c-MET抑制劑聯合EGFR-TKI對耐藥肺癌細胞增殖、凋亡的影響及其機制
玄香蘭,安昌善
(延邊大學附屬醫院,吉林延吉133000)
c-MET抑制劑聯合EGFR-TKI對耐藥肺癌細胞增殖、凋亡的影響及其機制
玄香蘭,安昌善
(延邊大學附屬醫院,吉林延吉133000)
目的觀察c-MET抑制劑SU11274聯合表皮生長因子-酪氨酸激酶抑制劑(EGFR-TKI)吉非替尼或埃羅替尼對人肺癌細胞株PC9/R(PC9細胞的獲得性吉非替尼EGFR-TKI耐藥株)增殖、凋亡的影響,并探討其作用機制。方法培養人肺癌細胞株PC9/R,將PC9/R細胞分為對照組(C組)、SU11274組(S組)、吉非替尼組(G組)、埃羅替尼組(E組)、吉非替尼聯合SU11274組(GS組)、埃羅替尼聯合SU11274組(ES組)。S組、G組、E組、GS組、ES組分別加入SU11274 5 μmol/L、吉非替尼1 μmol/L、埃羅替尼1 μmol/L、吉非替尼1 μmol/L+SU11274 5 μmol/L、埃羅替尼1 μmol/L+SU11274 5 μmol/L,C組不加藥物。于給藥72 h后采用MTT法檢測細胞增殖活力,流式細胞儀技術檢測細胞凋亡情況。采用Western blotting法檢測各組細胞中的p-EGFR、p-AKT、p-STAT3蛋白。結果GS組、ES組細胞存活率分別低于G組、E組(P均<0.05),聯合組細胞存活率均低于S組(P均<0.05),G組、E組、GS組、ES組細胞存活率均低于C組(P均<0.05)。GS組、ES組早期細胞凋亡比例分別高于G組、E組(P均<0.05),聯合組早期細胞凋亡比例均高于S組(P均<0.05),GS、ES、S組早期細胞凋亡比例均高于C組(P均<0.05)。GS組、ES組p-EGFR、p-AKT、p-STAT蛋白相對表達量分別低于G組、E組(P均<0.05),聯合組p-EGFR、p-AKT、p-STAT蛋白相對表達量均低于S組(P均<0.05),G組、E組、GS組、ES組蛋白相對表達量均低于C組(P均<0.05)。結論SU11274聯合吉非替尼或埃羅替尼可抑制EGFR-TKI耐藥的肺癌細胞增殖并促進其凋亡,作用機制可能與抑制EGFR信號通路功能有關。
非小細胞肺癌;肺癌細胞;耐藥;表皮生長因子-酪氨酸激酶抑制劑;c-MET抑制劑;細胞增殖;細胞凋亡
肺癌是發病率與病死率非常高的惡性腫瘤,其中非小細胞肺癌(NSCLC)占總數的85%~90%[1]。NSCLC治療除了傳統的手術、化療、放療外,還有靶向藥物治療。研究發現,表皮生長因子(EGFR)在NSCLC組織中的陽性率為53.1%~69.7%[2]。30%~40%的NSCLC患者存在EGFR突變[3],多數為腺癌患者,合并EGFR突變者預后往往不良。吉非替尼和埃羅替尼是第一代EGFR-酪氨酸激酶抑制劑(EGFR-TKI),已成為EGFR突變的晚期NSCLC患者的首選治療方案,然而在治療后期仍會不可避免地出現耐藥。研究[4,5]表明,c-MET通路異常激活是EGFR-TKI繼發耐藥的重要機制之一,因此c-MET成為了逆轉耐藥的新靶點。本研究將c-MET抑制劑SU11274與吉非替尼或埃羅替尼聯合作用于人肺腺癌細胞株PC9/R(PC9細胞的獲得性吉非替尼EGFR-TKI耐藥株),觀察細胞增殖、凋亡的變化,并探討其機制。
1 材料與方法
1.1 細胞與主要實驗材料 PC9、PC9/R均由上海市肺科醫院中心實驗室提供。吉非替尼和鹽酸埃羅替尼原料購自濟南匯豐達化工有限公司。SU11274購自美國Sigma公司。MTT試劑購自美國AMRESCO公司。細胞凋亡檢測試劑盒(FITC Annexin V Apoptosis Detection Kit 1)購自美國BD公司。兔抗人c-MET、p-EGFR,p-AKT、p-STAT3、GAPDH購自美國EPITMICS公司。辣根過氧化物酶標記的羊抗兔購自美國JECTION公司。
