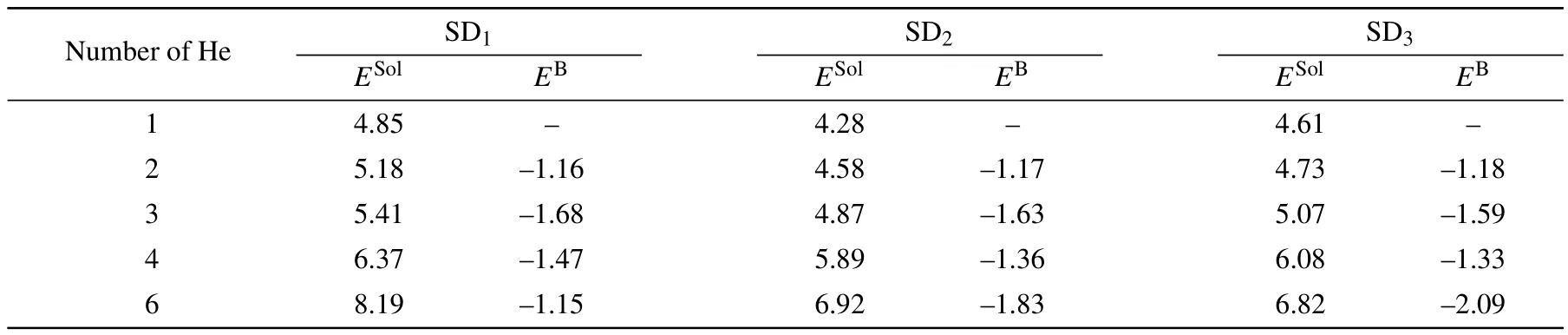
Table 4.Solution energies E Sol(in eV)and binding energies E B(in eV)of He clusters trapped in SDs with the increasing number of He atoms.
To access the possibility that isolated He atoms in OISs aggregate in one SD,we also calculated the binding energies (Table 4).All of the binding energies of He clusters in the SDs are negative,suggesting that the binding process is energetically favorable.For He clusters in SD1,the He trimer has the lowest binding energy and is the most stable cluster.For SD2and SD3,a He hexamer has the lowest binding energy, indicating that He atoms tend to form a large cluster in SD2and SD3.
4.Conclusion
We have performed first-principles calculations of He clustering in ThO2.As the concentration of He atoms in ThO2increases,the He atoms tend to form a cluster around an OIS. A He dimer is the most stable cluster in an OIS.However,one OIS is not large enough to accommodate a large cluster,such as a He hexamer.When He atoms are located in SDs,the strain induced by the He atoms is released and the incorporation and binding energies decrease.The negative binding energies indicate that He atoms located in isolated OISs can easily aggregate in a SD.A He trimer is the most stable cluster in SD1. Large He clusters,such as a He hexamer,can also form in SDs.For SD2and SD3,even large clusters(more than six He atoms)can exist according to the calculated binding energies. Finally,our results suggest that the growth of a larger He clusters may occur by the diffusion and aggregation of Schottky defects with He atoms.Our further studies may concern the formation and diffusion of these large defects in ThO2with a calculation using a larger supercell.The clustering behavior of He atoms will affect the mechanical properties of ThO2.The degradation of mechanical properties will also be investigated in further investigations.
Acknowledgements
We thank the Supercomputing Center of the Chinese Academy of Sciences(SCCAS)and the Shanghai Supercomputing Center for computer resources.
