由芳香羧酸和含氮配體構筑的兩個金屬鋅配合物的合成、晶體結構和熒光性質
肖伯安 陳水生
(1鄭州幼兒師范高等專科學校,鄭州450099)(2阜陽師范學院化學與材料工程學院,阜陽236041)
由芳香羧酸和含氮配體構筑的兩個金屬鋅配合物的合成、晶體結構和熒光性質
肖伯安1陳水生*,2
(1鄭州幼兒師范高等專科學校,鄭州450099)
(2阜陽師范學院化學與材料工程學院,阜陽236041)
以1-(4-咪唑基)-4-(四氮唑)苯(HL)與對苯二甲酸(H2pbda)或間苯二甲酸(H2mbda)作為混合配體,與硫酸鋅進行水熱合成反應,構筑了2個新的金屬配位配合物[Zn2(L)2(pbda)]n(1)和[Zn2(L)2(mbda)]n(2),并進行了元素分析、紅外、熱重、X射線粉末衍射及單晶衍射等表征。晶體結構分析表明:配合物1結構中,去質子化的L-與鋅離子結合成[Zn2(L)2]2+的二維結構,相鄰的二維層由pbda2-配體連接成二重貫穿層柱狀的具有dmc拓撲的三維結構,拓撲符號為(4·82)(4·85);配合物2是由2個羧酸基團與鋅離子形成的雙核[Zn2(COO)2]為基本單元連結而成的單一6連結具有(412·63)-pcu拓撲的三維結構。同時,對配合物1和2在室溫下的固體熒光性質進行了研究。
配位聚合物;合成;結構;熒光性質
0 Introduction
The fabrication of functional metal-organic frameworks(MOFs),are totally dependent on the judicious choiceofsuitableorganicspaceswithdifferent binding sites,directions,and lengths[1-3],and reaction conditions have a great influence on the resulting structures of the complexes[4-6].Therefore,in order to obtain desirable specific structures and functions of the architectures,it is vital to design the organic ligands and/or regulate the synthesis conditions.The poly-azaheteroaromaticligandsandmulticarboxylic acids with two or more coordination atoms,are versatile ligands to be employed to the assembly of various MOFs[7-12].As a consequence,most MOFs are constructed from metal ions and carboxylic acid-or nitrogen-containing ligands.Particularly,N-heterocyclic bridging ligands such as imidazolate,tetrazolate can exhibit more versatile coordination modes because the multi-N donor group can often act as anionic ligands by deprotonation of acidic N-H groups,in addition, they can also serve as neutral ones[13-14].Meanwhile, mixed ligands by incorporating multicarboxylates and N-donor ligands have been proved to be effective strategytobuilddesirableMOFs.Ourprevious research has revealed that the combination of O-donor carboxylate ligand and an N-donor ligand is facile to construct MOFs,and among them,unusual topology, exceptional entangled structures,selective adsorption of CO2molecules,and tunable luminescence properties[15-18].Take the favorable coordination ability of the multi-N donor groups into account,we design the multi-N organic ligands 1-(1H-imidazol-4-yl)-4-(4H-tetrazol-5-yl)benzene(HL)containingdifunction groups with 4-imidazolate and tetrazolate groups.Following such a mixed ligand strategy,we have performed the study on reactions of ligand HL together with different carboxylate ligands and ZnSO4·7H2O salts. Herein we report the syntheses,crystal structures and photoluminescent properties of two new zinccomplexes[Zn2(L)2(pbda)]n(1)and[Zn2(L)2(mbda)]n(2) obtainedbyreactionsofHLandtwodifferent carboxylic acids,respectively.
1 Experimental
1.1 Materials and measurements
The ligand HL was synthesized according to our previously reported literature[19].Elemental analyses were carried out with a Perkin-Elmer 240C Elemental Analyzer.IR spectra were recorded on a Bruker Vector 22 FT-IR spectrophotometer using KBr pellets. Thermogravimetric analyses(TGA)were performed on a simultaneous SDT 2960 thermal analyzer under nitrogen at a heating rate of 10℃·min-1.Powder X-ray diffraction(PXRD)patterns were measured on a Shimadzu XRD-6000 X-ray diffractometer with Cu Kα (λ=0.154 18 nm)radiation,in which the X-ray tube was operated at 40 kV and 40 mA at room temperature. The luminescence spectra for the powdered solid samples at room temperature were measured on an Aminco Bowman Series 2 spectrofluorometer with a xenon arc lamp as the light source with the pass width of emission and excitation spectra of 5 nm.
