Synthesis of diamonds in Fe C systems using nitrogen and hydrogen co-doped impurities under HPHT?
Shi-Shuai Sun(孫士帥),Zhi-Hui Xu(徐智慧),Wen Cui(崔雯),Xiao-Peng Jia(賈曉鵬),and Hong-An Ma(馬紅安)
1 College of Science,Tianjin University of Technology,Tianjin 300384,China
2 College of Physics and Materials Science,Tianjin Normal University Tianjin 300387,China
3 State Key Laboratory of Superhard Materials,Jilin University,Changchun 130012,China
Synthesis of diamonds in Fe C systems using nitrogen and hydrogen co-doped impurities under HPHT?
Shi-Shuai Sun(孫士帥)1,?,Zhi-Hui Xu(徐智慧)1,Wen Cui(崔雯)2,Xiao-Peng Jia(賈曉鵬)3,and Hong-An Ma(馬紅安)3
1 College of Science,Tianjin University of Technology,Tianjin 300384,China
2 College of Physics and Materials Science,Tianjin Normal University Tianjin 300387,China
3 State Key Laboratory of Superhard Materials,Jilin University,Changchun 130012,China
In this study,we investigate the effect of nitrogen and hydrogen impurities on colors,morphologies,impurity structures and synthesis conditions of diamond crystals in Fe–C systems with C3N6H6additives at pressures in the range 5.0–6.5 GPa and temperatures of 1400–1700°C in detail.Our results reveal that the octahedron diamond nucleation in a Fe–C system is evidently inhibited by co-doped N–H elements,thereby resulting in the increase of minimum pressure and temperature of diamond synthesis by spontaneous nucleation.The octahedron diamond crystals synthesized from a pure Fe–C system are colorless,while they become green in the system with C3N6H6additive.The surface defects of diamond will deteriorate when the nitrogen and hydrogen atoms simultaneously incorporate in the diamond growth environment in the Fe–C system.We believe that this study will provide some important information and be beneficial for the deep understanding of the crystallization of diamonds from different component systems.
high pressure and high temperature,diamond,N/H impurity,catalyst/solvent
1.Introduction
An increasing attention has been paid on the diamond growth under high pressure and high temperature(HPHT) during the last decades,owing to their exotic properties and demonstrated applications.[1–3]It has been established experimentally that catalyst/solvent is an important factor to affect the diamond nucleation and growth.[4–8]Although the studies of diamond crystallization in various catalyst/solvent systems provide further insight into the mechanism of diamond formation,many problems related to the general features and peculiarities of natural diamond remains open because the growth of natural diamond is a complex process.[9,10]Recently,adding impurities is found to be another key factor for controlling the crystallization,morphological characteristics, and optical properties of diamond crystals.[11–14]In order to perceive the mechanism of diamond nucleation and growth more clearly,and provide the potential possibility to synthesize diamonds with unique properties,considerable attention has been devoted on the synergistic effect of catalyst/solvent and impurity on diamond crystallization.
Considering the primary impurities of nitrogen and hydrogen in natural diamonds,the crystallization of diamonds from various catalyst/solvent systems with nitrogen or hydrogen impurity have been widely investigated.Experimental studies show that the nitrogen or hydrogen impurity introduced into catalyst/solvent system will induce drastic changes on the growth,morphology,and particularly the properties of diamonds.[15–17]Further,the simultaneous incorporation of nitrogen and hydrogen into diamond growth conditions is confirmed to be a reasonable approach for investigating the genesis of natural diamond.[18,19]In fact,many natural diamond crystals associated with the inhomogeneous distribution of defects and impurities are composed by{111}faces.Hence, investigating the crystallization of{111}octahedron diamond in the catalyst/solvent system co-doped with nitrogen and hydrogen impurities under HPHT conditions will be considerably beneficial for our further understanding of the genesis of natural diamond.In addition,among catalyst/solvent systems, Fe is a primary element in the earth crust and plays a significant role in the growth process of natural diamond.Simultaneously,the stable growth form of diamond synthesized from Fe–C system under HPHT is typically octahedron.[16]Hence, the study of the synthesis of diamond in the Fe–C system with nitrogen and hydrogen co-doped impurities will be beneficial for further revealing the formation of natural diamond.However,publication on this subject is scarce.
