白細(xì)胞介素2基因轉(zhuǎn)染細(xì)胞因子誘導(dǎo)的殺傷細(xì)胞對惡性黑素瘤細(xì)胞的殺傷作用
蘆蘭 謝叢華 張浩中 許綠葉 師幸偉 謝君 車彪 丁雯
430071武漢大學(xué)中南醫(yī)院腫瘤放化療科(蘆蘭、謝叢華);長江航運總醫(yī)院內(nèi)科(張浩中、許綠葉、師幸偉、丁雯),外科(謝君、車彪)
白細(xì)胞介素2基因轉(zhuǎn)染細(xì)胞因子誘導(dǎo)的殺傷細(xì)胞對惡性黑素瘤細(xì)胞的殺傷作用
蘆蘭 謝叢華 張浩中 許綠葉 師幸偉 謝君 車彪 丁雯
430071武漢大學(xué)中南醫(yī)院腫瘤放化療科(蘆蘭、謝叢華);長江航運總醫(yī)院內(nèi)科(張浩中、許綠葉、師幸偉、丁雯),外科(謝君、車彪)
目的探討白細(xì)胞介素2(IL?2)基因轉(zhuǎn)染細(xì)胞因子誘導(dǎo)的殺傷細(xì)胞(CIK)對惡性黑素瘤的殺傷能力。方法提取小鼠脾細(xì)胞,分離淋巴細(xì)胞,培養(yǎng)CIK細(xì)胞,用攜帶IL?2的質(zhì)粒PEGF?N1?IL?2轉(zhuǎn)染CIK細(xì)胞,熒光顯微鏡觀察質(zhì)粒轉(zhuǎn)染情況,反轉(zhuǎn)錄?聚合酶鏈反應(yīng)(RT?PCR)鑒定IL?2基因的表達(dá)。將效應(yīng)細(xì)胞(CIK細(xì)胞或IL?2轉(zhuǎn)染CIK細(xì)胞)和靶細(xì)胞(B16黑素瘤細(xì)胞)分別按照效靶比10∶1、20∶1和40∶1混合培養(yǎng),采用4 h乳酸脫氫酶釋放測定法,檢測兩種CIK細(xì)胞對B16細(xì)胞的細(xì)胞毒活性。按照效靶比40∶1混合,采用酶聯(lián)免疫吸附測定法(ELISA)檢測兩種CIK細(xì)胞IL?2、干擾素γ(IFN?γ)和腫瘤壞死因子α(TNF?α)水平。建立小鼠黑素瘤模型,將28只模型小鼠平均分為4組:對照組(瘤旁注射0.2 ml生理氯化鈉溶液)、IL?2組(瘤旁注射100 IU IL?2)、CIK組(瘤旁注射細(xì)胞數(shù)約1×106CIK細(xì)胞懸液)、IL?2轉(zhuǎn)染CIK組(瘤旁注射細(xì)胞數(shù)約1×106IL?2轉(zhuǎn)染CIK細(xì)胞懸液),通過腫瘤形態(tài)學(xué)和抑瘤率、細(xì)胞凋亡率來評價荷瘤小鼠腫瘤生長情況。兩組正態(tài)分布計量資料的比較行t檢驗,多組計量資料的比較采用方差分析,兩兩間多重比較采用LSD?t檢驗。結(jié)果熒光顯微鏡及RT?PCR均顯示IL?2轉(zhuǎn)染CIK細(xì)胞成功。效靶比實驗顯示,40∶1時IL?2轉(zhuǎn)染CIK細(xì)胞對B16細(xì)胞的毒性最強(qiáng),IL?2轉(zhuǎn)染CIK細(xì)胞組分泌IL?2(1107.26±6.49 pg/ml)、IFN?γ(50.01±3.35 pg/ml)和TNF?α(39.86±3.25 pg/ml)的能力明顯高于CIK細(xì)胞組(分別為51.09±3.85、32.71±2.43、30.11±3.08 pg/ml),兩組比較,t值分別為442.60、14.93和6.89,差異均有統(tǒng)計學(xué)意義(P<0.01)。動物實驗顯示,與干預(yù)前相比,干預(yù)后對照組小鼠腫瘤體積明顯增大(P<0.05),而IL?2組、CIK組和IL?2轉(zhuǎn)染CIK組小鼠腫瘤體積明顯減小(P<0.001),且IL?2轉(zhuǎn)染CIK組腫瘤體積顯著小于其他3組(均P<0.01),但I(xiàn)L?2組和CIK組差異無統(tǒng)計學(xué)意義(P>0.05)。CIK組、IL?2組和IL?2轉(zhuǎn)染CIK組的細(xì)胞凋亡率均顯著大于對照組(P<0.01),IL?2轉(zhuǎn)染CIK組的細(xì)胞凋亡率及抑瘤率均顯著大于IL?2組和CIK組(P<0.01),而IL?2組和CIK組細(xì)胞凋亡率及抑瘤率差異無統(tǒng)計學(xué)意義(P>0.05)。結(jié)論IL?2轉(zhuǎn)染CIK細(xì)胞對惡性黑素瘤有更強(qiáng)的殺傷作用。
白細(xì)胞介素2;細(xì)胞因子誘導(dǎo)殺傷細(xì)胞;轉(zhuǎn)染;黑色素瘤
