人黑素瘤藥物研究進展
付靖波, 于 斌, 張紅霞, 朱海英*
1. 海軍軍醫大學基礎部細胞生物學教研室,上海200433 2. 復旦大學生命科學院,上海200433
·綜述·
人黑素瘤藥物研究進展
付靖波1, 于 斌2, 張紅霞1, 朱海英1*
1. 海軍軍醫大學基礎部細胞生物學教研室,上海200433 2. 復旦大學生命科學院,上海200433
黑素瘤患者死亡率較高。其治療方法除了傳統的手術切除和放射治療外,化療一度成為主要的治療手段。近年來,隨著人們對黑素瘤發生發展分子機制的深入研究,基于靶向治療、生物免疫治療的方法和藥物不斷涌現。目前,除了已經在臨床治療中使用的藥物外,一些正在進行臨床研究的待批準藥物和正進行基礎研究的潛在藥物也表現出良好的應用前景。本文就目前治療黑素瘤的代表性藥物及潛在藥物的分子機制及治療效果作一綜述。
黑素瘤;多藥聯合治療;生物免疫治療;靶向治療
黑素瘤起源于皮膚、黏膜和色素膜的黑素細胞。雖然黑素瘤的發病率低于基底細胞癌和鱗狀細胞癌,但由于其易發生淋巴和血行轉移,且易轉移到肺、腦等器官,因此患者死亡率高。據統計,皮膚癌患者中僅4%的人罹患黑素瘤,但因皮膚癌死亡的患者中有80%為黑素瘤患者[1]。近年來,雖然多種腫瘤的發病增長率開始下降,但黑素瘤的發病增長率仍以每年3%增長[2]。廣泛切除聯合選擇性的淋巴清掃對于黑素瘤早期患者有較好的療效[3];對于黑素瘤晚期患者,多采用放射性治療及化療,但這兩種方法對患者傷害較大而且療效欠佳、不良反應大且患者易產生抗藥性。因此,需要尋找安全高效的黑素瘤治療方法。隨著人們對黑素瘤發病機制研究的深入,特別是相關突變基因的鑒定及關鍵免疫調節檢查點的發現,黑素瘤化療藥物治療、靶向治療、生物免疫治療及多藥物聯合治療手段得到不同程度的發展,本文就此作一綜述。
1 化療藥物
1975年,達卡巴嗪(dacarbazine, DITC)得到美國食品與藥物監督管理局(FDA)批準,成為第1個被用于黑素瘤臨床治療的化療藥物。此后,多種化療藥物先后出現,化療也一度成為黑素瘤臨床治療的主要手段,但各種化療藥物的治療效果參差不齊,相關藥物見表1。其中,替莫唑胺(temozolomide,TMZ)因有較好的穿透血腦屏障的能力而對治療腦轉移的黑素瘤顯示出更好的效果[4];亞硝基脲類藥物可作用于處于不同增殖期的黑素瘤細胞。然而,化療不良反應大、患者易產生抗藥性的缺陷使單一化療藥物難以成為黑素瘤治療的長遠選擇[5]。而以紫杉醇及長春花堿為代表的天然類藥物及新發現的天然藥物(如Honokiol、Forsythiae Fructus及P-Hydroxycinnamaldehyde)在良好地抑制黑素瘤的同時具有更低的毒性[6-8]。近年來,天然藥物與納米技術結合也取得了較大成效,納米材料荷載的天然藥物在具有更好的細胞吸收與靶向性的同時,具有更低的毒性。已有研究[9]證明,順鉑蛋白納米顆粒對黑素瘤細胞B16有明顯的抑制效果,提示納米分子與鉑類化合物共同作用可用于治療黑素瘤。白蛋白結合型紫杉醇已顯示出較好的療效,且與紫杉醇單藥相比有更低的致過敏性[10]。研究[11]表明,缺氧誘導因子-1α(HIF-1α)在腫瘤組織中的積累可促進黑素瘤的進展,導致患者生存率降低,而抗壞血酸(AA)和磷酸抗壞血酸-2(A2P)等可用以調節HIF-1α的積累和活性。此外,甲基砜可以抑制HIF-1α及血管內皮生長因子(VEGF)、促血管生成蛋白和轉鐵蛋白等促腫瘤轉移的調節因子的表達[12]。