1.2 Synthesis of complex[Zn2(L)2(pbda)]n(1)
A reaction mixture of HL(21.2 mg,0.1 mmol), ZnSO4·7H2O(28.7 mg,0.1 mmol),H2pbda(18.0 mg, 0.1 mmol)and H2O(8 mL)was adjusted to pH=6 with 0.5 mol·L-1NaOH solution.The mixture was then sealed into a 16 mL Teflon-lined stainless steel container and heated at 160℃for 3 days.After cooling to the room temperature,colorless needle crystals of 1 were collected by filtration and washed by water and ethanol for several times with a yield of 52%.Anal. Calcd.for C14H9N6O2Zn(%):C,46.88;H,2.53;N,23.43. Found(%):C,46.65;H,2.42;N,23.56.IR(KBr,cm-1): 3 325~3 086(m),1 607(vs),1 468(m),1 393(s),1 355 (vs),1 304(m),1 260(m),1 141(s),1 090(m),1 015 (w),946(w),851(s),826(vs),750(vs),643(s),599(w), 562(w).
1.3 Synthesis of complex[Zn2(L)2(mbda)]n(2)
Complex 2 was obtained by a hydrothermal procedure as that for preparation of 1 using H2mbda (16.6 mg,0.1 mmol)instead of H2pbda.Colorless needle crystals of 2 were collected in 68%yield after being washed with water and ethanol several times. Anal.Calcd.for C14H9N6O2Zn(%):C,46.88;H,2.53;N,23.43.Found(%):C,46.97;H,2.69;N,23.31.IR (KBr,cm-1):3 143~2 790(w),1 607(vs),1 556(vs), 1 456(vs),1 399(s),1 298(w),1 141(m),1 071(m), 977(m),838(m),813(m),763(s),713(m),643(s),593 (w),543(w).
1.4 Crystal structure determination