近年來,腫瘤過繼免疫治療成為生物治療中最活躍的研究領(lǐng)域,如白細(xì)胞介素2/淋巴因子激活殺傷細(xì)胞(IL?2/LAK)、CD3AK細(xì)胞、腫瘤浸潤性淋巴細(xì)胞(TIL)、自然殺傷細(xì)胞(NK細(xì)胞)、樹突細(xì)胞、細(xì)胞因子誘導(dǎo)的殺傷細(xì)胞(CIK細(xì)胞)等,在臨床上已成功用于治療多種惡性腫瘤。由于惡性黑素瘤有較強(qiáng)的免疫原性,免疫療法成為其治療領(lǐng)域熱點[1?2]。但同時又發(fā)現(xiàn)許多問題,如治療惡性黑素瘤時IL?2劑量過大、CIK細(xì)胞低效、簡單聯(lián)合效果差等,導(dǎo)致臨床應(yīng)用受到極大限制[3?4]。為此,我們通過將IL?2基因轉(zhuǎn)染至CIK細(xì)胞來增強(qiáng)CIK的細(xì)胞毒性,觀察其對荷瘤鼠的抑瘤和促細(xì)胞凋亡作用,探討IL?2基因轉(zhuǎn)染CIK細(xì)胞對惡性黑素瘤的殺傷能力。
材料與方法
一、材料
B16黑素瘤細(xì)胞株由華中科技大學(xué)同濟(jì)醫(yī)學(xué)院附屬協(xié)和醫(yī)院皮膚免疫實驗室保種傳代;清潔級近交系C57BL/6小鼠購于湖北省實驗動物中心[動物合格證號SCXK(鄂)2008?0005],在華中科技大學(xué)同濟(jì)醫(yī)學(xué)院附屬協(xié)和醫(yī)院動物飼養(yǎng)中心恒溫恒濕條件下飼養(yǎng),墊料、飼料和飲水均經(jīng)滅菌處理;取6~8周齡雌性實驗鼠(體重15~20 g)備用。胎牛血清(杭州四季青生物工程材料有限公司),脂質(zhì)體2000(美國Invitrogen公司),pEGFP?N1質(zhì)粒(湖南科愛醫(yī)療器械有限公司),質(zhì)粒DNA提取與純化試劑盒(上海華舜生物技術(shù)有限公司),細(xì)胞因子IL?2、干擾素γ(IFN?γ)及腫瘤壞死因子α(TNF?α)ELISA試劑盒(上海元象醫(yī)療器械有限公司);FACS?420型流式細(xì)胞儀(美國BD公司),熒光顯微鏡(日本Olympus公司)。
二、制備CIK細(xì)胞[5]
切取C57BL/6小鼠脾臟,分離脾細(xì)胞,分離淋巴細(xì)胞,無血清1640培養(yǎng)基洗滌2次,調(diào)整細(xì)胞為1× 106/ml。加入1 000 IU/ml IFN?γ,在含10%胎牛血清的1640培養(yǎng)液中于37℃、5%CO2條件下培養(yǎng)24 h。第2天加入50 ng/ml抗CD3單抗、100 IU/ml IL?1α、300 IU/ml IL?2。以后每2 d更換1次培養(yǎng)基并加300 IU/ml IL?2,第13天收獲CIK細(xì)胞,分為CIK細(xì)胞組和CIK細(xì)胞備用組。
三、IL?2轉(zhuǎn)染CIK細(xì)胞[6?7]
轉(zhuǎn)染前1 d,將CIK備用組細(xì)胞按1×106/ml接種于不含青霉素、鏈霉素、慶大霉素的1640培養(yǎng)液中培養(yǎng),用1 ml無血清Opti?MEM重懸。無菌條件下取C57BL/6小鼠脾臟,用RNA提取試劑盒提取總RNA。采用Genbank提供的小鼠IL?2引物序列,上游引物5′?CCTTGCTAATCACTCCTCAC?3′,下游引物5′?TATGTGTTGTAA GCAGGAGG?3′,產(chǎn)物510 bp,由生工生物工程(上海)股份有限公司設(shè)計合成。按反轉(zhuǎn)錄試劑盒和PCR試劑盒說明進(jìn)行擴(kuò)增,得到PCR擴(kuò)增產(chǎn)物,經(jīng)過1%瓊脂糖凝膠電泳鑒定PCR產(chǎn)物為IL?2 cDNA;通過凝膠DNA回收試劑盒回收PCR產(chǎn)物中的IL?2 cDNA。經(jīng)純化后,在多種酶切酶及連接酶作用下與質(zhì)粒pEGFP?N1(可發(fā)綠色熒光)融合形成pEGFP?N1?IL?2。用質(zhì)粒提取試劑盒提取質(zhì)粒,將1 μg融合質(zhì)粒用無血清Opti?MEM稀釋,與2 μl脂質(zhì)體2000在室溫下孵育,然后與前1 d接種好的CIK細(xì)胞混勻,最后加入2 ml含10%胎牛血清的1640培養(yǎng)液繼續(xù)培養(yǎng)待用。24 h后收集CIK細(xì)胞作為IL?2轉(zhuǎn)染CIK細(xì)胞組,取部分轉(zhuǎn)染后的細(xì)胞懸液在倒置熒光顯微鏡下觀察,取部分轉(zhuǎn)染后的CIK細(xì)胞懸液進(jìn)行RT?PCR,1%瓊脂糖凝膠電泳,確定IL?2基因是否轉(zhuǎn)染至CIK細(xì)胞。