1.2 細胞分組與給藥方法 將PC9/R細胞分為對照組(C組)、SU11274組(S組)、吉非替尼組(G組)、埃羅替尼組(E組)、吉非替尼聯合SU11274組(GS組)、埃羅替尼聯合SU11274組(ES組)。S組、G組、E組、GS組、ES組分別加入SU11274 5 μmol/L、吉非替尼1 μmol/L、埃羅替尼1 μmol/L、吉非替尼1 μmol/L+SU11274 5 μmol/L、埃羅替尼1 μmol/L+SU11274 5 μmol/L,C組不加入藥物。
1.3 細胞增殖觀察 取對數生長期的細胞用胰酶消化,將100 μL(含5×103個細胞)懸液接種于96孔板。待細胞貼壁后,各組加入相應藥物。72 h后,每孔內加入20 μL的MTT(5 mg/mL),放入細胞培養箱中孵育。4 h后,1 200 r/min離心10 min,棄上清液。每孔加入200 μL的DMSO,搖床上混勻30 min至結晶完全溶解。用酶標儀測量波長530 nm時的OD值。細胞存活率=(實驗組OD值-空白組OD值)/(對照組OD值-空白組OD值)×100%。實驗重復3次。
1.4 凋亡細胞檢測 取1×105個對數生長期細胞接種于6孔板,培養24 h。貼壁后棄原培養液,各組加入相應藥物。72 h后,胰酶消化并收集全部細胞到5 mL試管,1 500 r/min離心5 min,去上清液,生理鹽水洗滌1次。加入1×Binding Buffer調整細胞濃度為1×106/mL,取100 μL(1×105個細胞)到新的5 mL試管。各管內加入5 μL的FITC和5 μL的PI,室溫、避光15 min。上機前加入400 μL的1×Binding Buffer,1 h內上流式細胞儀觀察細胞凋亡情況。實驗重復3次。
1.5 細胞p-EGFR、p-AKT、p-STAT3蛋白檢測 取對數生長的細胞,各組分別加藥處理48 h,后立即冰上裂解細胞,4 ℃下12 000 r/min離心30 min,收集各組蛋白裂解液。用BCA蛋白定量法對蛋白定量。取30~40 μg蛋白經8%~10% SDS-PAGE電泳分離后,轉印至NC膜上,用5%脫脂奶粉封閉1 h,一抗孵育4 ℃過夜,TBST洗膜10 min×3次,二抗室溫搖床孵育1 h,TBST洗膜5 min×5次,ECL化學發光試劑顯色、曝光成像。以目的蛋白與內參蛋白灰度值的百分比表示蛋白相對表達量。
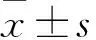
2 結果
2.1 各組細胞存活率比較 C組、S組、G組、E組、GS組、ES組細胞存活率分別為100%、99.5%±2.0%、82.5%±4.2%、60.2%±3.2%、68.0%±5.3%、45.6%±3.3%,GS組、ES組細胞存活率分別低于G組、E組(P均<0.05),聯合組細胞存活率均低于S組(P均<0.05),G組、E組、GS組、ES組細胞存活率均低于C組(P均<0.05),但S組與C組無統計學差異(P>0.05)。
2.2 各組細胞凋亡率比較 C組、S組、G組、E組、GS組、ES組早期凋亡細胞比例分別為2.7%±0.3%、10.2%±1.2%、5.1%±0.2%、3.3%±0.7%、39.3%±3.5%、23.4%±2.9%,晚期凋亡細胞比例分別為3.6%±1.0%、7.6±1.4%、4.5%±0.9%、8.4%±2.3%、6.5%±1.8%、12.0%±4.7%。GS組、ES組早期細胞凋亡比例分別高于G組、E組(P均<0.05),聯合組早期細胞凋亡比例均高于S組(P均<0.05),GS組、ES組、S組早期細胞凋亡比例高于C組(P均<0.05),但G組、E組與C組無統計學差異(P均>0.05)。
2.3 各組細胞p-EGFR、p-AKT、p-STAT蛋白表達比較 GS組、ES組p-EGFR、p-AKT、p-STAT蛋白相對表達量分別低于G組、E組(P均<0.05),聯合組p-EGFR、p-AKT、p-STAT蛋白相對表達量均低于S組(P均<0.05),G組、E組、GS組、ES組蛋白相對表達量均低于C組(P均<0.05),但S組與C組無統計學差異(P>0.05)。詳見圖1、表1。

圖1 各組細胞中p-EGFR、p-AKT、p-STAT蛋白表達情況