TheX-raydiffractionmeasurementsfortwo single crystals with dimensions of 0.24 mm×0.22mm× 0.21 mm(1)and 0.20 mm×0.18 mm×0.16 mm(2)were performed on Bruker Smart Apex CCD diffractometer with graphite-monochromatedMo Kα radiation(λ= 0.071 075 nm).The structures of complexes were solved by direct methods,and the non-hydrogen atoms were located from the trial structure and then refined anisotropically with SHELXTL using a full-matrix least-squares procedure based on F2values[20].All nonhydrogen atoms were refined anisotropically.Details of the crystal parameters,data collection and refinements for 1 and 2 are summarized in Table 1.Selected bond lengths and angles for 1 and 2 are listed in Table 2.
CCDC:1502275,1;1502276,2.
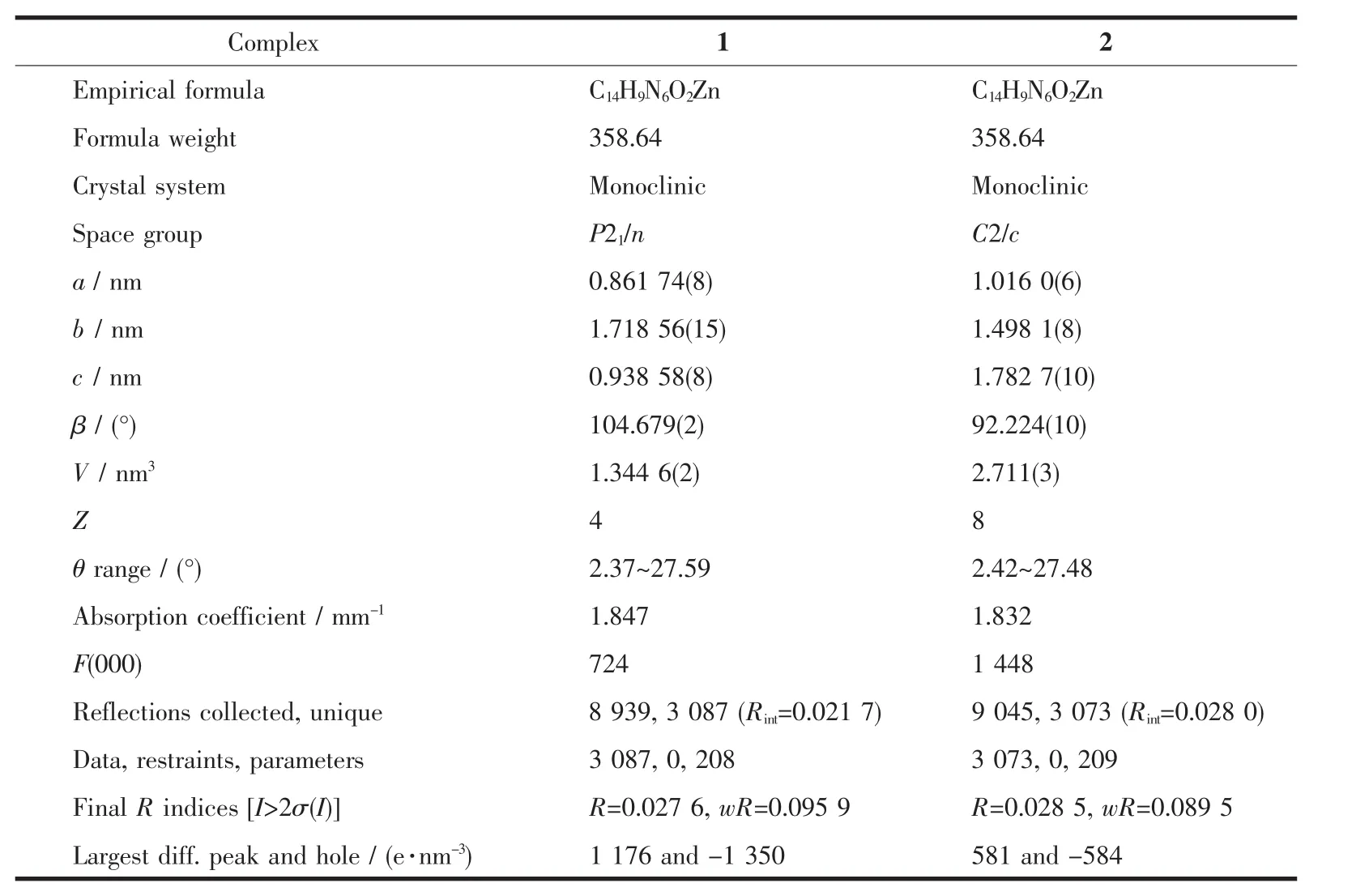
Table 1Crystallographic data of complexes 1 and 2
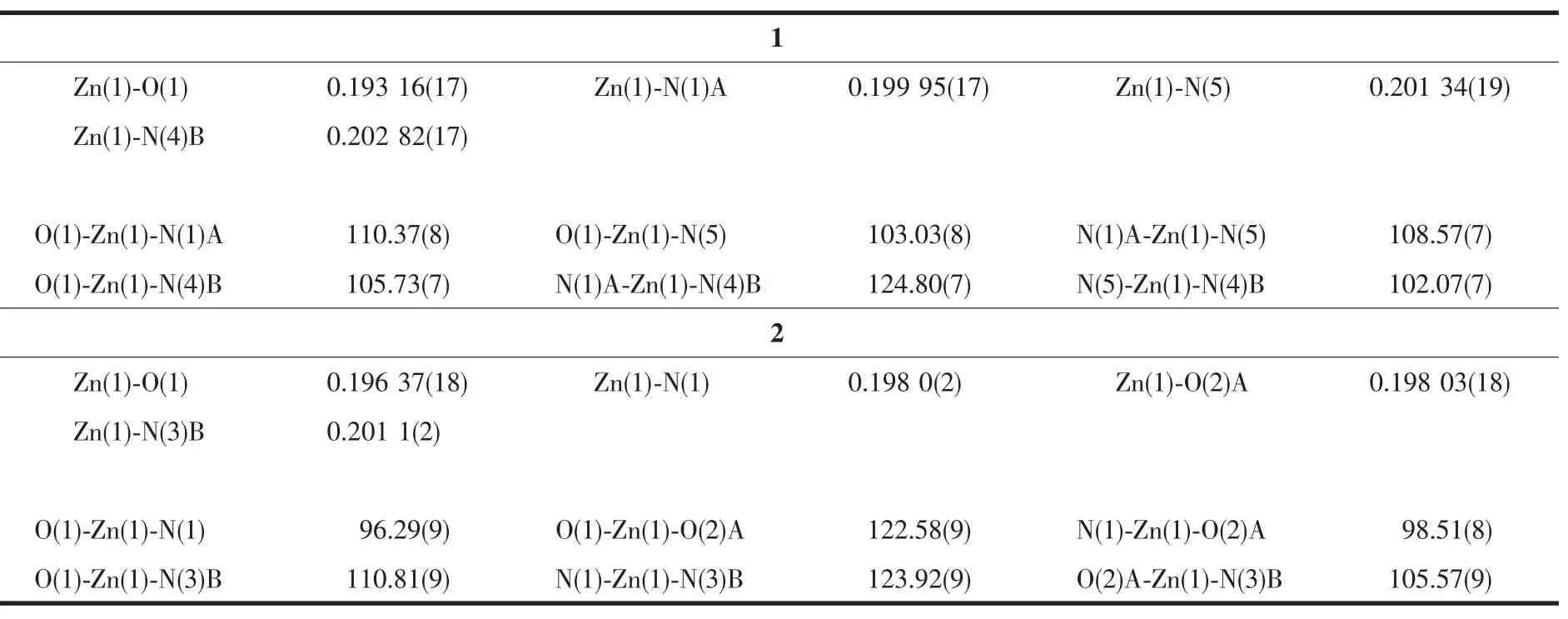
Table 2Selected bond lengths(nm)and bond angles(°)of complexes 1 and 2
2 Results and discussion
2.1 Crystal structure of 1
The complex 1 crystallizes in monoclinic form with space group of P21/n(Table 1)and the asymmetric unit consists of one deprotonated L-ligand,a half of pbda2-and one Znatom,.As shown in Fig.1,each Znatom is four-coordinated by three nitrogen(N5, N1A,N4B)atoms from three different HL ligands and one oxygen atom(O1)of one carboxylate group from a pbda2-,forming distorted tetrahedral coordination geometry.TheZn-O distances are 0.19316(17)nm while the Zn-N ones are 0.199 95(17),0.201 34(19)and 0.202 82(17)nm respectively,and the coordination angles