四、CIK細(xì)胞毒性檢測
取CIK細(xì)胞組和IL?2轉(zhuǎn)染CIK細(xì)胞組樣本各10個備用,以B16黑素瘤細(xì)胞株為靶細(xì)胞,CIK細(xì)胞為效應(yīng)細(xì)胞,調(diào)整靶細(xì)胞和效應(yīng)細(xì)胞均為1×106/ml,按照效靶比10∶1、20∶1和40∶1加入96孔細(xì)胞培養(yǎng)板中,置于37℃5%CO2培養(yǎng)箱中培養(yǎng)4 h,同時設(shè)自然釋放組(0.1 ml效應(yīng)細(xì)胞或靶細(xì)胞)和最大釋放組(0.1 ml靶細(xì)胞+0.1 ml 1%NP?40液),每組設(shè)3復(fù)孔。采用4 h乳酸脫氫酶(LDH)釋放測定法[8],檢測兩組CIK細(xì)胞對黑素瘤細(xì)胞的細(xì)胞毒活性,酶標(biāo)儀檢測490 nm處吸光度(A值)代表LDH釋放水平。細(xì)胞毒活性(%)=(實驗孔A值-效應(yīng)細(xì)胞自然釋放A值-靶細(xì)胞自然釋放A值)/(靶細(xì)胞最大釋放A值-靶細(xì)胞自然釋放A值)×100%。
五、CIK細(xì)胞分泌的細(xì)胞因子檢測
效應(yīng)細(xì)胞與靶細(xì)胞按照40∶1混合48 h后,采用雙抗體夾心ELISA法檢測CIK細(xì)胞組和IL?2轉(zhuǎn)染CIK細(xì)胞組IL?2、IFN?γ和TNF?α水平。取100 μl混合樣本離心后的上清液和標(biāo)準(zhǔn)品分別加入酶標(biāo)板,通過孵育、加抗、洗滌、顯色等步驟,最后用酶標(biāo)儀在450 nm處測定A值,計算各樣品中IL?2、IFN?γ和TNF?α濃度。
六、建立動物模型[9]
體外培養(yǎng)B16黑素瘤細(xì)胞株,分別取對數(shù)生長期細(xì)胞懸液0.1 ml(1×106個細(xì)胞)接種于28只C57BL/6小鼠背部皮下。接種9d后出現(xiàn)直徑6~9mm腫瘤。按隨機(jī)數(shù)字表法將28只小鼠平均分為4組,每組7只,分別于瘤旁注射0.2 ml生理氯化鈉溶液(對照組)、0.2 ml 500 IU/ml IL?2(IL?2組)、0.1 ml 1× 107/ml CIK細(xì)胞懸液(CIK組)、0.1 ml 1×107/ml IL?2轉(zhuǎn)染CIK細(xì)胞懸液(IL?2轉(zhuǎn)染CIK組),每隔3 d注射1次,連續(xù)3次。
七、計算抑瘤率和細(xì)胞凋亡率
在小鼠接種B16細(xì)胞9 d后,使用游標(biāo)卡尺于體外測量腫瘤直徑。腫瘤近似體積=ab2/2(a為最大直徑,b為最小直徑),單位mm3。各組小鼠均于最后1次注射治療3 d后斷頸取瘤,測量腫瘤重量(g)。抑瘤率(%)=(1-實驗組平均瘤重/對照組平均瘤重)×100%。然后將瘤組織分解、研磨、離心,經(jīng)高濃度碘化丙錠(PI)染色后,熒光倒置顯微鏡下觀察黑素瘤細(xì)胞的形態(tài),流式細(xì)胞儀檢測細(xì)胞凋亡情況,根據(jù)PI熒光直方圖上亞二倍體峰(凋亡峰)來計算各樣本凋亡細(xì)胞百分率。
八、統(tǒng)計學(xué)方法
采用SPSS18.0統(tǒng)計軟件,兩組正態(tài)分布計量資料的比較行t檢驗,多組計量資料的比較采用方差分析,兩兩間多重比較采用LSD?t檢驗,P<0.05為差異有統(tǒng)計學(xué)意義。
結(jié)果
一、IL?2轉(zhuǎn)染CIK細(xì)胞和鑒定
轉(zhuǎn)染融合質(zhì)粒pEGFP?N1?IL?2的CIK細(xì)胞在倒置熒光顯微鏡下觀察,轉(zhuǎn)染有目的基因的細(xì)胞發(fā)出綠色熒光(圖1A)。轉(zhuǎn)染過的CIK細(xì)胞經(jīng)過RT?PCR,瓊脂糖凝膠電泳可見510 bp的DNA條帶,符合小鼠IL?2 cDNA的電泳遷移率,表明IL?2轉(zhuǎn)染成功(圖1B)。