[1]Lombardi C,Luzzi L,Padovani E and Vettraino F 2008 Prog.Nucl. Energy 50 944
[2]Breza J,Daí? lek P and Ne?as V 2010 Ann.Nucl.Eng.37 685
[3]Mirvakili S M,Gholamzadeh Z and Feghhi S A H 2016 Nucl.Sci.Tech. 27 79
[4]Liu Z B,Liu Y,Liu G M and Hou J 2016 Nucl.Sci.Tech.27 123
[5]Roudil D,Bonhoure J,Pik R,Cuney M,Jégou C and Gauthier-Lafaye F 2008 J.Nucl.Mater.378 70
[6]Ansarifar G R and Akhavan H R 2016 Nucl.Sci.Tech.27 28
[7]Sattonnay G,Vincent L,Garrido F and Thome L 2006 J.Nucl.Mater. 355 131
[8]Grimes R W,Miller R H and Catlow C 1990 J.Nucl.Mater.172 123
[9]Ronchi C and Hiernaut J 2004 J.Nucl.Mater.325 1
[10]Han H,Wickramaratne D,Huang Q,Dai J,Li T,Wang H,Zhang W and Huai P 2016 RSC Advances 6 84262
[11]Wang H,Han H,Yin G,Wang C Y,Hou Y Y,Tang J,Dai J X,Ren C L,Zhang W and Huai P 2017 Materials 10 103
[12]Han H,Yin G,Wang H,Wang C,Shao K,Zhang W,Dai J and Huai P 2017 Comput.Mater.Sci.133 159
[13]Counts W,Wolverton C and Gibala R 2010 Acta Mater.58 4730
[14]Wang B T,Shi H,Li W D and Zhang P 2010 J.Nucl.Mater.399 181
[15]Lu Y,Yang Y and Zhang P 2012 J.Phys.:Condens.Matter 24 225801
[16]Thompson A E and Wolverton C 2011 Phys.Rev.B 84 134111
[17]Ma L and Ray A K 2012 Phys.Lett.A 376 1499
[18]Brillant G,Gupta F and Pasturel A 2011 J.Nucl.Mater.412 170
[19]Yun Y,Eriksson O and Oppeneer P M 2009 J.Nucl.Mater.385 72
[20]Dabrowski L and Szuta M 2014 J.Alloys Compd.615 598
[21]Wang C Y,Han H,Shao K,Cheng C and Huai P 2015 Chin.Phys.B. 24 097101
[22]Kresse G and Furthmüller J 1996 Phys.Rev.B 54 11169
[23]Kresse G and Joubert D 1999 Phys.Rev.B 59 1758
[24]Bl?chl P E 1994 Phys.Rev.B 50 17953
[25]Perdew J P,Chevary J,Vosko S,Jackson K A,Pederson M R,Singh D and Fiolhais C 1992 Phys.Rev.B 46 6671
[26]Perdew J P,Burke K and Ernzerhof M 1996 Phys.Rev.Lett.77 3865
[27]Alexandrov V,Gr?nbech-Jensen N,Navrotsky A and Asta M 2010 Phys.Rev.B 82 174115
[28]Geng H Y,Chen Y,Kaneta Y,Kinoshita M and Wu Q 2010 Phys.Rev. B 82 094106
[29]Dudarev S,Manh D N and Sutton A 1997 Philos.Mag.B 75 613
[30]Henkelman G,Arnaldsson A and Jónsson H 2006 Comput.Mater.Sci. 36 354
[31]Monkhorst H J and Pack J D 1976 Phys.Rev.B 13 5188
[32]Zhang G,Xiang X,Yang F,Peng X,Tang T,Shi Y and Wang X 2016 Phys.Chem.Chem.Phys.18 1649
[33]Zhang P,Zhao J and Wen B 2012 J.Nucl.Mater.423 164
[34]de Lara-Castells M P,Stoll H and Mitrushchenkov A O 2014 J.Phys. Chem.A 118 6367
[35]Olsen J S,Gerward L,Kanchana V and Vaitheeswaran G 2004 J.Alloys Compd.381 37
[36]Dorado B,Freyss M and Martin G 2009 Eur.Phys.J.B 69 203
[37]Yun Y,Kim H,Kim H and Park K 2008 J.Nucl.Mater.378 40
[38]Henkelman G,Uberuaga B P and Jónsson H 2000 J.Chem.Phys.113 9901
[39]Da?browski L and Szuta M 2014 J.Alloys Compd.615 598
[40]Matzke H 1987 J.Chem.Soc.,Faraday Trans.283 1121
[41]Lidiard A 1966 J.Nucl.Mater.19 106
31 March 2017;revised manuscript
18 May 2017;published online 18 July 2017)
10.1088/1674-1056/26/9/097101
?Project supported by the Program of International Samp;T Cooperation,China(Grant No.2014DFG60230),the National Natural Science Foundation of China (Grant Nos.11605273,21571185,U1404111,11504089,21501189,and 21676291),the Shanghai Municipal Science and Technology Commission,China (Grant No.16ZR1443100),and the Strategic Priority Research Program of the Chinese Academy of Sciences(Grant No.XDA02040104).
?Corresponding author.E-mail:hanhan@sinap.ac.cn
?Corresponding author.E-mail:huaiping@sinap.ac.cn
?2017 Chinese Physical Society and IOP Publishing Ltd http://iopscience.iop.org/cpb http://cpb.iphy.ac.cn
- Chinese Physics B的其它文章
- Relationship measurement between ac-Stark shift of 40Ca+clock transition and laser polarization direction?
- Air breakdown induced by the microwave with two mutually orthogonal and heterophase electric field components?
- Collective motion of active particles in environmental noise?
- Temperature dependence of heat conduction coefficient in nanotube/nanowire networks?
- Analysis of dynamic features in intersecting pedestrian flows?
- Heat transfer enhancement in MOSFET mounted on different FR4 substrates by thermal transient measurement?