around the Zn1 are in the range of 102.07(7)°~124.80(7)°(Table 2).In 1,the deprotonated tetrazole fragment of L-coordinating with Znatoms form a one-dimensional(1D)chain structure,which is further connected by the 4-imidazolyl groups of L-to give a two-dimentional(2D)double-layer fes network with 4· 82topology in the bc plane(Fig.2)[21].And the Zn-L-2D sheets are further pillared by rod-type pbda2-to form a three-dimensional(3D)framework with Zn…Zn separation of 1.127 nm between two adjacent 2D layers (Fig.3).Topologically,each L-links three Znatoms, which can be regarded as a 3-connected node.As for each Znatom,it in turn links two L-and two pbda2-, hence,it can be treated as a 4-connector.Topology analysis calculated by TOPOS program[22]suggests thatthe resulting structure of the complex 1 is a binodal (3,4)-connected dmc net with point Schl?fli symbol of(4·82)(4·85)(Fig.4a)[23].When it is viewed along the c axis,the structure contains 1.425 nm×1.566 nm channels,which is occupied by another interpenetrating independent framework,in return,the complex 1 is a rare 2-fold interpenetrating dmc network(Fig.4b).
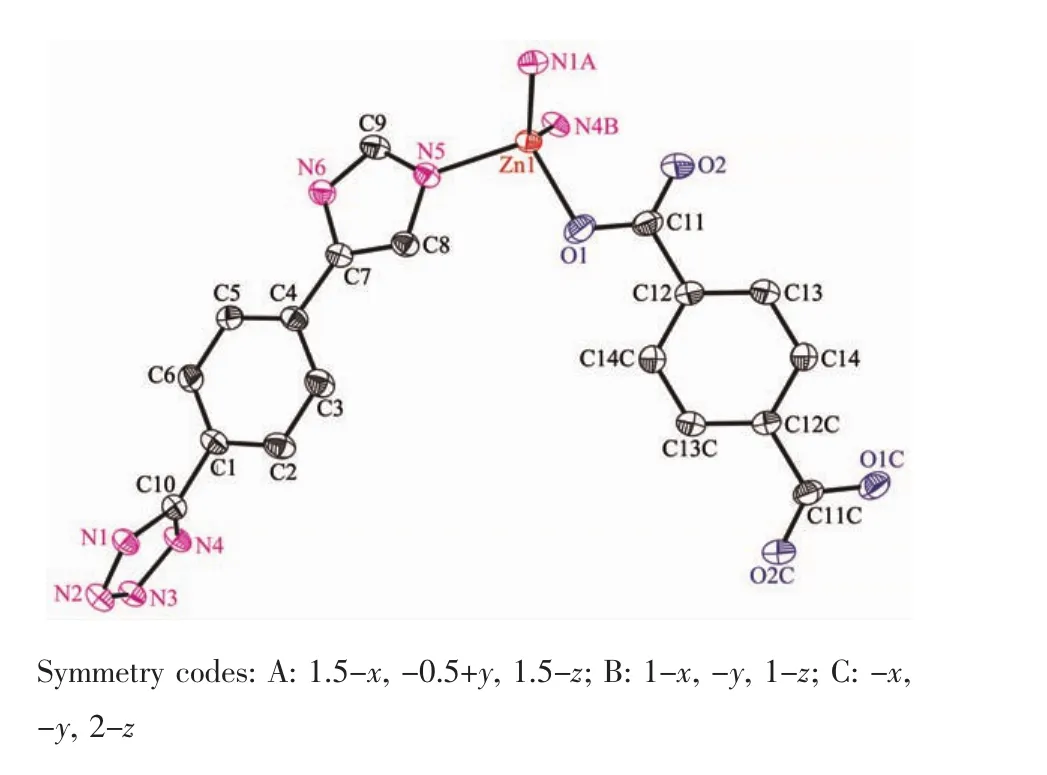
Fig.1View of the coordination environment of Znatom with thermal ellipsoids drawn at the 50% probability level for the complex 1
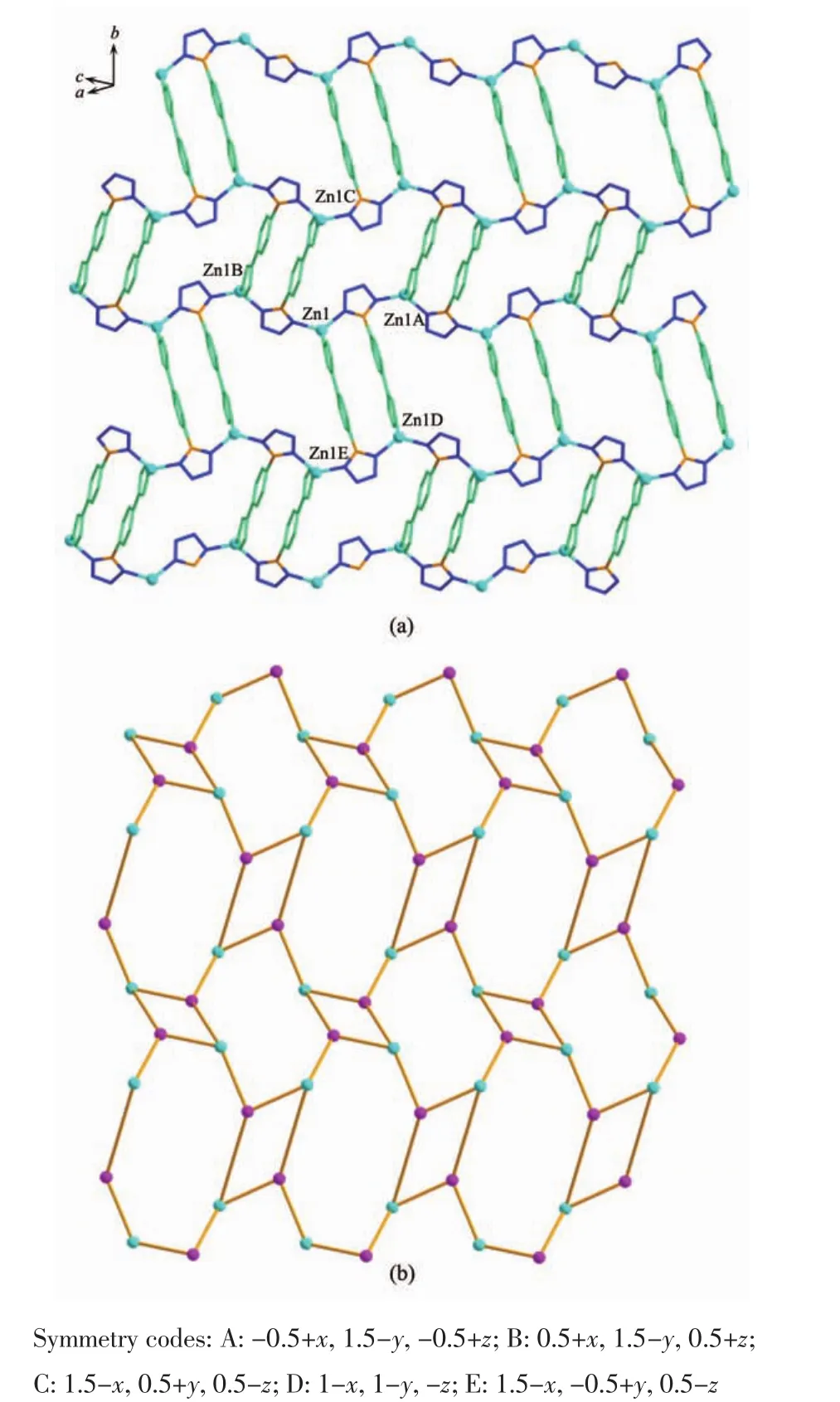
Fig.2(a)2D double-layer of 1 formed by Znatoms and deprotonated L-ligands;(b)Simplified 2D fes net of 1