二、CIK細(xì)胞的細(xì)胞毒性檢測
隨效靶比的增大,CIK細(xì)胞和IL?2轉(zhuǎn)染的CIK細(xì)胞對B16細(xì)胞的細(xì)胞毒活性皆逐漸增大,均在效靶比為40∶1時最強(qiáng)(P<0.01)。在相同效靶比下,IL?2轉(zhuǎn)染CIK組對B16細(xì)胞的細(xì)胞毒活性皆強(qiáng)于CIK組,兩組差異均有統(tǒng)計學(xué)意義(P<0.01),見表1。
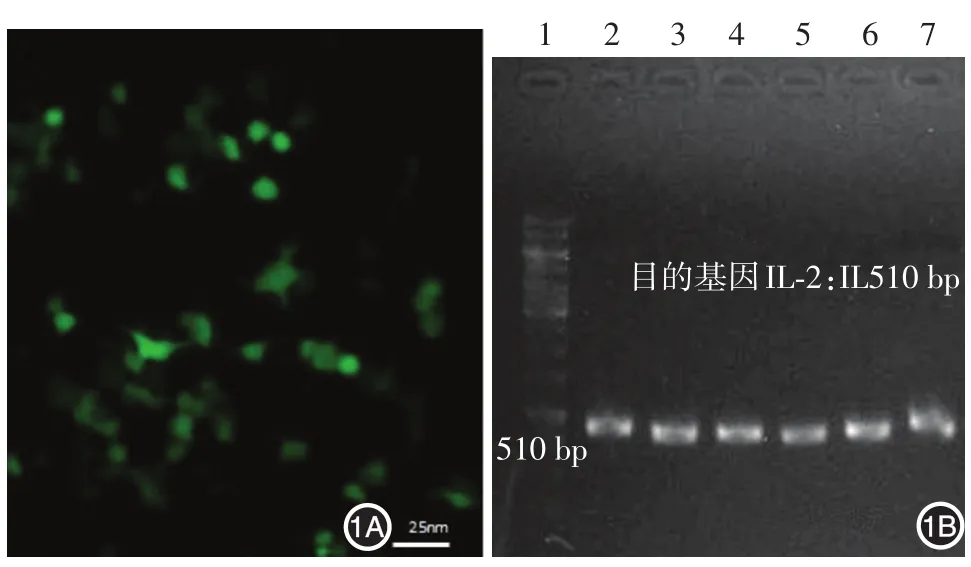
圖1 白細(xì)胞介素2(IL?2)轉(zhuǎn)染細(xì)胞因子誘導(dǎo)的殺傷細(xì)胞(CIK細(xì)胞)的鑒定 1A:攜帶質(zhì)粒pEGFP?N1?IL?2的CIK細(xì)胞顯示綠色熒光;1B:IL?2轉(zhuǎn)染CIK細(xì)胞的RT?PCR,1:標(biāo)準(zhǔn)參照物(100~15 000 bp),2~7:IL?2 cDNA
表1 不同CIK細(xì)胞對B16黑素瘤細(xì)胞株的毒性(%,±s)
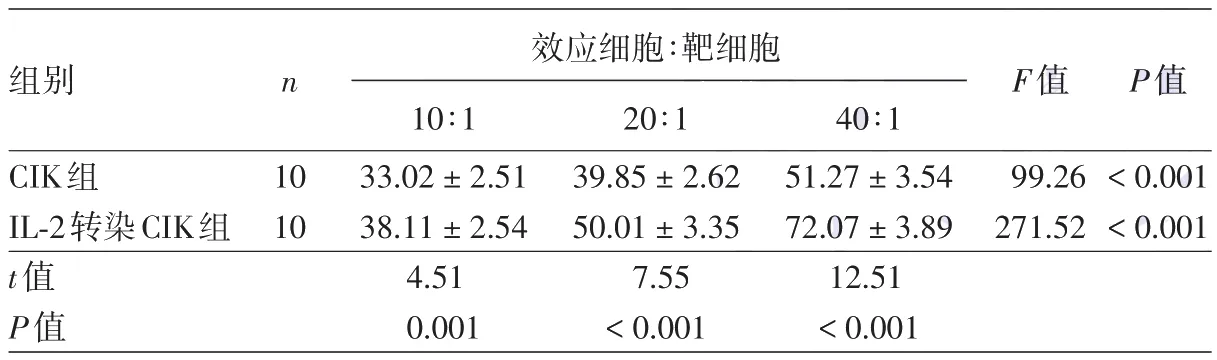
表1 不同CIK細(xì)胞對B16黑素瘤細(xì)胞株的毒性(%,±s)
注:CIK:細(xì)胞因子誘導(dǎo)的殺傷細(xì)胞;IL?2:白細(xì)胞介素2
組別CIK組IL?2轉(zhuǎn)染CIK組t值P值n 10 10效應(yīng)細(xì)胞∶靶細(xì)胞10∶1 33.02±2.51 38.11±2.54 4.51 0.001 20∶1 39.85±2.62 50.01±3.35 7.55<0.001 40∶1 51.27±3.54 72.07±3.89 12.51<0.001 F值99.26 271.52 P值<0.001<0.001
三、細(xì)胞因子的表達(dá)
在效靶比40∶1時IL?2轉(zhuǎn)染CIK組IL?2、IFN?γ和TNF?α濃度均明顯高于CIK組,差異均有統(tǒng)計學(xué)意義(P<0.01),見表2。
表2 效應(yīng)細(xì)胞與靶細(xì)胞比40∶1時不同CIK細(xì)胞細(xì)胞因子表達(dá)水平比較(±s) pg/ml
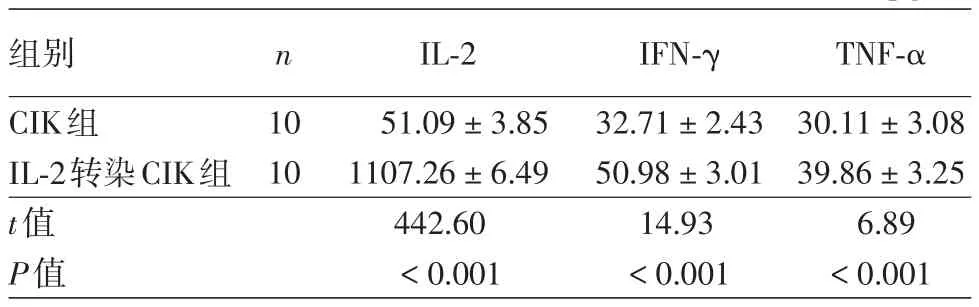
表2 效應(yīng)細(xì)胞與靶細(xì)胞比40∶1時不同CIK細(xì)胞細(xì)胞因子表達(dá)水平比較(±s) pg/ml
注:CIK:細(xì)胞因子誘導(dǎo)的殺傷細(xì)胞;IL?2:白細(xì)胞介素2;IFN?γ:干擾素γ;TNF?α:腫瘤壞死因子α
組別CIK組IL?2轉(zhuǎn)染CIK組t值P值n 10 10 IL?2 51.09±3.85 1107.26±6.49 442.60<0.001 IFN?γ 32.71±2.43 50.98±3.01 14.93<0.001 TNF?α 30.11±3.08 39.86±3.25 6.89<0.001
四、荷瘤小鼠形態(tài)學(xué)比較
干預(yù)治療前可見對照組、IL?2組、CIK組和IL?2轉(zhuǎn)染CIK組荷瘤小鼠背部皮下有大小近乎一致的腫瘤結(jié)節(jié)。干預(yù)治療9 d后,4組小鼠的腫瘤形態(tài)發(fā)生了不同的變化,其中對照組腫瘤變大,其他3組腫瘤均變小,IL?2轉(zhuǎn)染CIK組腫瘤變小最明顯,見圖2。
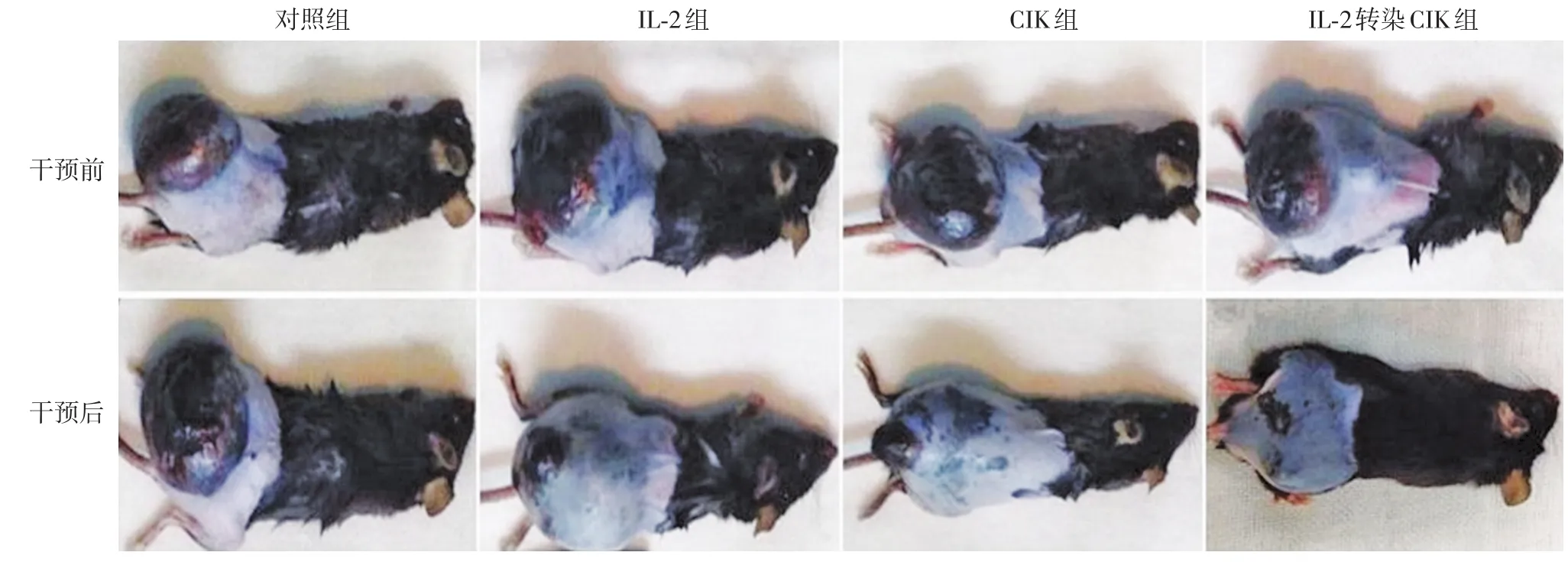
圖2 四組荷瘤小鼠干預(yù)治療前后腫瘤形態(tài)變化 干預(yù)治療前,4組荷瘤小鼠背部皮下腫瘤結(jié)節(jié)大小近乎一致;干預(yù)治療9 d后,對照組腫瘤變大,其他3組腫瘤變小,IL?2轉(zhuǎn)染CIK組腫瘤變小最明顯。IL?2:白細(xì)胞介素2;CIK:細(xì)胞因子誘導(dǎo)的殺傷細(xì)胞
五、四組抑瘤效果評價