In the present study,we investigated the diamond crystallization in the system of pure Fe catalyst with additive C3N6H6.The effect of simultaneous incorporation of nitrogen and hydrogen elements on colors,morphologies,impurity structures,and synthesis conditions of octahedron diamond crystals in the Fe–C system is investigated in detail.We be-lieve that this study will provide some important information and be beneficial for the deep understanding of the crystallization of diamond from different component systems.
2.Experimental
The diamond crystallization experiments were conducted using a china-type large volume cubic high-pressure apparatus(CHPA)(SPD-6×1200)with a sample chamber of 13-mm edge length.The design of the high-pressure cell for diamond synthesis is shown in Fig.1.A Pt RH/Pt Rh6 thermocouple was used for measuring the temperature in each experiment, whose junction was located near the crystallization sample. The pressure was calibrated by the pressure-induced phase transitions of bismuth,thallium,and barium.Crude scalelike graphite powder and pure Fe powder(200 mesh)were used as carbon source and solvent–catalyst,respectively.In addition,0.1 wt.%C3N6H6powders were used as the additive for providing the sources of nitrogen and hydrogen.
After the experiment,the sample was dissolved in boiling H2SO4and HNO3for eliminating the remaining graphite and metal on the diamond surface.Then,the morphologies and the structures of the obtained sample were characterized by optical microscopy(OM),SEM,and Fourier transform infrared (FTIR).
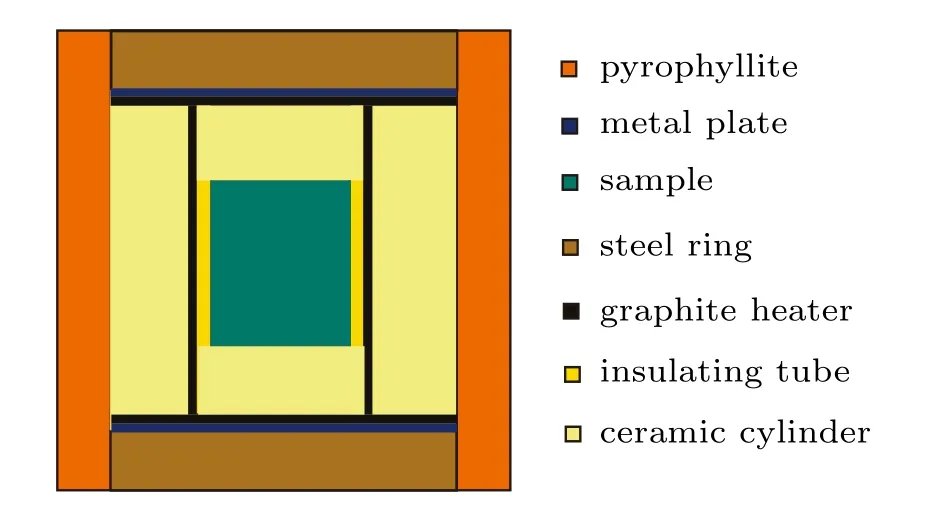
Fig.1.(color online)High-pressure cell for diamond synthesis.
3.Results and discussion
3.1.Diamond crystallization in Fe–C system with C3N6 H6 additive
The diamond crystallization from a Fe–C system with and without C3N6H6additive runs at 5.0–6.5 GPa and 1400–1700°C conditions.Our results reveal that the minimum pressure and temperature for diamond crystallization in the Fe–C–C3N6H6system(6.1 GPa,1500°C)is evidently higher than that without an additive system(5.5 GPa,1400°C).In order to illuminate the effect of C3N6H6additive on diamond crystallization in the Fe–C system clearly,we draw the P–T diagram(Fig.2(a))of diamond growth under different systems based on our experimental results(Table 1).The results indicate that the V shape of diamond growth moves up when the C3N6H6additive is added into the Fe–C system.It is reported that adding C3N6H6will release N and H impurities simultaneously under HPHT.[16,18]Hence,when a large number of N and H impurities exist in the diamond growth environment from the Fe–C system,the characteristics of Fe catalyst will change resulting in the change of diamond synthesized conditions.