表1 黑素瘤化療藥物的作用機制及療效
PFS(progression-free-survival):無進展生存期;ORR(objective response rate):客觀緩解率;OS(overall survival):總生存期;OR(odd ratio):比值比;OSR(overall survival rate):總存活率;DCR(disease control rate):疾病控制率
2 生物治療
黑素瘤為免疫原性較強的腫瘤,但因其具有“掩藏”抗原的特性,故常顯示出免疫逃逸性質。因此,通過激發機體的免疫系統以減少瘤體微環境中調節性T淋巴細胞數量、增加細胞毒性T淋巴細胞數量進而增強局部抗腫瘤免疫力在較長一段時間內是黑素瘤治療的研究熱點。1991年,特異細胞毒性T細胞識別的人類黑素瘤抗原被成功分離。近些年,T細胞活化模式日益清晰,隨著T細胞活化的“雙信號模式”的闡明、樹突狀細胞(DC)免疫生物學的進展和人類白細胞抗原(HLA)基因測序的完成,黑素瘤的生物治療得到進一步發展[19],其主要包括基因治療、細胞因子治療、過繼免疫治療以及疫苗治療。
2.1 細胞因子治療 自IL-2作為治療晚期/轉移性黑素瘤的非特異性免疫調節劑而得到FDA批準后,干擾素(INF)、貝伐單抗等因其對免疫系統的調節功能而多用于黑素瘤的免疫治療以及生物化學治療。其中,貝伐單抗更是常與免疫抑制劑及化療藥物聯合使用,且取得較好效果。而白細胞介素-2(IL-2)與IL-12除單藥使用外,在基因治療中也療效較好。研究[20]使用體內電穿孔方法(EP)將IL-2與IL-12基因導入轉移性黑素瘤細胞內,在19例患者中,2例完全緩解。進一步將IFN-γ與IL-2、IL-12在小鼠體內聯用,CD8+的CTL細胞溶解酶活性增強[21],這一結果為EP/IL-2、IL-12療法的進一步發展提供了參考(表2)。
2.2 免疫檢查點 細胞因子治療可在短期內增強體內的免疫效應,常效果不明顯且持續時間較短。隨著免疫檢查點的發現,特異性靶向免疫檢查點的單克隆抗體抑制了腫瘤免疫逃逸效應,從而彌補了細胞因子治療的缺點(表3)。免疫檢查點參與T細胞的負調控,其在腫瘤患者的T細胞中表達相對升高,是造成腫瘤免疫逃逸現象的主要原因。目前已有兩大類免疫檢查點抑制劑被美國FDA批準應用于黑素瘤的臨床治療,分別為抗CTLA-4和抗PD-1/PD-L1的抗體。然而,腫瘤免疫檢查點抑制劑易產生較大的不良反應,可在抑制腫瘤達到的同時殺傷正常細胞。
近年來新發現的免疫檢查點除CTLA-4和PD-1/L1外,還包括T細胞免疫球蛋白結構域-3(TIM-3)、殺傷細胞抑制性受體(KIRs)、B7-H3等。目前以TIM-3的研究最為深入。其主要表達于輔助性T細胞(Th1)表面,與癌胚抗原相關細胞黏附分子1(CEACAM1)相互作用而誘發腫瘤免疫逃逸。在對轉移性的結腸癌細胞的TIM-3進行敲除及對其功能進行抑制后,調節性T細胞(Treg)與T細胞受到的抑制明顯減弱[27],故TIM-3或可成為黑素瘤免疫治療的潛在靶點。