組別p-EGFR蛋白p-STAT3蛋白p-AKT蛋白C組79.5±3.450.7±4.851.0±6.8S組70.1±4.545.3±6.655.1±5.0G組67.1±3.333.0±2.857.6±7.2GS組49.9±2.9*24.8±4.7*42.8±6.4*E組69.6±4.636.8±5.852.8±3.6ES組44.4±5.1#26.2±3.7#39.4±2.5#
注:與G組相比,*P<0.05;與E組相比,#P<0.05。
3 討論
EGFR是細胞表面跨膜蛋白,以同源或異源二聚體形式活化,通過激活細胞內PI3K-AKT、MEK/ERK、STAT3等信號通路,促進細胞增殖、分化、發育[6]。c-MET是由MET編碼的一類具有自主磷酸化活性的跨膜受體,可激活細胞內多種信號通路,如PI3K-AKT、Ras-MAPK、STAT3通路等[7,8],從而促進腫瘤細胞間的相互作用、基質黏附、細胞遷移、侵襲和血管生成。約25%的NSCLC患者存在c-MET蛋白過表達,2%~4%的肺腺癌和肺鱗癌患者存在c-MET擴增[9,10],而c-MET擴增或過表達均與NSCLC的不良預后相關[11]。
吉非替尼和埃羅替尼用于治療EGFR突變的NSCLC患者,已取得良好的療效,但最終可因耐藥導致腫瘤復發或進展。研究發現,在EGFR突變的EGFR-TKI獲得性耐藥的NSCLC患者中,約20%的患者存在MET擴增[12],且往往伴隨著c-MET蛋白高表達[13]。SU11274可選擇性抑制c-MET活化,對其他酪氨酸激酶無作用。據研究[15]報道,在c-MET擴增的耐藥肺癌細胞中,c-MET抑制劑可明顯提高EGFR-TKI耐藥肺癌細胞的敏感性,但單用c-MET抑制劑無明顯作用。
本研究所用的人肺腺癌細胞株PC9/R具有EGFR 19外顯子E746~A750的缺失突變,是由PC9細胞株進行誘變和篩選獲得的耐藥性細胞株[14]。本研究將c-MET抑制劑與吉非替尼或埃羅替尼聯合作用于PC9/R細胞,結果顯示,GS組、ES組細胞存活率分別低于G組、E組,聯合組細胞存活率均低于S組,G組、E組、GS組、ES組細胞存活率均低于C組,而S組與C組無差異。GS組、ES組早期細胞凋亡比例分別高于G組、E組,聯合早期細胞凋亡比例均高于S組,GS組、ES組、S組早期細胞凋亡比例高于C組,而G組、E組與C組無統計學差異。這說明單用SU11274對PC9/R細胞株的增殖抑制和促凋亡作用并不明顯,但與吉非替尼或埃羅替尼聯用可顯著抑制細胞增殖、促進細胞凋亡,提示SU11274可增強EGFR-TKI對獲得性耐藥肺癌細胞的敏感性。
腫瘤的發生及發展通過多種信號的作用,下游信號分子可以重疊,所以抑制單一信號通路在實體腫瘤可能不會產生明顯的抑瘤作用[16]。EGFR突變及EGFR活化,激活細胞內PI3K/AKT、STAT3、MEK/ERK等EGFR下游信號通路,促進腫瘤細胞增殖、侵襲、血管生成。本研究結果顯示,單用吉非替尼、埃羅替尼或SU11274對EGFR、AKT、STAT3的磷酸化無明顯影響,吉非替尼或埃羅替尼聯合SU11274能夠顯著抑制EGFR及下游信號分子AKT及STAT3的磷酸化,提示吉非替尼或埃羅替尼聯合SU11274對PC9/R的細胞增殖抑制作用可能與抑制EGFR信號通路功能有關。
總之,SU11274聯合吉非替尼或埃羅替尼對PC9/R細胞具有顯著抑制增殖和促凋亡作用,可阻斷EGFR通路下游分子AKT、STAT3的活化。上述研究結果為c-MET抑制劑聯合EGFR-TKI方案的應用研究奠定了基礎。有研究[17,18]表明,c-MET抑制劑對于原發MET擴增的患者效果明顯,與EGFR-TKI聯合用藥的效果并不優于c-MET抑制劑單藥方案[19~21]。對于EGFR-TKI耐藥后MET擴增,患者的EGFR通路功能仍然活躍,需要采取與EGFR-TKI聯合用藥。目前c-MET抑制劑及MET單克隆抗體如Capmatinib(ICN280)聯合吉非替尼[22]、Emibetuzumab(LY2875358)聯合埃羅替尼[23]的臨床試驗正在進行中。
[1] DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014[J]. CA Cancer J Clin, 2014,64(4):252-271.