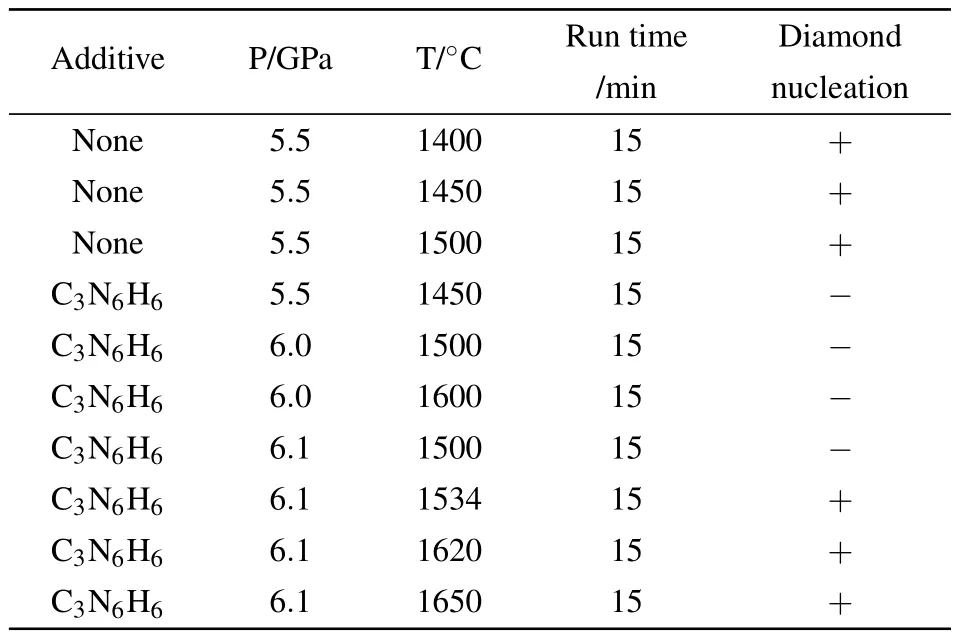
Table 1.Partial experimental results of diamond growth in Fe–C systems.
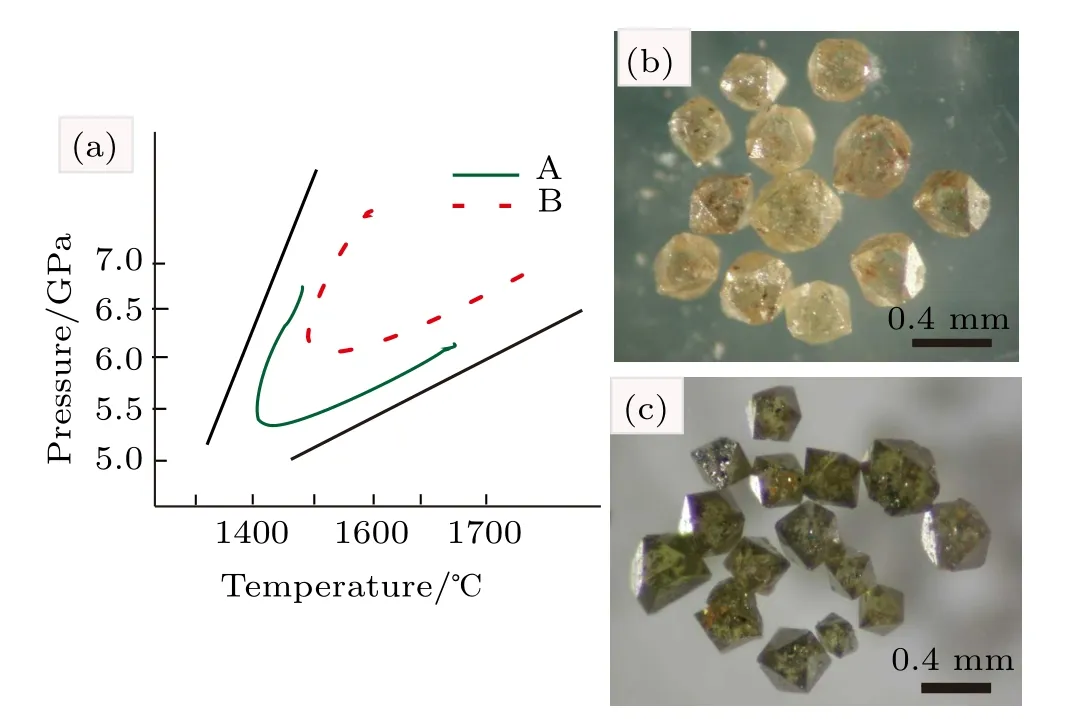
Fig.2.(color online)(a)P–T diagram of diamond synthesized in Fe–C system,A:without additive,B:with C3 N6H6 additive;(b)OM photographs of diamond crystals synthesized from Fe–C system;(c)OM photographs of diamond crystals synthesized from Fe–C system with 0.1 wt.%C3N6H6 additive.