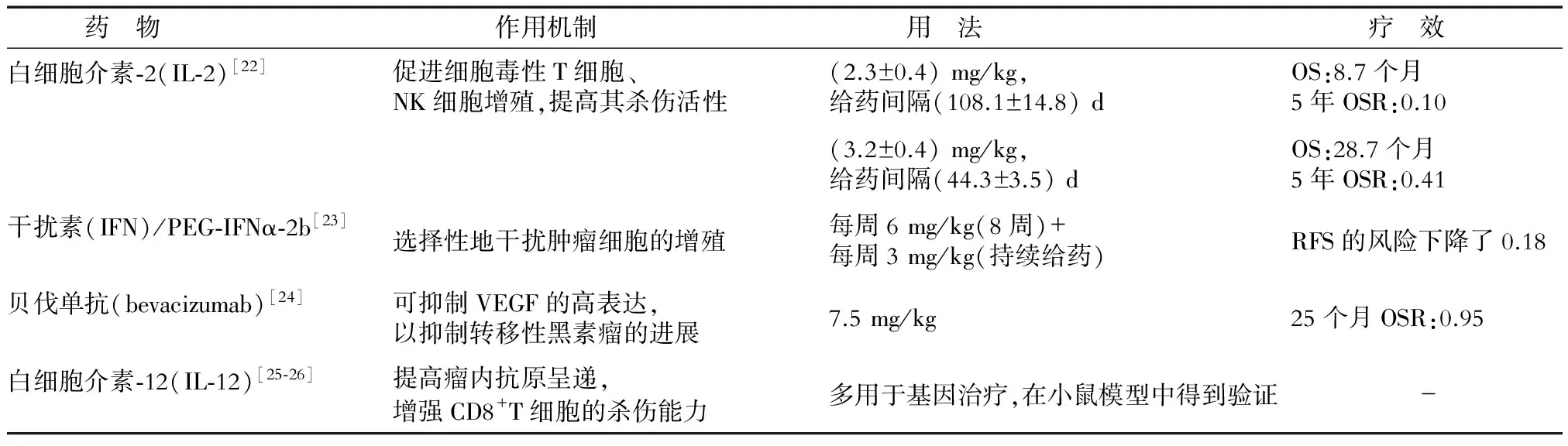
表2 黑素瘤細胞因子藥物的作用機制和療效
OS(overall survival):總生存期;OSR(overall survival rate):總存活率;RFS(recurrence-free survival):無復發生存期
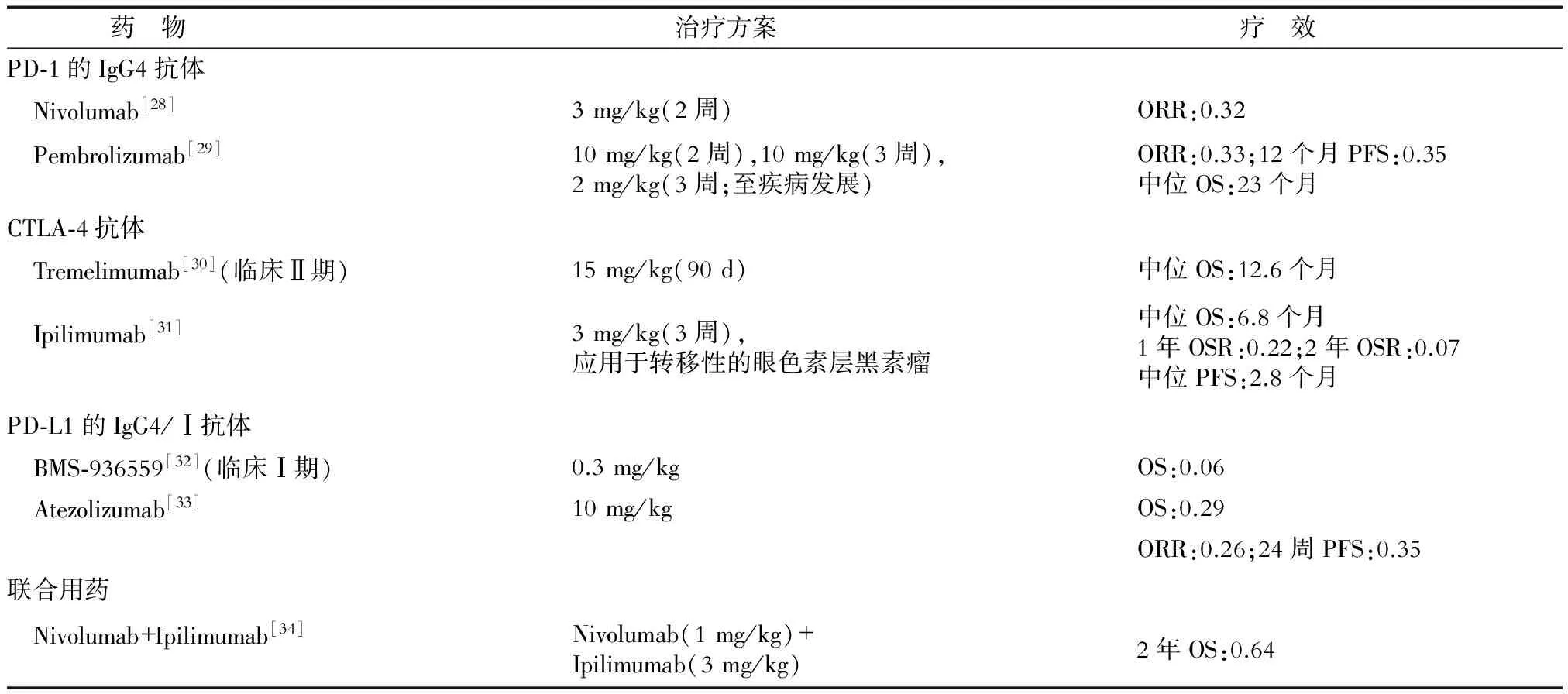
表3 免疫檢查點抑制劑的作用機制及治療效果
ORR(objective response rate):客觀緩解率;OS(overall survival):總生存期;OSR(overall survival rate):總存活率;PFS(progression-free survival):無進展生存期
2.3 過繼性免疫治療(ACI) ACI中,腫瘤浸潤淋巴細胞(tumor-infiltrating lymphocytes,TIL)與嵌合抗原受體淋巴細胞(chimeric antigen receptor lymphocyte, CAR-T)因具有比腫瘤疫苗更為直接的抗瘤效果,成為近年來腫瘤免疫治療中的主力,在黑素瘤治療中的應用也得到發展(表4)。TIL療法已在臨床試驗中,而CAR-T療法也先后在體外及小鼠體內證明了其有效性,曾被批準進行臨床試驗,但治療效果因人、因病而異(表4)。雖然ACI在體表現出獨特的腫瘤治療效果,但易引起不良反應(患者常伴有持續發熱),患者治療后常反復發作,加之其在固體腫瘤中的療效弱于對血液瘤的療效,因此其在黑素瘤中的進一步應用受到阻礙。

表4 ACI的作用機制及治療效果
ORR(objective response rate):客觀緩解率
3 靶向治療
黑素瘤的發展與細胞增殖、分化和細胞死亡的關鍵信號通路中分子的畸變有關。如表5所示,50%~60%的黑素瘤有RAS/RAF/MEK/ERK(MAPK)通路的改變,這使該途徑成為主要治療靶點。KIT的變異較不常見且主要發生在黏膜、肢端、皮膚以及MAPK抑制劑治療后的抗藥性黑素瘤中,約占黑素瘤總體突變種類的1%[40]。Buparlisib為PI3K-AKT通路的另一種抑制劑,最近有研究[41]表明,Buparlisib抑制PI3K-AKT通路在體內與體外均起到對黑素瘤腦轉移的治療效果,提示PI3K-AKT通路可作為黑素瘤腦轉移的又一作用靶點。
細胞信號轉導系統極其復雜,且黑素瘤中各種細胞處于不同的生長階段。此外,轉移起始細胞的存在使黑素瘤治療后黑素瘤細胞仍會殘留甚至轉移。因此,針對某一單獨途徑的靶向治療的前期效果明顯,但治療后常有黑素瘤的復發,且復發的黑素瘤多對該藥物產生一定抗藥性。因此,在黑素瘤治療中,靶向藥物的使用應當同時針對黑素瘤細胞的多個代謝途徑,且應與其他種類藥物聯合使用,以達到更徹底的治療效果。