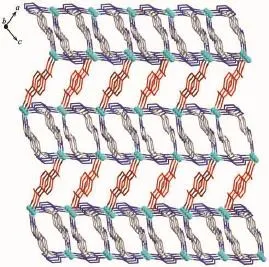
Fig.33D structure of 1 constructed from 2D networks pillared by pbda2-ligands
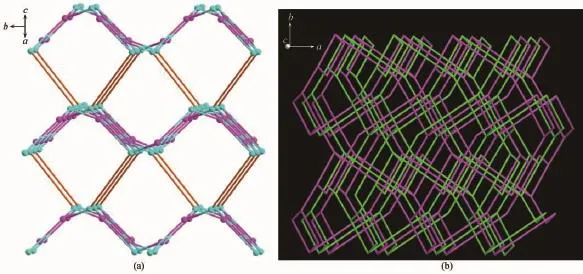
Fig.4(a)Topological view of the dmc topology of complex 1;(b)Schematic representation of the 2-fold interpenetrated 3D dmc net of 1
2.2 Crystal structure of 2
When 1,3-benzenedicarboxylic acid,instead of 1,4-benzenedicarboxylic acid,was employed in the reaction of 1,2 with a different structure was obtained. Single-crystal X-ray diffraction analysis reveals that complex 2 crystallizes in the monoclinic space group C2/c(Table 1)with one HL-,a half of mbda2-and one Znin the asymmetric unit.The crystallographically independent Zn1 shows a slightly distorted tetrahedral coordinationgeometry,beingcoordinatedbyone imidazole N atom,one tetrazole N atom from two L-ligands and two O atoms from different mbda2-(Fig.5). In this complex,both of each mbda2-ligand adopt μ2-η1∶η1-bridging coordination mode to coordinate with Znatoms,in return,each ligand acts as μ4-bridge to link four Znatoms.Two such carboxylate groups from different mbda2-ligands bridge two Znatoms to give a binuclear[Zn2(COO)2]motif,with the Zn…Zn distance of 0.347 nm.Meanwhile,each Zn2(COO)2binuclear unit links two mbda2-ligands and four L-to form a 6-connected node as secondary building unit (SBU)(Fig.6).As for the L-and mbda2-,both of them act as μ2-bridges