干預(yù)前,4組小鼠腫瘤體積比較,差異無統(tǒng)計學(xué)意義(F=0.08,P>0.05)。干預(yù)后,對照組小鼠腫瘤體積與干預(yù)前相比明顯增大(P<0.05),而IL?2組、CIK組和IL?2轉(zhuǎn)染CIK組明顯減小(均P<0.001)。干預(yù)后4組小鼠腫瘤體積比較,差異有統(tǒng)計學(xué)意義(P<0.001);LSD?t檢驗顯示,IL?2組、CIK組、IL?2轉(zhuǎn)染CIK組小鼠腫瘤體積明顯小于對照組(均P<0.01),IL?2轉(zhuǎn)染CIK組小于IL?2組和CIK組(均P<0.01),而IL?2組和CIK組比較差異無統(tǒng)計學(xué)意義(P>0.05)。見表3。
干預(yù)后4組瘤重比較差異有統(tǒng)計學(xué)意義(P<0.001);LSD?t檢驗顯示,IL?2組、CIK組和IL?2轉(zhuǎn)染CIK組瘤重均顯著低于對照組(均P<0.01),IL?2轉(zhuǎn)染CIK組低于IL?2組和CIK組(均P<0.01),而IL?2組和CIK組比較差異無統(tǒng)計學(xué)意義(P>0.05)。此外,IL?2組、CIK組和IL?2轉(zhuǎn)染CIK組抑瘤率比較,差異有統(tǒng)計學(xué)意義(P<0.001),其中IL?2轉(zhuǎn)染CIK組顯著大于IL?2組和CIK組(均P< 0.01),而IL?2組和CIK組差異無統(tǒng)計學(xué)意義(P>0.05)。見表3。
表3 四組荷瘤小鼠不同干預(yù)措施抑瘤效果的評價(±s)
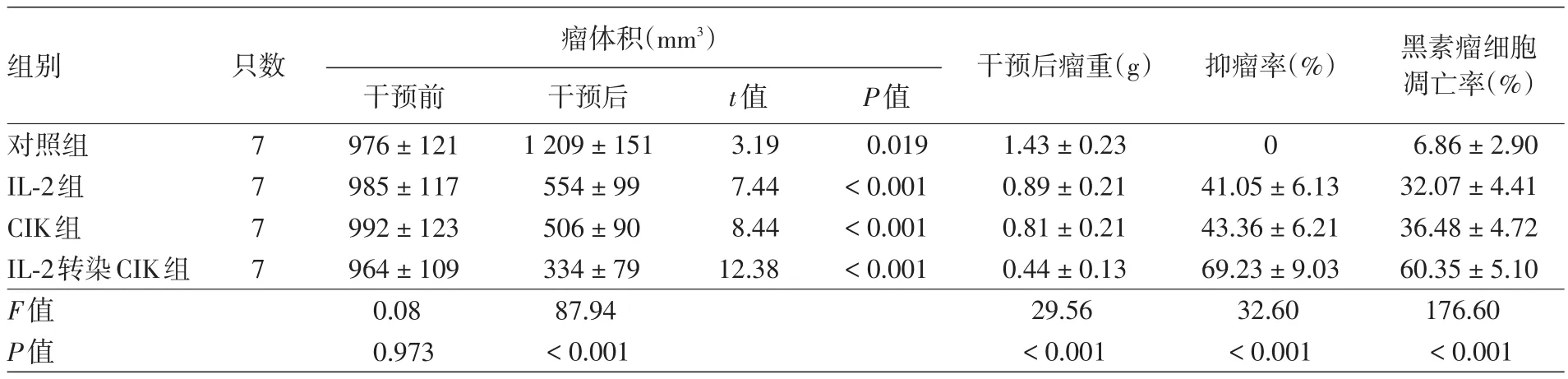
表3 四組荷瘤小鼠不同干預(yù)措施抑瘤效果的評價(±s)
注:IL?2:白細(xì)胞介素2;CIK:細(xì)胞因子誘導(dǎo)的殺傷細(xì)胞
組別 只數(shù) 瘤體積(mm3) 干預(yù)后瘤重(g) 抑瘤率(%)對照組IL?2組CIK組IL?2轉(zhuǎn)染CIK組F值P值7 7 7 7干預(yù)前976±121 985±117 992±123 964±109 0.08 0.973干預(yù)后1 209±151 554±99 506±90 334±79 87.94<0.001 t值3.19 7.44 8.44 12.38 P值0.019<0.001<0.001<0.001 0 1.43±0.23 0.89±0.21 0.81±0.21 0.44±0.13 29.56<0.001 41.05±6.13 43.36±6.21 69.23±9.03 32.60<0.001黑素瘤細(xì)胞凋亡率(%)6.86±2.90 32.07±4.41 36.48±4.72 60.35±5.10 176.60<0.001
六、檢測細(xì)胞凋亡
熒光顯微鏡下凋亡細(xì)胞核呈紅色,可見4組出現(xiàn)不同程度核固縮等細(xì)胞凋亡征象:對照組有少量的細(xì)胞凋亡,其余3組的凋亡征象均較對照組明顯,IL?2轉(zhuǎn)染CIK組的凋亡征象尤為明顯。見圖3。

圖3 碘化丙錠(PI)染色檢測各組黑素瘤細(xì)胞凋亡情況 對照組有少量的細(xì)胞凋亡,其余3組的凋亡均較對照組明顯,白細(xì)胞介素2(IL?2)轉(zhuǎn)染細(xì)胞因子誘導(dǎo)的殺傷細(xì)胞(CIK)組的凋亡尤為明顯。3A:對照組;3B:IL?2組;3C:CIK組;3D:IL?2轉(zhuǎn)染CIK組
流式細(xì)胞儀檢測經(jīng)PI染色的各組樣本,結(jié)果顯示,4組細(xì)胞凋亡率差異有統(tǒng)計學(xué)意義(P<0.001),CIK組、IL?2組和IL?2轉(zhuǎn)染CIK組細(xì)胞凋亡率均顯著大于對照組(P<0.01),IL?2轉(zhuǎn)染CIK組亦顯著大于IL?2組和CIK組(均P<0.01),而IL?2組和CIK組差異無統(tǒng)計學(xué)意義(P>0.05)。見表3。
討論
細(xì)胞過繼免疫治療是通過輸注免疫活性細(xì)胞增強(qiáng)腫瘤患者的免疫功能以達(dá)到抗腫瘤的效果。近年來,體內(nèi)外研究發(fā)現(xiàn),細(xì)胞過繼免疫治療的療效并不理想,如何使過繼的免疫活性細(xì)胞在體內(nèi)發(fā)揮持續(xù)的作用,從而引起體內(nèi)腫瘤免疫記憶并激發(fā)新一輪抗腫瘤效應(yīng),是目前迫切需要解決的問題,也是提高腫瘤生物治療療效的關(guān)鍵。