此外,針對黑素瘤起始細胞的治療近年來也逐步得到重視。已有研究[50]表明,人ATP-結合盒(ABC)轉運蛋白在黑素瘤的多重耐藥中起重要作用。相關蛋白ABCB5可被視為具有高致瘤能力的黑素瘤起始細胞的標志物[51],而解旋酶HAGE在ABCB5黑素瘤起始細胞介導的腫瘤發生中起關鍵作用[52],故HAGE可作為特異性靶點用于針對黑素瘤起始細胞的靶向治療。
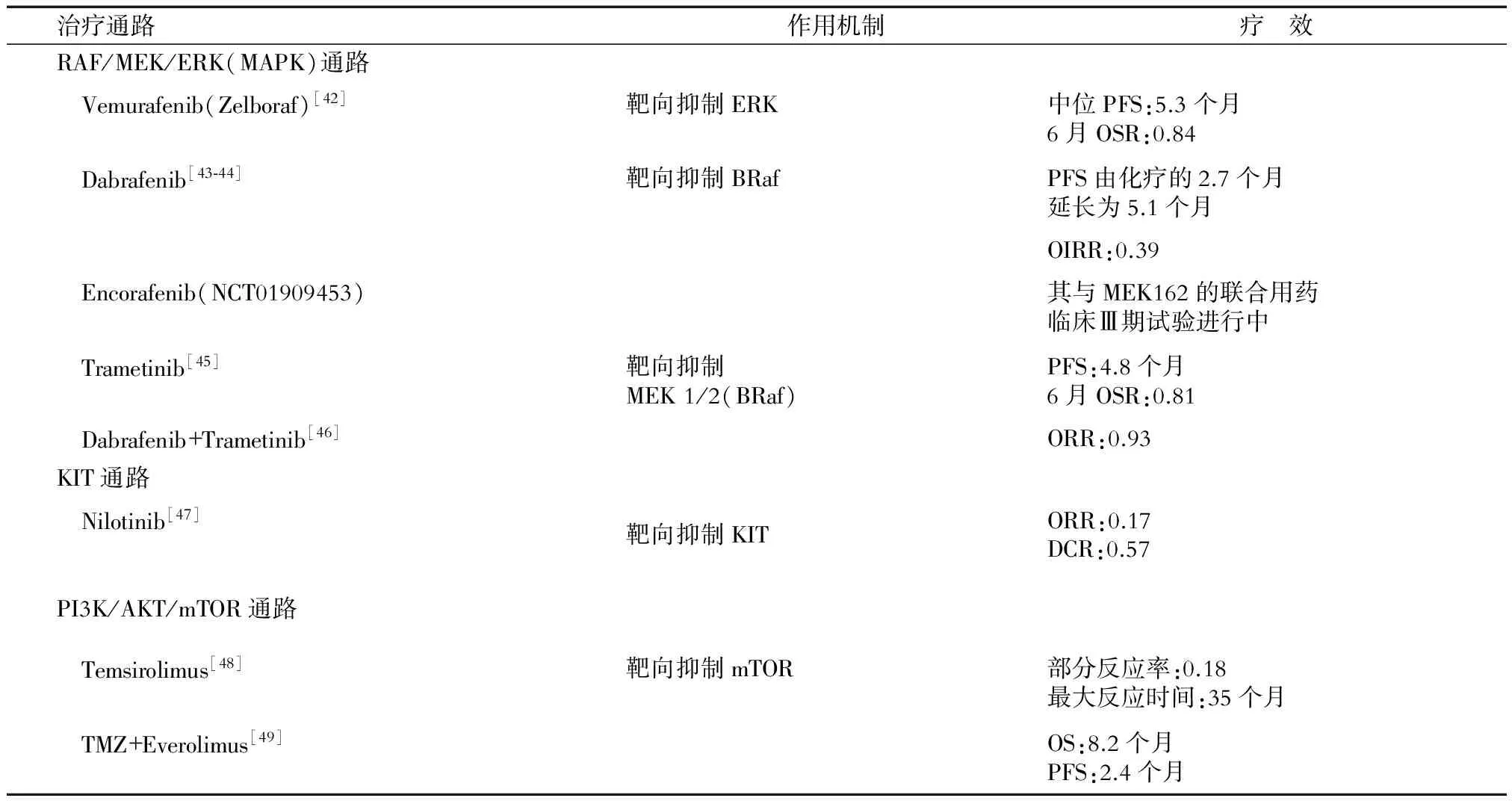
表5 靶向治療的作用機制及治療效果
PFS(progression-free survival):無進展生存期;OSR(overall survival rate):總存活率;OIRR(overall intracranial response rate ):顱內總體反應率;ORR(objective response rate):客觀緩解率;DCR(disease control rate):疾病控制率;OS(overall survival):總生存期
4 多藥聯合治療
多藥聯合治療主要包括多種同類藥物同時使用以及不同種類藥物聯合使用的治療(表6)。將化療藥物和生物制劑聯合應用以同時對黑素瘤中的多個代謝途徑進行抑制,比單藥治療有更高的客觀緩解率及更低的復發率。目前免疫檢查點抑制劑與其他藥物的聯合使用仍未得到充分發展。

表6 多藥物聯合的治療效果
RFS(recurrence-free survival):無復發生存期;OS(overall survival):總生存期;ORR(objective response rate):客觀緩解率;PFS(progression-free survival):無進展生存期;OSR(overall survival rate):總存活率
5 國內黑素瘤治療藥物及研發
雖然黑素瘤在我國發病率較低,但是仍保持著較高的增長率,應引起臨床工作者的重視。雖然近些年來我國在一些抗黑素瘤藥物如淋巴毒素-α衍生物[59]、納米氧化亞銅[60]、Aspartyl-chlorin p6 dimethylester(7b)[61]等的研發上取得了一定成效,但是我國對于黑素瘤的臨床藥物研究仍有所欠缺,尤其是抗黑素瘤藥物針對中國人的效果以及不良反應的研究缺乏充足的數據支持[62](表7)。因此,針對中國人的黑素瘤藥物的臨床試驗仍需進一步開展。
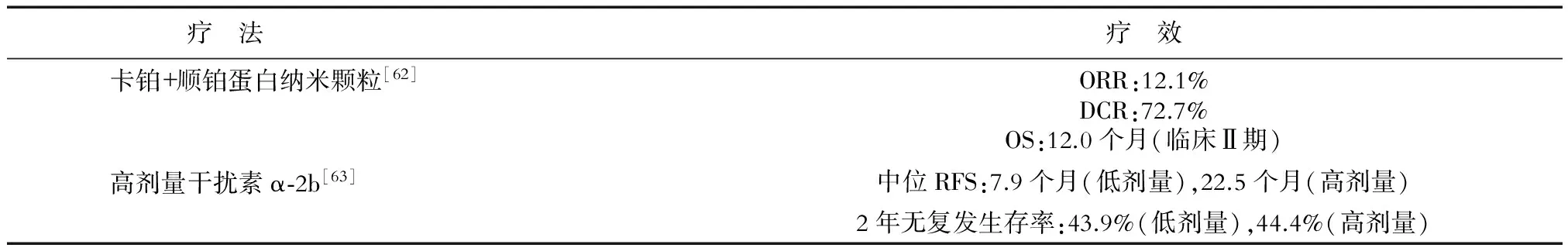
表7 部分藥物在中國的臨床應用效果
ORR(objective response rate):客觀緩解率;DCR(disease control rate):疾病控制率;RFS(recurrence-free survival):無復發生存期
6 展 望