[2] 王洋,梁岳培.抗VEGF和抗EGFR靶向治療非小細胞肺癌的研究進展[J].臨床醫學工程,2011,18(2):312-314.
[3] 羅承遜,陳易華.非小細胞肺癌的靶點及靶向治療藥物的應用[J].西南軍醫,2017,19(1):63-65.
[4] Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling[J]. Science, 2007,316(5827): 1039-1043.
[5] Soh J, Okumura N, Lockwood WW, et al. Oncogene mutations, copy number gains and mutant allele specific imbalance (MASI) frequently occur together in tumor cells[J]. PLoS One, 2009,4(10):7464.
[6] Turke AB, Zejnullahu K, Wu YL, et al.Preexistence and clonal selection of MET amplication in EGFR mutant NSCLC[J]. Cancer Cell, 2010,17(1):77-88.
[7] Goyal L, Muzumdar MD, Zhu AX. Targeting the HGF/c-MET pathway in hepatocellular carcinoma[J].Clin Cancer Res, 2013,19(9): 2310-2318.
[8] Trovato M, Torre ML, Ragonese M, et al. HGF/c-met system targeting PI3K/AKT and STAT3/phosphorylated-STAT3 pathways in pituitary adenomas: an immunohistochemical characterization in view of targeted therapies[J]. Endocrine, 2013,44(3): 735-743.
[9] Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers[J]. Nature, 2012,489(7417):519-525.
[10] Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma[J]. Nature, 2014,511(7511):543-550.
[11] Christensen JG, Burrows J, Salgia R. c-Met as a target for human cancer and characterization of inhibitors for therapeutic intervention[J]. Cancer Lett, 2005,225(1):1-26.
[12] Bean J, Brennan C, Shih JY, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib[J]. Proc Natl Acad Sci U S A, 2007,104(52): 20932-20937.
[13] Park S, Koh J, Kim DW, et al. MET amplification, protein expression, and mutations in pulmonary adenocarcinoma[J]. Lung Cancer, 2015,90(3): 381-387.
[14] 鞠立霞,周彩存,唐亮,等.吉非替尼獲得性耐藥的非小細胞肺癌細胞株PC9/AB2相關耐藥機制研究[J].中華結核和呼吸雜志,2010,33(5):354-358.
[15] Suda K, Tomizawa K, Osada H, et al. Conversion from the “oncogene addiction” to “drug addiction” by intensive inhibition of the EGFR and MET in lung cancer with activating EGFR mutation[J]. Lung Cancer, 2012,76(3): 292-299.
[16] Liu X, Yao W, Newton RC, et al. Targeting the c-MET signaling pathway for cancer therapy[J]. Expert Opin Investig Drugs, 2008,17(7): 997-1011.
[17] Noonan SA, Berry L, Lu X, et al. Identifying the appropriate FISH criteria for defining MET copy number-driven lung adenocarcinoma through oncogene overlap analysis[J]. J Thorac Oncol, 2016,11(8): 1293-1304.
[18] Rabeau A, Rouquette I, Vantelon JM, et al. Interest of crizotinib in a lung cancer patient with de novo amplification of MET[J]. Rev Mal Respir, 2017,34(1):57-60.
[19] Neal JW, Dahlberg SE, Wakelee HA, et al. Erlotinib, cabozantinib, or erlotinib plus cabozantinib as second-line or third-line treatment of patients with EGFR wild-type advanced non-small-cell lung cancer (ECOG-ACRIN 1512): a randomised, controlled, open-label, multicentre, phase 2 trial[J]. Lancet Oncol, 2016,17(12):1661-1671.