The optimal synthetic conditions for high quality diamond growth from different systems are shown in Table 2.The OM photographs of synthetic diamond crystals are shown in Figs.2(b)and 2(c).The size of diamond crystals from Fe–C and Fe–C–C3N6H6systems is approximately 0.4 mm in diameter.Moreover,the stable growth forms with and without additive C3N6H6are octahedron.However,the crystal color changes from colorless to green when the C3N6H6is added into the Fe–C system.It is well known that the type and concentration of impurities incorporated into a diamond structure affect the color of the diamond.[11,13]Hence,our results reveal that the diamonds synthesized from Fe–C and Fe–C–C3N6H6systems exhibit different types and concentrations of impurities incorporated into the diamond structure.
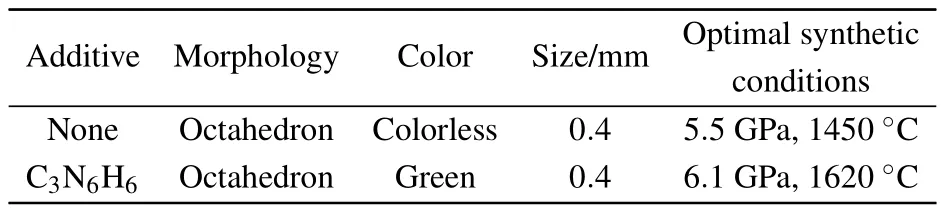
Table 2.Synthetic conditions for high quality diamond growth from Fe–C systems.
3.2.FTIR spectra of synthesized diamonds
In order to clarify the states of impurities incorporated into the diamond structure,the FT-IR absorption is used for quantitative measurements.Typical FTIR spectra of the synthesized diamond from the Fe–C system with and without C3N6H6additive are shown in Fig.3.Previous reports reveal that the nitrogenrelated absorption spectra is primarily located at the position of 1130,1280,and 1344 cm?1.[15,20]However, nitrogen-related absorption peaks in the FTIR spectra of diamond synthesized form Fe–C system are not found(Fig.3(b)). This phenomenon indicates that the concentration of nitrogen impurity in the structure of diamond from the Fe–C system is low,thereby inducing the colorless of the synthesized diamond.However,the strength of nitrogen related absorption peaks in the form of single substitutional nitrogen atoms(positions located at 1130 and 1344 cm?1)increase when C3N6H6is added into the Fe–C system(Fig.3(a)).Based on the following formula:[15]
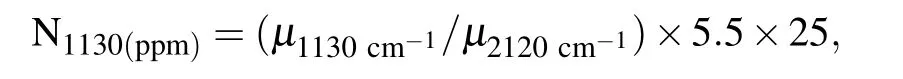
whereμ(1130 cm?1)andμ(2120 cm?1)represent the absorption intensities of 1130 cm?1and the dip at 2120 cm?1, respectively,the calculated concentration of nitrogen impurities are 325 ppm for the synthesized diamond from Fe–C–C3N6H6system.Simultaneously,the samples also exhibit some absorption peaks related to hydrogen impurity at 2850 and 2920 cm?1,corresponding to a–CH2–group.[17–19]The FTIR results reveal that the nitrogen and hydrogen atoms simultaneously incorporated into diamond growth conditions will significantly affect the inner structure of the diamond.