目前,隨著人們對黑素瘤生物學特性認識的逐漸深入及新型生物材料的不斷涌現,在黑素瘤的治療方面,一些新型藥物和療法也展現出很好的發展前景。如納米材料因其低毒性、高靶向性、高效性以及易溶解性而對黑素瘤有較好的療效[60]。一些納米材料如納米氧化亞銅對黑素瘤治療的效果較好[64]。但是,納米材料在藥物荷載能力及保存方面明顯不足,加之缺乏完善的藥物毒理評價體系,導致納米類藥物在臨床的進一步應用受到阻礙[65]。而光敏劑在特定波長下產生的細胞毒性活性氧可引起腫瘤細胞的凋亡與壞死[66]。其中,卟吩姆鈉(porfimer sodium)為經典的光敏劑,主要通過改變線粒體膜電位而誘導細胞凋亡,從而達到對黑素瘤的治療效果[67];Aspartyl-chlorin p6 dimethylester (7b)為水溶性光敏劑,在小鼠體內取得良好的黑素瘤抑制效果且具有低毒性[61]。此外,由于黑素瘤惡性程度、表型以及病情發展在年齡分布上有一定差異[68],所以相關抗黑素瘤藥物研發應當針對不同年齡的患者群體進行。
[ 1 ] SHAH D J, DRONCA R S. Latest advances in chemotherapeutic, targeted, and immune approaches in the treatment of metastatic melanoma[J]. Mayo Clin Proc, 2014,89(4):504-519.
[ 2 ] TRIPP M K, WATSON M, BALK S J, et al. State of the science on prevention and screening to reduce melanoma incidence and mortality: The time is now[J]. CA Cancer J Clin, 2016.
[ 3 ] 向 陽,朱 凱.腹股溝淋巴結清掃范圍對下肢惡性黑色素瘤患者預后的影響[J].中國臨床醫學, 2009,16(4):634-635.
[ 4 ] MIDDLETON M R, GROB J J, AARONSON N, et al. Randomized phase Ⅲ study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma[J]. J Clin Oncol, 2000, 18(1):158-166.
[ 5 ] KALIKI S, SHIELDS C L. Uveal melanoma: relatively rare but deadly cancer[J]. Eye (Lond), 2017,31(2):241-257.
[ 6 ] GUILLERMO-LAGAE R, SANTHA S, THOMAS M, et al. Antineoplastic effects of honokiol on melanoma[J]. Biomed Res Int, 2017,2017:5496398.
[ 7 ] ZHAO L M, SUN G G, HAN L N, et al. P-Hydroxycinnamaldehyde induces B16-F1 melanoma cell differentiationviathe RhoA-MAPK signaling pathway[J]. Cell Physiol Biochem, 2016,38(6):2247-2260.