linking two neighboring Zn2(COO)2units to form 3D framework(Fig.7).Topologically,theycan be considered as 2-connected nodes and not counted as node,and the complex 2 exhibits a uninodal 6-connected 3D architecture with(412·63)-pcu topology (Fig.8)[24].
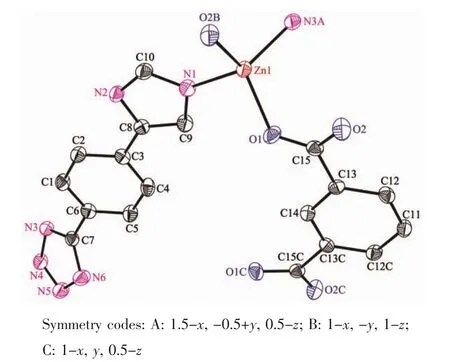
Fig.5View of the coordination environment of Znatom with thermal ellipsoids drawn at the 50% probability level for the complex 2
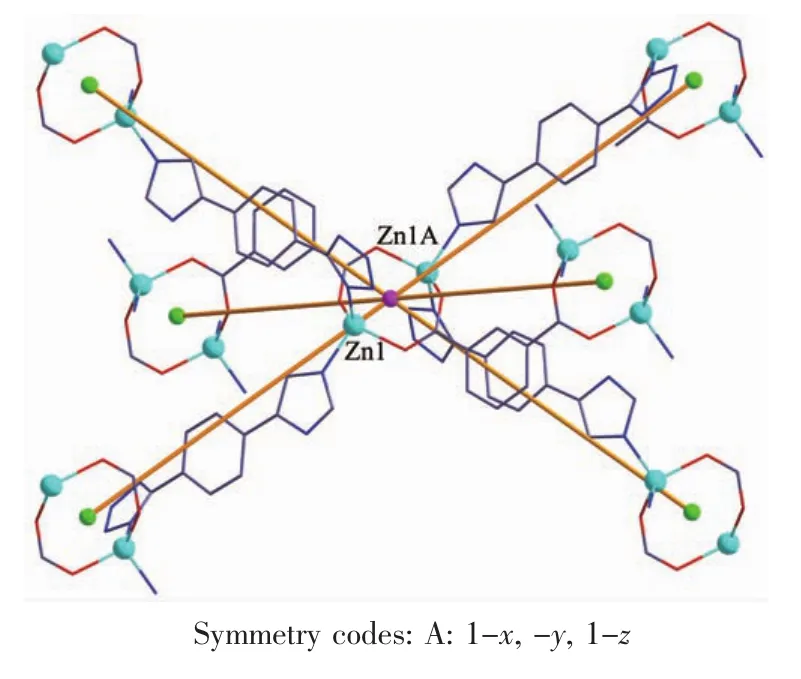
Fig.6View of the linkages of a binuclear Znnodes with six adjacent identical cores of 2
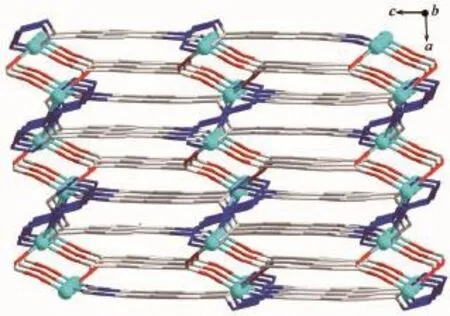
Fig.73D framework of 2
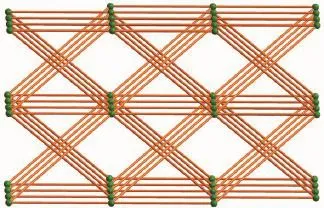
Fig.8Schematic representation of 3D α-Po net of 2
2.3 Thermal stabilities and powder X-ray diffraction
Complexes 1 and 2 were subjected to ascertain thestabilityofsupramoleculararchitectureby thermogravimetric analysis(TGA),shown in Fig.9.No obvious weight loss was found for 1 and 2 before the decomposition of the framework occurred at about 450 and 325℃respectively.Particularly,the residual percentage weight(Obsd.23.02%)at the end of the decomposition of the complex 1 is in accordance with the formation of ZnO(Calcd.22.68%).Powder XRD experiment was performed to confirm the phase purity of bulk sample,and the experimental patterns of the as-synthesized sample are well consistent with the simulated ones,indicating the phase purity of the sample(Fig.10).