IL?2由活化的T細(xì)胞分泌,半衰期短,以自分泌方式發(fā)揮作用,通過增強(qiáng)免疫細(xì)胞的細(xì)胞毒性和抗原特異性,促進(jìn)細(xì)胞增殖,增強(qiáng)顆粒酶和細(xì)胞穿孔素等的表達(dá)來發(fā)揮抗腫瘤作用[10]。研究發(fā)現(xiàn)[11],單純IL?2在體外并不具有殺瘤作用,但聯(lián)合應(yīng)用或在體內(nèi)時可增強(qiáng)免疫效應(yīng)細(xì)胞的殺瘤活性,并延長免疫效應(yīng)細(xì)胞的存活時間。腫瘤患者的IL?2含量低于健康人,細(xì)胞毒性T淋巴細(xì)胞(CTL)、NK細(xì)胞、LAK、CIK等免疫細(xì)胞難以被活化和增殖,不能有效清除逃逸的腫瘤細(xì)胞。因此,提供外源IL?2可提高腫瘤患者的免疫監(jiān)視功能。1998年FDA批準(zhǔn)IL?2用于不能手術(shù)切除的惡性黑素瘤治療,隨后的研究發(fā)現(xiàn),IL?2在有效劑量范圍內(nèi)的毒副作用較大,從而限制了其在臨床上的應(yīng)用[12]。目前研究聚焦在IL?2與其他治療聯(lián)合應(yīng)用上,聯(lián)合方式有局部注射、靜脈滴注、基因轉(zhuǎn)染等,其中基因轉(zhuǎn)染最受推崇。基因轉(zhuǎn)染有電穿孔、脂質(zhì)體轉(zhuǎn)染、病毒轉(zhuǎn)染、納米轉(zhuǎn)染等,目前脂質(zhì)體和納米轉(zhuǎn)染應(yīng)用較多[13?14]。
CIK兼有T淋巴細(xì)胞強(qiáng)大的抗瘤活性和NK細(xì)胞的無組織相容性限制的殺瘤特點,被稱為NK樣T淋巴細(xì)胞。CIK治療被認(rèn)為是新一代抗腫瘤過繼免疫治療首選,是繼手術(shù)、化療、放療治療腫瘤的第4種模式[14]。但CIK細(xì)胞在健康人外周血中極其罕見,僅1%~5%,其增殖、細(xì)胞毒性的強(qiáng)度、持續(xù)時間等問題成為制約其臨床療效的關(guān)鍵。CD3+CD56+為CIK的主要表型,且細(xì)胞毒活性僅存于CD3+CD56+細(xì)胞中,其比例增加預(yù)示CIK細(xì)胞毒活性增強(qiáng)。如何提高CIK細(xì)胞中CD3+CD56+細(xì)胞的比例是目前研究的熱點[15]。CIK體外增殖需要外源性細(xì)胞因子如IL?2、IL?12、IL?7等的輔助,其中IL?2加入的時間和方式對CIK的增殖及細(xì)胞毒活性意義重大[16]。外源性IL?2誘導(dǎo)的免疫反應(yīng)都是非腫瘤特異性的,毒副反應(yīng)大,但將IL?2基因轉(zhuǎn)染免疫細(xì)胞,在轉(zhuǎn)染細(xì)胞的局部分泌IL?2,不僅可以避免全身性副作用,而且可以誘導(dǎo)機(jī)體產(chǎn)生抗腫瘤免疫反應(yīng)。
本研究顯示,IL?2基因轉(zhuǎn)染的CIK細(xì)胞在熒光顯微鏡下可見綠色熒光,提示質(zhì)粒轉(zhuǎn)染成功,轉(zhuǎn)染后的CIK細(xì)胞基因經(jīng)過RT?PCR擴(kuò)增后,瓊脂糖凝膠電泳可見510 bp的DNA條帶,再次證明IL?2轉(zhuǎn)染成功。3種效靶比評價IL?2轉(zhuǎn)染的CIK對B16惡性黑素瘤細(xì)胞的細(xì)胞毒活性,顯示效靶比為40∶1時其細(xì)胞毒活性最強(qiáng);且IL?2轉(zhuǎn)染的CIK細(xì)胞分泌IL?2等細(xì)胞因子的能力要強(qiáng)于單純CIK細(xì)胞。動物實驗顯示,轉(zhuǎn)染IL?2的CIK細(xì)胞具有更強(qiáng)的抑瘤和促凋亡作用,且顯著強(qiáng)于單純IL?2或CIK細(xì)胞的治療。