[ 8 ] BAO J, DING R, ZOU L, et al. Forsythiae fructus inhibits B16 melanoma growth involving MAPKs/Nrf2/HO-1 mediated anti-oxidation and anti-inflammation[J]. Am J Chin Med, 2016, 44(5):1043-1061.
[ 9 ] SHRIKHANDE S S, JAIN D S, ATHAWALE R B, et al. Evaluation of anti-metastatic potential of cisplatin polymeric nanocarriers on B16F10 melanoma cells[J]. Saudi Pharm J, 2015,23(4):341-351.
[10] KOTTSCHADE L A, SUMAN V J, AMATRUDA T 3rd, et al. A phase Ⅱ trial of nab-paclitaxel (ABI-007) and carboplatin in patients with unresectable stage Ⅳ melanoma : a North Central Cancer Treatment Group Study, N057E(1)[J]. Cancer, 2011,117(8):1704-1710.
[11] FISCHER A P, MILES S L. Ascorbic acid, but not dehydroascorbic acid increases intracellular vitamin C content to decrease hypoxia inducible factor -1 alpha activity and reduce malignant potential in human melanoma[J]. Biomed Pharmacother, 2017,86:502-513.
[12] CARON J M, CARON J M. Methyl sulfone blocked multiple hypoxia- and non-hypoxia-induced metastatic targets in breast cancer cells and melanoma cells[J]. PLoS One, 2015,10(11):e0141565.
[13] ZHU W, ZHOU L, QIAN J Q, et al. Temozolomide for treatment of brain metastases: a review of 21 clinical trials[J]. World J Clin Oncol, 2014,5(1):19-27.
[14] AVRIL M F, AAMDAL S, GROB J J, et al. Fotemustine compared with dacarbazine in patients with disseminated malignant melanoma: a phase Ⅲ study[J]. J Clin Oncol, 2004, 22(6):1118-1125.
[15] EVANS L M, CASPER E S, ROSENBLUTH R. PhaseⅡ trial of carboplatin in advanced malignant melanoma[J]. Cancer Treat Rep, 1987,71(2):171-172.
[16] GLOVER D, GLICK J H, WEILER C, et al. WR-2721 and high-dose cisplatin: an active combination in the treatment of metastatic melanoma[J]. J Clin Oncol, 1987,5(4):574-578.
[17] RAO R D, HOLTAN S G, INGLE J N, et al. Combination of paclitaxel and carboplatin as second-line therapy for patients with metastatic melanoma[J]. Cancer, 2006,106(2):375-382.
[18] HERSH E M, DEL VECCHIO M, BROWN M P, et al. A randomized, controlled phase Ⅲ trial of nab-paclitaxel versus dacarbazine in chemotherapy-na?ve patients with metastatic melanoma[J]. Ann Oncol, 2015,26(11):2267-2274.
[19] 汪文君, 劉向輝. 惡性黑色素瘤的生物治療研究進展[J]. 口腔醫學, 2014,34(3):225-227.
[20] DAUD A I, DECONTI R C, ANDREWS S, et al. PhaseⅠtrial of interleukin-12 plasmid electroporation in patients with metastatic melanoma[J]. J Clin Oncol, 2008,26(36):5896-5903.
[21] SIN J I, PARK J B, LEE I H, et al. Intratumoral electroporation of IL-12 cDNA eradicates established melanomas by Trp2(180-188)-specific CD8+CTLs in a perforin/granzyme-mediated and IFN-γ-dependent manner: application of Trp2(180-188) peptides[J]. Cancer Immunol Immunother, 2012,61(10):1671-1682.
[22] GREENE J M, SCHNEBLE E J, JACKSON D O, et al. A phase Ⅰ/Ⅱa clinical trial in stage Ⅳ melanoma of an autologous tumor-dendritic cell fusion (dendritoma) vaccine with low dose interleukin-2[J]. Cancer Immunol Immunother, 2016,65(4):383-392.
[23] BOTTOMLEY A, COENS C, SUCIU S, et al. Adjuvant therapy with pegylated interferon alfa-2b versus observation in resected stage Ⅲ melanoma: a phase Ⅲ randomized controlled trial of health-related quality of life and symptoms by the European Organisation for Research and Treatment of Cancer Melanoma Group[J]. J Clin Oncol, 2009,27(18):2916-2923.
[24] CORRIE P G, MARSHALL A, DUNN J A, et al. Adjuvant bevacizumab in patients with melanoma at high risk of recurrence (AVAST-M): preplanned interim results from a multicentre, open-label, randomised controlled phase 3 study[J]. Lancet Oncol, 2014,15(6):620-630.
[25] GALVAN D L, O’NEIL R T, FOSTER A E, et al. Anti-tumor effects after adoptive transfer of IL-12 transposon-modified murine splenocytes in the OT-I-melanoma mouse model[J]. PLoS One, 2015,10(10):e0140744.
[26] ANDRIJAUSKAITE K, SURIANO S, CLOUD C A, et al. IL-12 conditioning improves retrovirally mediated transduction efficiency of CD8+T cells[J]. Cancer Gene Ther, 2015,22(7):360-367.
[27] HUANG Y H, ZHU C, KONDO Y, et al. CEACAM1 regulates TIM-3-mediated tolerance and exhaustion[J]. Nature, 2015,517(7534):386-390.