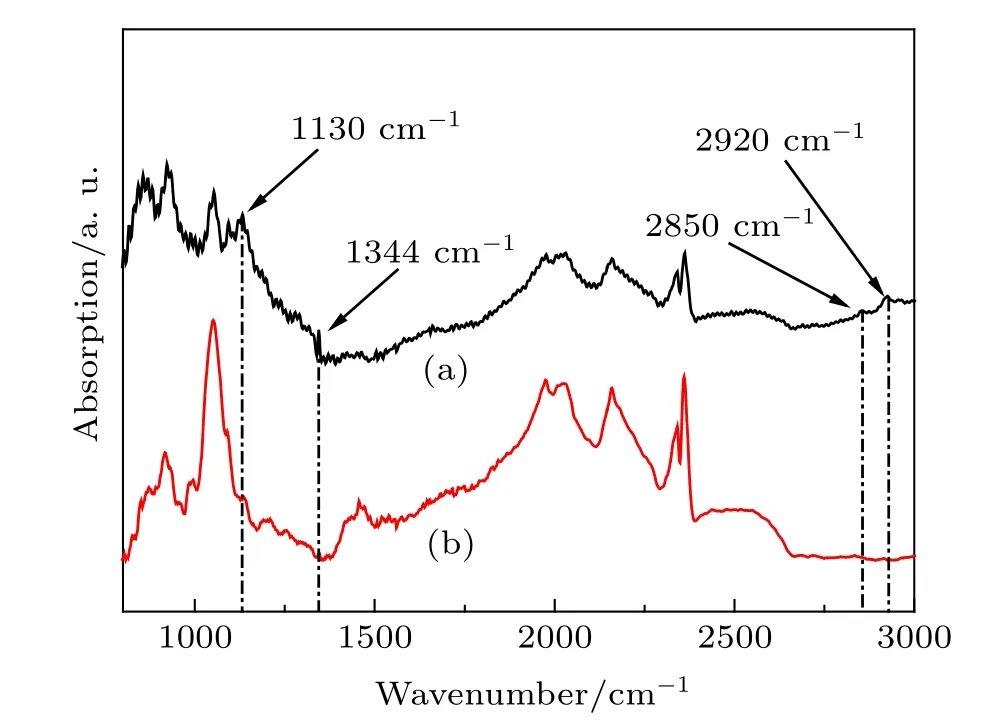
Fig.3.(color online)Typical FTIR spectra of synthesized diamond from Fe–C system:(a)with C3N6H6 additive;(b)without C3N6H6 additive.
3.3.Surface characters of synthetic diamond crystals
The SEM photographs for diamond crystals synthesized from Fe–C system are shown in Fig.4.It is found that the diamond crystals synthesized from the Fe–C system exhibit octahedral shape with predominantly{111}faces(Fig.4(a)).In addition,there are many triangle cavities shape defects in the {111}surfaces(Figs.4(b)and 4(c)).The cavities of triangular pyramid shown in the same lattice plane maintain the same triangular orientation,that is,each edge of the triangle pyramid is parallel.Meanwhile the orientation of triangular pyramid is opposite to that of the{111}crystal plane.In order to analyze the surface characters of the synthetic diamond crystals from the Fe–C–C3N6H6system in detail,the SEM photographs of diamond crystals synthesized from different conditions in the Fe–C–C3N6H6system are shown in Figs.5–7.It is clear that the stable growth face of a diamond synthesized from the Fe–C–C3N6H6system is also{111}surfaces,which is similar with that of the Fe–C system.However,the surface characters of the diamond synthesized from the Fe–C–C3N6H6system evidently differ from the diamond obtained from the pure Fe–C system.At relatively low temperatures,the diamond surface is rather rough with some foothills shape defect (Fig.5).As the temperature increases,although the{111} surfaces become relatively smooth,the large depressions or hollows are observed at the surface and vertices of the octahedral diamond crystal(Fig.6).In some experiments,which are performed at higher temperatures,larger number and bigger size of triangle shape defects appear on the diamond surface (Fig.7(b)).During the diamond growth process,the surface defects are easily formed owing to the impurity incorporation into the crystal structure.Considering the difficult incorporation of iron atoms into the diamond structure in Fe–C–C3N6H6system,[16,17]the simultaneous incorporation of the nitrogen and hydrogen atoms play an important role in the morphology change of the diamond surface,which is consistent with the result of FTIR(Fig.3).