綜上所述,IL?2轉(zhuǎn)染CIK細(xì)胞可被成功構(gòu)建,效靶比40∶1時IL?2轉(zhuǎn)染CIK細(xì)胞對惡性黑素瘤細(xì)胞毒活性增大,IL?2轉(zhuǎn)染的CIK細(xì)胞能分泌更多的IL?2、IFN?γ和TNF?α,促進(jìn)CIK細(xì)胞的成熟,增強(qiáng)CIK細(xì)胞的腫瘤殺傷能力。通過IL?2轉(zhuǎn)染可以增強(qiáng)CIK細(xì)胞對惡性黑素瘤的殺傷作用。同時我們也關(guān)注到其他學(xué)者應(yīng)用IL?12、IFN?γ等替代或聯(lián)合IL?2來增強(qiáng)CIK細(xì)胞的殺瘤作用,這將是我們以后努力的方向。
[1]CSCO黑色素瘤專家委員會.中國黑色素瘤診治指南(2015版)[M].北京:人民衛(wèi)生出版社,2015,40?41.
[2]Olszanski AJ.Current and future roles of targeted therapy and immunotherapy in advanced melanoma[J].J Manag Care Spec Pharm,2014,20(4):346?356.DOI:10.18553/jmcp.2014.20.4. 346.
[3]Otáhal P,Trněny M.Current approaches in cancer immuno?therapy[J].Klin Onkol,2015,28 Suppl 3:3S105?111.DOI:10.14735/ amko20153S105.PMID:26489509.
[4]錢其軍,吳孟超.腫瘤精準(zhǔn)細(xì)胞免疫治療:夢想照進(jìn)現(xiàn)實[J].中國腫瘤生物治療雜志,2015,22(2):151?158.DOI:10.3872/j. issn.1007?385X.2015.02.003.
[5]Huang X,Cui S,Shu Y.Cisplatin selectively downregulated the frequency and immunoinhibitory function of myeloid?derived suppressor cells in a murine B16 melanoma model[J].Immunol Res,2016,64(1):160?170.DOI:10.1007/s12026?015?8734?1.
[6]張家模,吳小候,張翾,等.白細(xì)胞介素2錨定的exosomes的制備及其對膀胱癌細(xì)胞的誘導(dǎo)殺傷效應(yīng)[J].中華腫瘤雜志, 2011,33(8):564?569.DOI:10.3760/cma.j.issn.0253?3766.2011. 08.002.
[7]李安娜.IL?2基因轉(zhuǎn)染的CIK細(xì)胞聯(lián)合樹突狀細(xì)胞的抗肝癌實驗研究[D].南寧:廣西醫(yī)科大學(xué),2008.
[8]張蕾,李芳秋,張士新,等.LDH釋放法檢測腫瘤患者PBMC和CIK細(xì)胞的細(xì)胞毒活性[J].現(xiàn)代檢驗醫(yī)學(xué)雜志,2008,23(6): 27?29.DOI:10.3969/j.issn.1671?7414.2008.06.008.
[9]錢悅,蔣蘋,褚淑娟,等.攜帶MIA基因單核細(xì)胞增生性李斯特菌抑制惡性黑素瘤的研究[J].中華皮膚科雜志,2009,42(6): 399?401.DOI:10.3760/cma.j.issn.0412?4030.2009.06.011.
[10]Carmenate T,Pacios A,Enamorado M,et al.Human IL?2 mutein with higher antitumor efficacy than wild type IL?2[J].J Immunol, 2013,190(12):6230?6238.DOI:10.4049/jimmunol.1201895.
[11]Vacca P,Martini S,Garelli V,et al.NK cells from malignant pleural effusions are not anergic but produce cytokines and display strong antitumor activity on short?term IL?2 activation[J]. EurJImmunol,2013,43(2):550?561.DOI:10.1002/eji.201242783.