[20] Scagliotti G, von Pawel J, Novello S, et al. Phase III multinational,randomized, double-blind, placebo-controlled study of tivantinib (ARQ197) plus erlotinib versus erlotinib alone in previously treated patients with locally advanced or metastatic nonsquamous non-small-cell lung cancer[J]. J Clin Oncol, 2015,33(24):2667-2674.
[21] Yoshioka H, Azuma K, Yamamoto N, et al. A randomized, double-blind, placebo-controlled, phase III trial of erlotinib with or without a c-Met inhibitor tivantinib (ARQ 197) in Asian patients with previously treated stage IIIb/IV nonsquamous nonsmall-cell lung cancer harboring wild-type epidermal growth factor receptor (ATTENTION study)[J]. Ann Oncol, 2015,26(10):2066-2072.
[22] Rybkin II, Smit E, Kopp HG, et al. PS01.60: Ph Ib/II, Trial of INC280 ± Erlotinib vs Platinum + Pemetrexed in Adult pts with EGFR-Mutated, cMET amplified, EGFR TKI Resistant, Advanced NSCLC: Topic: Medical Oncology[J]. J Thorac Oncol, 2016,11(11):S307-S308.
[23] Rosen LS, Goldman JW, Algazi AP, et al. A First-in-Human Phase I Study of a Bivalent MET Antibody, Emibetuzumab (LY2875358), as Monotherapy and in Combination with Erlotinib in Advanced Cancer[J]. Clin Cancer Res, 2017,23(8):1910-1919.
Effectsofc-METinhibitorcombinedwithEGFR-TKIonproliferationandapoptosisofdrug-resistantlungcancercells
XUANXianglan,ANChangshan
(TheAffiliatedHospitalofYanbianUniversity,Yanji133000,China)
ObjectiveTo observe the effects of c-MET inhibitor SU11274 combined with the epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) gefitinib or erlotinib on the proliferation and apoptosis of human lung cancer cell line PC9/R (acquired gefitinib EGFR-TKI-resistance cell line PC9 cells) and to investigate the mechanism.MethodsWe cultured the human lung cancer cell line PC9/R and divided them into six groups: the control group (group C) , SU11274 group (group S), gefitinib group (group G), erlotinib group (group E), gefitinib combined with SU11274 group (group GS), and erlotinib combined with SU11274 group (group ES). The groups S, G, E, GS, and ES were added with 5 umol/L SU11274, 1 umol/L gefitinib, 1 umol/L erlotinib, 1 μmol/L gefitinib+5 μmol/L SU11274, and 1 μmol/L erlotinib+5 μmol/L SU11274; group C was not added any drugs. At 72 h after administration, the cell proliferation was detected by using MTT method, and the apoptosis was detected by using flow cytometry. The protein expression of p-EGFR, p-STAT3, and p-AKT was detected by Western blotting.ResultsThe cell survival rates were lower in the groups GS and ES than in the groups G and E, and the cell survival rates of the combination groups were lower than that of group S (allP<0.05). The cell survival rates of groups G, E, GS, and ES were lower than that of group C (allP<0.05), The early apoptosis rates of the groups GS and ES were higher than those of the groups G and E respectively, and both groups were lower than group S (allP<0.05). The early apoptosis rates of groups GS, ES, and S were higher than that of the group C (allP<0.05). The protein expression of p-EGFR, p-STAT3, and p-AKT of the groups GS and ES were lower than those of the groups G and E, respectively (allP<0.05). The protein expression of p-EGFR, p-STAT3, and p-AKT of the combination group was lower than that of the group S, and the protein expression of p-EGFR, p-STAT3, and p-AKT of the groups G, E, GS, and ES was lower than that of the group C (allP<0.05).ConclusionsSU11274 combined with gefitinib or erlotinib can inhibit the proliferation and promote apoptosis of the EGFR-TKI resistance lung cancer cells by inhibiting the function of EGFR pathway.
non-small-lung cancer; lung cancer cells; drug resistance; epidermal growth factor receptor-tyrosine kinase inhibitor; c-MET inhibitor; cell proliferation; apoptosis
10.3969/j.issn.1002-266X.2017.39.004
R734.2
A
1002-266X(2017)39-0015-04
玄香蘭(1983-),女,碩士,主要研究方向為肺癌的基礎與臨床。E-mail: xuanxianglan101@hotmail.com
2017-08-25)