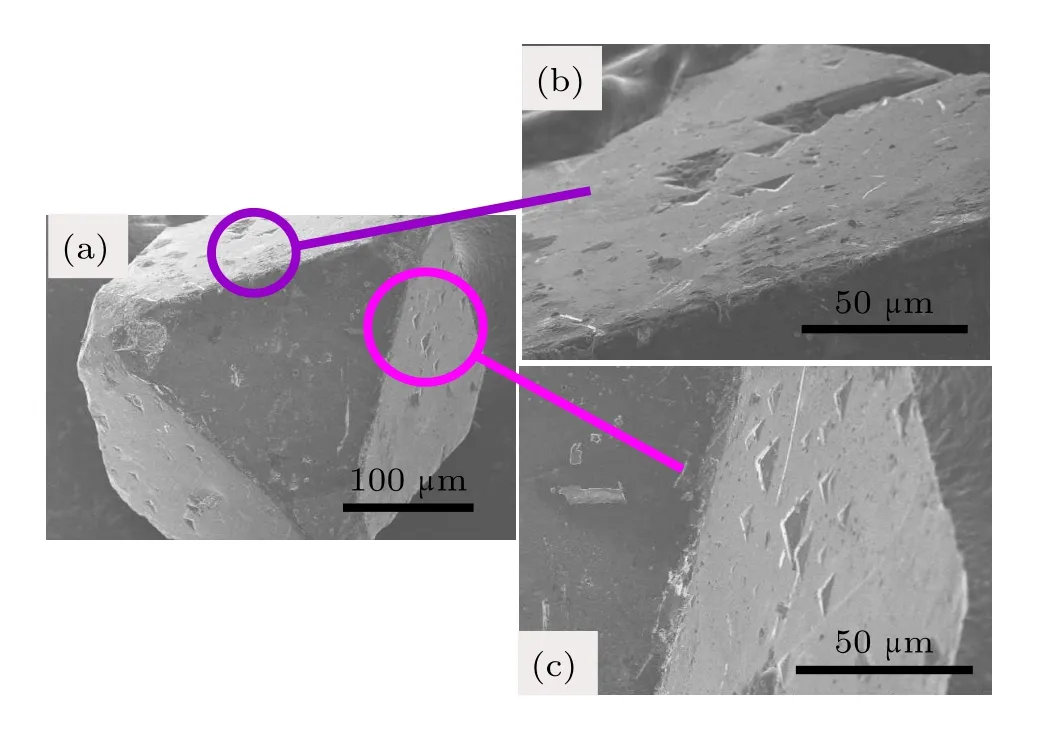
Fig.4.(color online)SEM photographs of diamond crystals synthesized from Fe–C system under 5.5 GPa,1450°C.
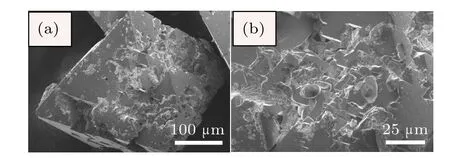
Fig.5.(color online)SEM photographs of diamond crystals synthesized from Fe–C system with C3N6H6 additive under 6.1 GPa,1534°C.
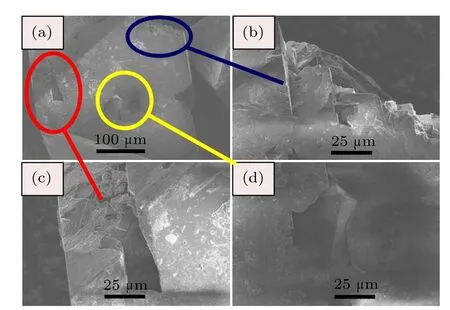
Fig.6.(color online)SEM photographs of diamond crystals synthesized from Fe–C system with C3N6H6 additive under 6.1 GPa,1620°C.