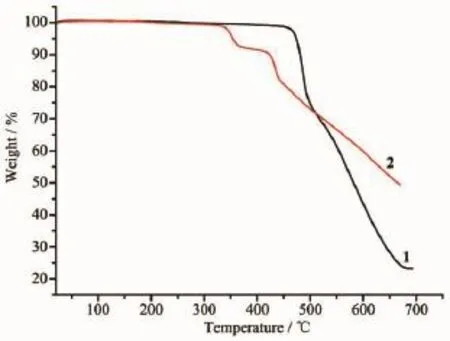
Fig.9TG curves of 1 and 2
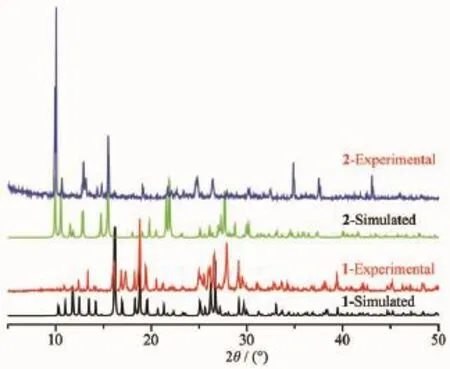
Fig.10Simulated and experimental PXRD patterns of 1 and 2
2.4 Luminescent properties
Compounds constructed by d10metal centers and conjugated organic linkers can be promising candidates employed as chemical sensors and photochemistry[25].The solid-state photoluminescent properties of complexes 1 and 2 have been investigated together with free HL ligands at room temperature(Fig.11). The free HL ligand shows blue photoluminescence emission at 385 nm upon excitation at 338 nm,which is probably attributable to the π*→n or π*→π transitions[26].As previously reported[27],the emission bands of solid-state benzenecarboxylate ligands can be assigned to the π*→n transition,but fluorescent emission of benzenedicarboxylate ligands resulting from the π*→n transition is very weak compared with that of the π*→π transition of the HL ligand,so benzenecarboxylate ligands almost have no contribution to the fluorescent emission of as-synthesized coordination polymers[28].On complexation of theseligands with Znatoms,excitation of the microcrystalline samples leads to the generation of strong blue fluorescentemissions,withthemaximalpeaks occurring at 406 nm(λex=340 nm)for 1,424 nm(λex= 345,375 nm)for 2.In contrast to the case for the free HL ligand,the emission bands of complexes 1 and 2 have red-shift of 21 and 39 nm,respectively.Such broademissionbandsofthecomplexesmainly originate from ligand-based luminescence,corresponding shifts originated from ligand-to-metal charge transfer[29-30].