[28] U.S. Food and Drug Administration. Opdivo (nivolumab) prescribing information[R].U.S. Food and Drug Administration, 2016.
[29] RIBAS A, HAMID O, DAUD A, et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma[J]. JAMA, 2016,315(15):1600-1609.
[30] RIBAS A, KEFFORD R, MARSHALL M A, et al. Phase Ⅲrandomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma[J]. J Clin Oncol, 2013,31(5):616-622.
[31] ZIMMER L, VAUBEL J, MOHR P, et al. PhaseⅡDeCOG-study of ipilimumab in pretreated and treatment-na?ve patients with metastatic uveal melanoma[J]. PLoS One, 2015,10(3):e0118564.
[32] BRAHMER J R, TYKODI S S, CHOW L Q, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer[J]. N Engl J Med, 2012,366(26):2455-2465.
[33] HAMID O, SOSMAN J A, LAWRENCE D P, et al. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic melanoma (mM)[J]. J Clin Oncol, 2013,31(15).
[34] HODI F S, CHESNEY J, PAVLICK A C, et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial[J]. Lancet Oncol, 2016,17(11):1558-1568.
[35] ZIKICH D, SCHACHTER J, BESSER M J. Predictors of tumor-infiltrating lymphocyte efficacy in melanoma[J]. Immunotherapy, 2016,8(1):35-43.
[36] KHAMMARI A, KNOL A C, NGUYEN J M, et al. Adoptive TIL transfer in the adjuvant setting for melanoma: long-term patient survival[J]. J Immunol Res, 2014,2014:186212.
[37] YVON E, DEL VECCHIO M, SAVOLDO B, et al. Immunotherapy of metastatic melanoma using genetically engineered GD2-specific T cells[J]. Clin Cancer Res, 2009,15(18):5852-5860.
[38] GARGETT T, YU W, DOTTI G, et al. GD2-specific CAR T cells undergo potent activation and deletion following antigen encounter but can be protected from activation-induced cell death by PD-1 blockade[J]. Mol Ther, 2016,24(6):1135-1149.
[39] GELDRES C, SAVOLDO B, HOYOS V, et al. T lymphocytes redirected against the chondroitin sulfate proteoglycan-4 control the growth of multiple solid tumors bothinvitroandinvivo[J]. Clin Cancer Res, 2014,20(4):962-971.
[40] SHTIVELMAN E, DAVIES M Q, HWU P, et al. Pathways and therapeutic targets in melanoma[J]. Oncotarget, 2014,5(7):1701-1752.
[41] NIESSNER H, SCHMITZ J, TABATABAI G, et al. PI3K pathway inhibition achieves potent antitumor activity in melanoma brain metastasesinvitroandinvivo[J]. Clin Cancer Res, 2016,22(23):5818-5828.
[42] CHAPMAN P B, HAUSCHILD A, ROBERT C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation[J]. N Engl J Med, 2011,364(26):2507-2516.
[43] HAUSCHILD A, GROB J J, DEMIDOV L V, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial[J]. Lancet, 2012,380(9839):358-365.
[44] LONG G V, TREFZER U, DAVIES M A, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial[J]. Lancet Oncol, 2012,13(11):1087-1095.
[45] FLAHERTY K T, ROBERT C, HERSEY P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma[J]. N Engl J Med, 2012,367(2):107-114.
[46] LONG G V, STROYAKOVSKIY D, GOGAS H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma[J]. N Engl J Med, 2014,371(20):1877-1888.
[47] LEE S J, KIM T M, KIM Y J, et al. PhaseⅡtrial of nilotinib in patients with metastatic malignant melanoma harboring KIT gene aberration: a multicenter trial of Korean Cancer Study Group (UN10-06)[J]. Oncologist, 2015,20(11):1312-1319.
[48] SLINGLUFF C L JR, PETRONI G R, MOLHOEK K R, et al. Clinical activity and safety of combination therapy with temsirolimus and bevacizumab for advanced melanoma: a phaseⅡtrial (CTEP 7190/Mel47)[J]. Clin Cancer Res, 2013,19(13):3611-3620.
[49] DRONCA R S, ALLRED J B, PEREZ D G, et al. PhaseⅡstudy of temozolomide (TMZ) and everolimus (RAD001) therapy for metastatic melanoma: a North Central Cancer Treatment Group study, N0675[J]. Am J ClinOncol, 2014,37(4):369-376.
[50] 徐文博, 張江安, 于建斌,等. ABC轉運蛋白ABCG2在皮膚黑素瘤中的表達[J]. 中國皮膚性病學雜志, 2010,24(5):395-397.
[51] SCHATTON T, MURPHY G F, FRANK N Y, et al. Identification of cells initiating human melanomas[J]. Nature, 2008,451(7176):345-349.
[52] LINLEY A J, MATHIEU M G, MILES A K, et al. The helicase HAGE expressed by malignant melanoma-initiating cells is required for tumor cell proliferationinvivo[J]. J Biol Chem, 2012,287(17):13633-13643.
[53] FLAHERTY L E, OTHUS M, ATKINS M B, et al. Southwest Oncology Group S0008: a phase Ⅲ trial of high-dose interferon alfa-2b versus cisplatin, vinblastine, and dacarbazine, plus interleukin-2 and interferon in patients with high-risk melanoma--an intergroup study of cancer and leukemia Group B, Children’s Oncology Group, Eastern Cooperative Oncology Group, and Southwest Oncology Group[J]. J ClinOncol, 2014,32(33):3771-3778.