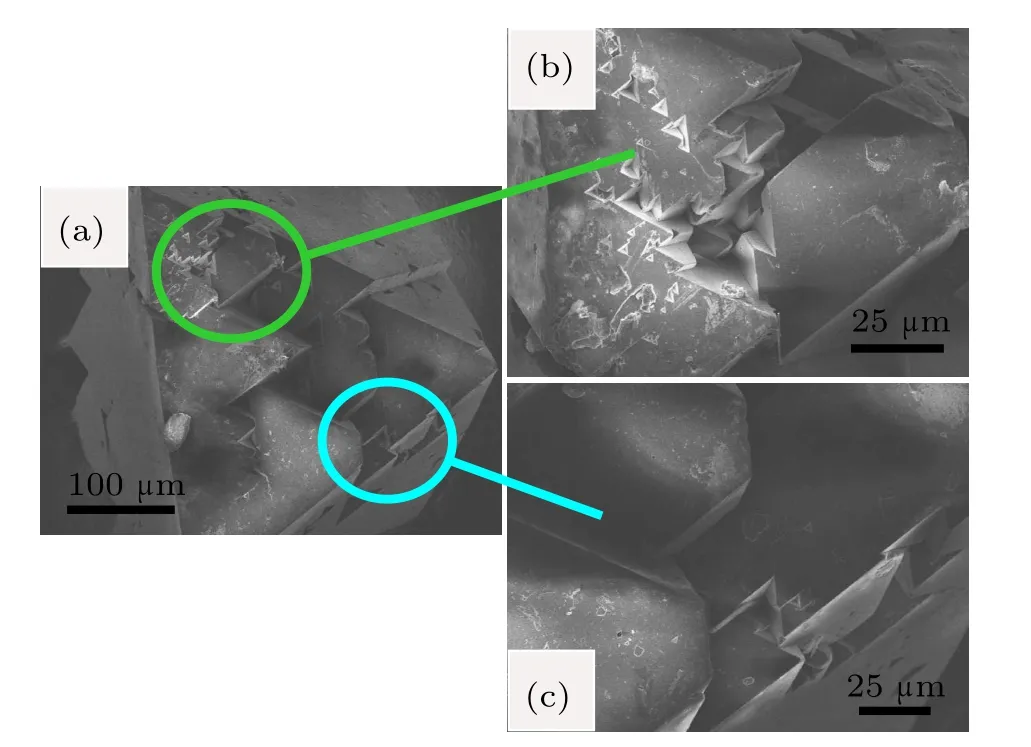
Fig.7.(color online)SEM photographs of diamond crystals synthesized from Fe–C system with C3N6H6 additive under 6.1 GPa,1650°C.
The surface texture of the most natural octahedron diamonds is diverse,which is similar to the observed above mentioned phenomenon.Analysis of the data on the interior structure of natural octahedron diamonds,it can be found that the nitrogen and hydrogen are typically the primary impurities.Hence,the color and surface morphology of natural octahedron diamonds are related to the incorporation of nitrogen and hydrogen impurities into the diamond growth environments,which is similar to the phenomenon in our experiments. Therefore,it should be noted that the states of nitrogen and hydrogen impurities in our produced octahedron diamonds are still evidently different from natural octahedron diamonds.In fact,a longer time is essential for the formation of natural diamonds,while only more than 1 min is required for man made diamonds.[21,22]Hence,although the nitrogen and hydrogen are easily incorporated into the diamond structure,more stable states of these impurities,such as the aggregated nitrogen forms and the structure of>C=CH2(located at 3107 cm?1), require further evolution.
4.Conclusion
In this study,we successfully synthesized diamond crystals with octahedron shape from Fe–C systems with and without C3N6H6additive under HPHT conditions.Our results revealed that the diamond nucleation in a Fe–C system is evidently inhibited by co-doped N–H elements,thereby resulting in the increase of minimum pressure and temperature of diamond synthesis by spontaneous nucleation.Moreover,the color and surface morphology are evidently changed when C3N6H6is added into the Fe–C system.The results of FTIR studies on synthesized diamonds indicate that the concentration of nitrogen impurity in diamond crystals with C3N6H6additive is higher than that of the system without additive.Simultaneously,the hydrogen atoms can enter the diamond lattice when N–H co-doped into diamond growth environments. We believe that our study will be considerably beneficial for further investigation on the genesis of natural diamond.