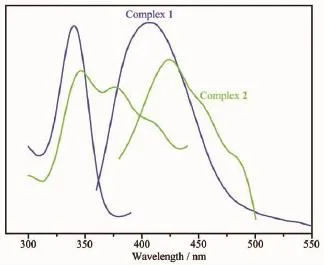
Fig.11Excitation(left)and emission(right)spectra of complex 1 and 2
[1]Cook T R,Zheng Y R,Stang P J,et al.Chem.Rev.,2013, 113:734-777
[2]Chen S S,Chen M,Takamizawa S,et al.Chem.Commun., 2011,47:4902-4904
[3]Li X J,Jiang F L,Wu M Y,et al.Inorg.Chem.,2012,51: 4116-4122
[4]Chen S S,Liu Q,Zhao Y,et al.Cryst.Growth Des.,2014, 14:3727-3741
[5]Karmakar A,Martins L M D R S,Hazra S,et al.Cryst.Growth Des.,2016,16:1837-1849
[6]Chen S S,Wang P,Takamizawa S,et al.Dalton Trans.,2014, 43:6012-6020
[7]Chen S S,Qiao R,Sheng L Q,et al.Z.Anorg.Allg.Chem., 2013,639:1808-1814
[8]Xiao L,Li W D,Fang X,et al.Chin.J.Struct.Chem.,2016, 35:781-788
[9]Guo X Q,Wang M,Meng,F,et al.CrystEngComm,2016,18: 5616-5619
[10]Liu S J,Cao C,Xie C C,et al.Dalton Trans.,2016,45:9209 -9215
[11]Maity D K,Halder A,Bhattacharya B,et al.Cryst.Growth Des.,2016,16:1162-1167
[12]Hu Z Y,Zhu J J,Chen S S,et al.Chin.J.Struct.Chem., 2015,34:899-905
[13]Chen S S.CrystEngComm,2016,18:6543-6565
[14]Chen S S,Chen M,Takamizawa S,et al.Chem.Commun., 2011,47:752-754
[15]Chen S S,Sheng L Q,Liu Z D,et al.Cryst.Growth Des., 2016,16:229-241
[16]Chen S S,Chen Z H,Fan J,et al.Cryst.Growth Des.,2012, 12:2315-2326
[17]Chen S S,Zhao Y,Fan J,et al.CrystEngComm,2012,14: 3564-3576
[18]Qiao R,Chen S S,Sheng L Q,et al.J.Solid State Chem., 2015,228:199-207
[19]Chen S S,Qiao R,Sheng L Q,et al.CrystEngComm,2013, 15:5713-5725
[20]Bruker 2000,SMART Ver.5.0,SAINT-plus Ver.6,SHELXTL Ver.6.1 and SADABS Ver.2.03,Bruker AXS Inc.,Madison, WI,2000.
[21]Xie L X,Hou X W,Fan Y T,et al Cryst.Growth Des.,2012, 12:1282-1291
[22]Blatov V A.TOPOS,A Multipurpose Crystallochemical Analysis with the Program Package,Samara State University, Russia,2009.
[23]Blatov V A,Carlucci L,Ciani G,et al.CrystEngComm, 2004,6:378-395
[24]Yang Q,Zhang X F,Zhao J P,et al.Cryst.Growth Des., 2011,11:2839-2845
[25]Kreno L E,Leong K,Farha O K,et al.Chem,Rev.,2012, 112:1105-1125
[26]Meng F,Zhang M,Shen K,et al.Dalton Trans.,2015,44: 1412-1419
[27]Hua J A,Zhao Y,Liu Q,et al.CrystEngComm,2014,16: 7536-7546
[28]Li Y W,Ma H,Chen Y Q,et al.Cryst.Growth Des.,2012, 12:189-196
[29]Wan X Y,Jiang F L,Chen L,et al.CrystEngComm,2015, 17:3829-3837
[30]Song S Y,Song X Z,Zhao S N,et al.Dalton Trans.,2012, 41:10412-10421
Syntheses,Structures and Photoluminescence Properties of Two ZnComplexes Constructed from Mixed Carboxylate and N-Donor Ligands
XIAO Bo-An1CHEN Shui-Sheng*,2
(1Zhengzhou Preschool Education College,Zhengzhou 450099,China)
(2College of Chemistry&Chemical Engineering,Fuyang Normal University,Fuyang,Anhui 236041,China)
Two Zncoordination polymers[Zn2(L)2(pbda)]n(1)and[Zn2(L)2(mbda)]n(2)were synthesized by reactions of ZnSO4·7H2O with rigid ligand 1-(1H-imidazol-4-yl)-4-(4H-tetrazol-5-yl)benzene(HL)and different carboxylic acids of 1,4-benzenedicarboxylic acid(H2pbda)or 1,3-benzenedicarboxylic acid(H2mbda).The complexes have characterized by single-crystal X-ray diffraction,elemental analysis,IR spectroscopy,photoluminescence spectrum,TG and PXRD.Polymer 1 possesses[Zn2(L)2]2+sheets pillared by the pbda2-ligand to form a 2-fold interpenetrating three-dimensional(3D)dmc net with point Schl?fli symbol of(4·82)(4·85),while 2 exhibits a uninodal 6-connected 3D architecture with(412·63)-pcu topology based on the binuclear Znsecondary building units(SBUs).Solid state luminescent properties of 1 and 2 have been investigated.CCDC:1502275,1; 1502276,2.
coordination polymer;synthesis;structure;photoluminescence property
O614.24+1
A
1001-4861(2017)02-0347-07
10.11862/CJIC.2017.014
2016-09-05。收修改稿日期:2016-11-06。
國家自然科學基金(No.21171040)資助項目。
*通信聯系人。E-mail:chenss@fync.edu.cn