[54] ALRWAS A, PAPADOPOULOS N E, CAIN S, et al. PhaseⅠtrial of biochemotherapy with cisplatin, temozolomide, and dose escalation of nab-paclitaxel combined with interleukin-2 and interferon-α in patients with metastatic melanoma[J]. Melanoma Res, 2014,24(4):342-348.
[55] PAPADOPOULOS N E, BEDIKIAN A, RING S, et al. Phase Ⅰ/Ⅱ study of a cisplatin-taxol-dacarbazine regimen in metastatic melanoma[J]. Am J ClinOncol, 2009,32(5):509-514.
[56] FERRUCCI P F, MINCHELLA I, MOSCONI M, et al. Dacarbazine in combination with bevacizumab for the treatment of unresectable/metastatic melanoma: a phaseⅡstudy[J]. Melanoma Res, 2015,25(3):239-245.
[57] FLAHERTY K T, HAMILTON B K, ROSEN M A, et al. PhaseⅠ/Ⅱtrial of imatinib and bevacizumab in patients with advanced melanoma and other advanced cancers[J]. Oncologist, 2015,20(8):952-959.
[58] SPITLER L E, BOASBERG P, O’DAY S, et al. PhaseⅡstudy of nab-paclitaxel and bevacizumab as first-line therapy for patients with unresectable stage Ⅲ and Ⅳmelanoma[J]. Am J ClinOncol, 2015,38(1):61-67.
[59] WANG F H, LI Y H, LI S, et.al. Phase I clinical trial of intravenous recombinant human lymphotoxin-alpha derivative[J]. Ai Zheng, 2006,25(4):501-504.
[60] 于 斌, 連海燕, 王 野, 等. 納米材料應用于腫瘤治療的研究進展[J]. 中國細胞生物學學報, 2015,(4):594-598.
[61] MENG Z, ZHANG B, LIU B, et al. High carotenoids content can enhance resistance of selected Pinctadafucata families to high temperature stress[J]. Fish Shellfish Immunol, 2017,61:211-218.
[62] GUO Y Q, DING Y, LI D D, et.al. Efficacy and safety of nab-paclitaxel combined with carboplatin in Chinese patients with melanoma[J]. Med Oncol, 2015,32(9):234.
[63] MAO L, SI L, CHI Z, et.al. A randomised phaseⅡtrial of 1 monthversus1 year of adjuvant high-dose interferon α-2b in high-risk acral melanoma patients[J]. Eur J Cancer, 2011,47(10):1498-1503.
[64] WANG Y, YANG F, ZHANG H X, et al. Cuprous oxide nanoparticles inhibit the growth and metastasis of melanoma by targeting mitochondria[J]. Cell Death Dis, 2013,4:e783.
[65] RIGON R B, OYAFUSO M H, FUJIMURA A T, et al. Nanotechnology-Based Drug Delivery Systems for melanoma antitumoral therapy: a review[J]. Biomed Res Int, 2015,2015:841817.
[66] ROGERS L, SERGEEVA N N, PASZKO E, et al. Lead structures for applications in photodynamic therapy. 6. temoporfin anti-inflammatory conjugates to target the tumor microenvironment forinvitroPDT[J]. PLoS One, 2015,10(5):e0125372.
[67] CHOROMAN'SKA A, SACZKO J, KULBACKA J, et al. The potential role of photodynamic therapy in the treatment of malignant melanoma--an in vitro study[J]. Adv Clin Exp Med, 2012,21(2):179-185.
[68] 馬陽陽, 許建芳, 陳 蓮, 等.兒童黑色素瘤的病理分析:附4例報告[J].中國臨床醫學,2014,21(2):192-195.
Clinical treatment of human melanoma: recent progress
FU Jing-bo1, YU Bin2, ZHANG Hong-xia1, ZHU Hai-ying1*
1. Department of Cell Biology, College of Basic Medicine Sciences, Navy Military Medical University, Shanghai 200433, China 2. Department of Life Science, Fudan University, Shanghai 200433, China
The mortality rate of melanoma is high. Chemotherapy became the main treatment during the past years besides traditional surgical excision and radiotherapy.In recent years, with the in-depth study of the molecular mechanism of the occurrence and development of melanoma, new treatment methods and medicines basing on the target therapy and immune therapy have emerged constantly. At present, in addition to the drugs that have been used in clinical treatment, some of the drugs that are being approved for clinical research and the potential drugs which are still in basic research show good application prospects. In this paper, the molecular mechanism and therapeutic effect of the representative drugs or potential drugs in the treatment of melanoma were reviewed.
melanoma; multi-drug combination therapy; biological immunotherapy; targeted therapy
2017-03-17接受日期2017-06-08
國家自然科學基金(31471284). Supported by National Natural Science Foundation of China(31471284).
付靖波,海軍軍醫大學2015級臨床醫學專業本科學員. E-mail: fujingboi@163.com
*通信作者(Corresponding author). Tel: 021-81870944(0), E-mail: zinnia69@163.com
10.12025/j.issn.1008-6358.2017.20170223
R 739.5
A
[本文編輯] 廖曉瑜, 賈澤軍