[1]Huang Q,Yu D,Xu B,Hu W T,Ma Y M,Wang Y B,Zhao Z S,Wen B,He J L,Liu Z Y and Tian Y J 2014 Nature 510 250
[2]Wang Z Y,Dong L H,Wang D S and Dong Y H 2012 Precision Engineering 36 162
[3]Lu H C,Peng Y C,Lin M Y,Chou S L,Lo J I and Cheng B M 2017 Carbon 111 835
[4]Li Y,Jia X P,Feng YG,Fang C,Fan L J,Li Y D,Zeng X and Ma H A 2015 Chin.Phys.B 24 088104
[5]Palyanov Y N,Borzdov Y M,Kupriyanov I N,Bataleva Y V, Khokhryakov A F and Sokol A G 2015 Cryst.Growth Des.15 2539
[6]Liu W Q,Ma H A,Li X L,Liang Z Z,Li R and Jia X 2007 Diamond Relat.Mater.16 1486
[7]Kupriyanov I N,Khokhryakov A F,Borzdov Y M and Palyanov Y N 2016 Diamond Relat.Mater 69 198
[8]Yamaoka S,Kumar M D,Kanda H and Akaishi M 2002 Diamond Relat.Mater.11 1496
[9]Stachel T and Harris J W 2009 J.Phys.Condens.Mat.21 364206
[10]Palyanov Y N,Khokhryakov A F,Borzdov Y M and Kupriyanov I N 2013 Cryst.Growth Des.13 5411
[11]Li Y,Li Z B,Song M S,Wang Y,Jia X P and Ma H A 2016 Acta Phys. Sin.65 118103(in Chinese)
[12]Hu M H,Bi N,Li S S,Su T C,Zhou A G,Hu Q,Jia X P and Ma H A 2015 Chin.Phys.B 24 038101
[13]Liang Z Z,Jia X,Zang C Y,Zhu P W,Ma H A and Ren G Z 2005 Diamond Relat.Mater.14 243
[14]Lu Y G,Turner S,Ekimov E A,Verbeeck J and Tendelooa G V 2015 Carbon 86 156
[15]Liang Z Z,Jia X,Ma H A,Zang C Y,Zhu P W,Guan Q F and Kanda H 2005 Diamond Relat.Mater.14 1932
[16]Sun S S,Liu M N,Cui W,Jia X P,Ma H A and Yang L Y 2016 Int.J. Refract.Met H.61 79
[17]Sun S S,Jia X P,Yan B M,Wang F B,Chen N,Li Y D and Ma H A 2014 Cryst.Eng.Comm.16 2290
[18]Liu X B,Jia X P,Fang C and Ma H A 2016 Cryst.Eng.Comm.18 8506
[19]Sun S S,Jia X P,Yan B M,Wang F B,Li Y D,Chen N and Ma H A 2014 Diamond Relat.Mater.42 21
[20]Zhang Y F Zang C Y,Ma H A,Liang Z Z,Zhou L Li S S and Jia X P 2008 Diamond Relat.Mater.17 209
[21]Zhang Z F,Jia X P,Liu X B,Hu M H,Li Y,Yan B M and Ma H A 2012 Chin.Phys.B 21 038103
[22]Xiao H Y,Liu L N,Qin Y K,Zhang D M,Zhang Y S,Sui Y M and Liang Z Z 2016 Acta Phys.Sin.65 050701(in Chinese)
5 May 2017;revised manuscript
25 May 2017;published online 31 July 2017)
10.1088/1674-1056/26/9/098101
?Project supported by the National Natural Science Foundation of China(Grant Nos.11504267,11504269,and 51172089),the Open Project of State Key Laboratory of Superhard Materials,Jilin University,China(Grant No.201504),and the Doctoral Fund of Tianjin Normal University,China(Grant No.52XB1518).
?Corresponding author.E-mail:sssdashuai@163.com
?2017 Chinese Physical Society and IOP Publishing Ltd http://iopscience.iop.org/cpb http://cpb.iphy.ac.cn
- Chinese Physics B的其它文章
- Relationship measurement between ac-Stark shift of 40Ca+clock transition and laser polarization direction?
- Air breakdown induced by the microwave with two mutually orthogonal and heterophase electric field components?
- Collective motion of active particles in environmental noise?
- Temperature dependence of heat conduction coefficient in nanotube/nanowire networks?
- Analysis of dynamic features in intersecting pedestrian flows?
- Heat transfer enhancement in MOSFET mounted on different FR4 substrates by thermal transient measurement?