[12]Hughes T,Klairmont M,Broucek J,et al.The prognostic significance of stable disease following high?dose interleukin?2(IL?2)treatment in patients with metastatic melanoma and renal cell carcinoma[J].Cancer Immunol Immunother,2015,64(4): 459?465.DOI:10.1007/s00262?014?1652?6.
[13]劉秋霞.真核表達(dá)載體PEGFP?N1?IL?2構(gòu)建及CIK細(xì)胞的體外擴(kuò)增[D].承德:承德醫(yī)學(xué)院,2007.
[14]Rossowska J,Pajtasz?Piasecka E,Rysnik O,et al.Generation of antitumor response by IL?2?transduced JAWS II dendritic cells[J].Immunobiology,2011,216(10):1074?1084.DOI:10.1016/j. imbio.2011.05.006.
[15]Guo Y,Han W.Cytokine?induced killer(CIK)cells:from basic research to clinical translation[J].Chin J Cancer,2015,34(3): 99?107.DOI:10.1186/s40880?015?0002?1.
[16]Wang W,Meng M,Zhang Y,et al.Global transcriptome?wide analysis of CIK cells identify distinct roles of IL?2 and IL?15 in acquisition of cytotoxic capacity against tumor[J].BMC Med Genomics,2014,7:49.DOI:10.1186/1755?8794?7?49.
Cytotoxic effects of cytokine?induced killer cells transfected with the interleukin?2 gene on malignant melanoma cells
Lu Lan,Xie Conghua,Zhang Haozhong,Xu Lyuye,Shi Xingwei,Xie Jun,Che Biao,Ding Wen
Department of Radiation and Chemotherapy,Zhongnan Hospital of Wuhan University,Wuhan 430071, China(Lu L,Xie CH);Department of Internal Medicine,General Hospital of the Yangtze River Shipping, Wuhan 430010,China(Zhang HZ,Xu LY,Shi XW,Ding W);Department of Surgery,General Hospital of
Xie Conghua,Email:chxie_65@whu.edu.cn
ObjectiveTo evaluate cytotoxic effects of cytokine?induced killer cells(CIK cells)transfected with the interleukin?2(IL?2)gene on malignant melanoma cells.MethodsMouse spleen cells were extracted,lymphocyte cells were separated,and CIK cells were prepared from these lymphocyte cells. PEGF?N1 plasmids containing IL?2 gene(PEGF?N1?IL?2)were transfected into CIK cells.Fluorescence microscopy was used to observe transfection products,and reverse transcriptase?polymerase chain reaction(RT?PCR)was conducted to determine the IL?2 mRNA expression.Then,effector cells such as CIK cells and IL?2?transfected CIK cells were separately co?cultured with target cells(B16 melanoma cells)at effector?target ratios of 10∶1,20∶1 and 40∶1,then 4?hour lactate dehydrogenase release assay was performed to evaluate cytotoxic effects of the two kinds of CIK cells on B16 cells.After effector cells were co?cultured with target cells at the effector?target ratio of 40∶1 for 48 hours,enzyme?linked immunosorbent assay(ELISA)was conducted to detect levels of IL?2,interferon?γ(IFN?γ)and tumor necrosis factor?α(TNF?α)in the supernatant of the two kinds of CIK cells.Finally,mouse models of melanoma were established,and a total of 28 melanoma?bearing mice were divided into 4 groups to be peritumorally injected with 0.2 ml sodium chloride physiological solution(control group),100 IU IL?2 solution(IL?2 group),CIK cell suspension at a cell density of 1×106cells per milliliter(CIK group)and IL?2?transfected CIK cell suspension at a cell density of 1×106cells per milliliter(IL?2?transfected CIK group)respectively.Tumor morphology,tumor inhibition rate and cell apoptosis rate were used to evaluate tumor growth in the above groups.If data were normally distributed,t?test was used for comparing means between two groups,and analysis of variance and least significant difference(LSD)?ttest were used for comparing means among multiple groups.ResultsFluorescence microscopy and RT?PCR both showed that IL?2 was successfully transfected into CIK cells.The cytotoxic effect of IL?2?transfected CIK cells on B16 cells was strongest at the effector?target ratio of 40∶1.Levels of IL?2,IFN?γ and TNF?α were also significantly higher in the supernatant of IL?2?transfected CIK cells[(1107.26±6.49)pg/ml,(50.01±3.35)pg/ml,(39.86± 3.25)pg/ml]than those in that of CIK cells[(51.09±3.85)pg/ml,(32.71±2.43)pg/ml,(30.11±3.08)pg/ml,t=442.60,14.93,6.89,allP<0.01].Animal experiments showed that the tumor volume obviously increased in the control group(P<0.05),but significantly decreased in the IL?2 group,CIK group and IL?2?transfected CIK group(allP<0.001)after intervention compared with those before intervention. Furthermore,the tumor volume in the IL?2?transfected CIK group was significantly less than that in the other three groups(allP<0.01),but no significant difference was observed between the IL?2 group and CIK group(P>0.05).In addition,the apoptosis rate was significantly higher in the IL?2 group,CIK group, and IL?2?transfected CIK group than that in the control group(allP<0.01).The apoptosis rate and tumor inhibition rate were significantly higher in the IL?2?transfected CIK group than those in the IL?2 group and CIK group(allP<0.01),but insignificantly different between the IL?2 group and CIK group(P>0.05).ConclusionIL?2?transfected CIK cells had stronger killing effects on malignant melanoma.
Interleukin?2;Cytokine?induced killer cells;Transfection;Melanoma
謝叢華,Email:chxie_65@whu.edu.cn
10.3760/cma.j.issn.0412?4030.2017.04.006
武漢市衛(wèi)計委臨床醫(yī)學(xué)科研課題(WX13D16)
the Yangtze River Shipping,Wuhan 430010,China(Xie J,Che B)
Fund program:Clinical Research Project of Health and Family Planning Commission of Wuhan Municipality(WX13D16)
2016?08?03)
(本文編輯:周良佳 顏艷)

