基于3,5-二(氧乙酸基)苯甲酸構建的多核錳髤配位聚合物的合成、晶體結構和氧化-還原性質
呂靈芝 王曉娟 姜獻榮 馮云龍
基于3,5-二(氧乙酸基)苯甲酸構建的多核錳髤配位聚合物的合成、晶體結構和氧化-還原性質
呂靈芝 王曉娟 姜獻榮 馮云龍*
(浙江師范大學化學與生命科學學院,浙江省固體表面化學重點實驗室,金華 321004)
采用1,3-二(4-吡啶基)丙烷為模板劑,水熱法合成了3個以3,5-二(氧乙酸基)苯甲酸(H3BOABA)為配體的多核Mn髤配位聚 合 物 :{(H3O)[Mn4(BOABA)2(OH)3(H2O)]·H2O}n(1),{(H3O)4[Mn7(BOABA)4(OH)6(H2O)2]}n(2),{(H3O)4[Mn7(BOABA)4(OH)4(Cl)2(H2O)2]·2H2O}n(3)。通過X射線衍射,元素分析、紅外光譜和熱重分析進行了結構表征。結構分析表明,1為正交Pbcm空間群,由四核錳簇單元[Mn4(μ3-OH)3(COO)10]構成具有(5,12)-連接的3D網絡結構,拓撲符號為(32.47.5)2(38.423.514.621)。配位聚合物2與3為異質同構化合物,屬正交Ccma空間群,分別由七核錳簇單元[Mn7(OH)6(COO)16]和[Mn7(OH)4Cl2(COO)16]構成的具有(3,12)-連接的2D網絡結構,拓撲符號為(43)4(430.624.812)。測定了聚合物的氧化-還原電位。
配位聚合物;3,5-二(氧乙酸基)苯甲酸;晶體結構;循環伏安
During the past decade,interest has grown tremendously in the design and synthesis of crystalline materials constructed from molecular clusters linked by extended groups of atoms in view of their intriguing crystalline architectures and potential applications as functional materials[1-5].Most notable are metalorganic frameworks(MOFs),also known as coordination polymers(CPs),that are constructed from inorganic metal-containing nodes(also known as secondary building units,SBUs)and organic linkers[6].A universal strategy for the construction of CPs depends primarily on inorganic metal-containing nodes or coordination bonds,organic ligand design and post-synthetic modification on linkers[7-10].Among various organic ligands,multicarboxylate ligandsare selected as multifunctional organic ligands,not only because of theirvariouscoordination modesto metalions,resulting from completely or partially deprotonated sites allowing for the large diversity of topologies,but also becauseoftheirabilitytoactasH-bond acceptors and donors to assemble supramolecular structures[1,8-9,11-13].We successfully designed a multicarboxylate ligand,3,5-bis-oxyacetate-benzoic acid(H3BOABA),which possesses one rigid carboxyl group and two flexible carboxyl groups(Scheme 1).We have engaged in the research of these kinds of polymers by using H3BOABA ligand[14-16].Ever since,some CPs based on H3BOABA ligand have been explored[17-20].Herein we reportthe syntheses and structural characterizations of a family of Mn髤coordination polymers:{(H3O)[Mn4(BOABA)2(OH)3(H2O)]·H2O}n(1),{(H3O)4[Mn7(BOABA)4(OH)6(H2O)2]}n(2)and{(H3O)4[Mn7(BOABA)4(OH)4(Cl)2(H2O)2]·2H2O}n(3).The results show that the BOABA3-ligand not only has versatile traits of coordination chemistry,but also can form various network topologies[14,16].
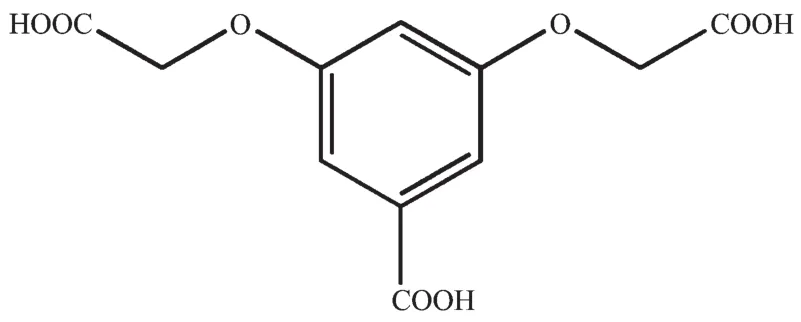
Scheme 1 Structure of 3,5-bis-oxyacetate-benzoic acid(H3BOABA)
1 Experimental
1.1 Materials and general methods
The ligand H3BOABA was prepared according to the literature method[14].All reagents were purchased commercially and used without further purification.The elemental analyses were performed on a Perkin-Elmer 2400 element analyzer.XRPD patterns were collected on a Philips PW3040/60 automated powder diffractometer,using Cu Kα radiation(λ=0.154 2 nm,U=36 kV,I=30 mA)with a 2θ range of 5°~50°.IR spectra were measured in KBr pellets on a Nicolet 5DX FT-IR spectrometer.The thermogravimetric measurements were performed on pre-weighed samples under air atmosphere using a Netzsch STA449C apparatus with a heating rate of 10℃·min-1from room temperature to 800℃.The electrochemical experiments were performed on CHI660c Electrochemical Workstation with a conventional three-electrode cell.A glassy carbon electrode was used as working electrode,platinum electrode as the auxiliary electrode,saturated calomel electrode (SCE)as the reference electrode,0.1 mol·L-1KCl solution as the supporting electrolyte solution under nitrogen stream.
1.2 Synthesis of{(H3O)[Mn4(BOABA)2(OH)3(H2O)]·H2O}n(1)
A mixture ofH3BOABA(0.5 mmol,0.135 g),Mn(CH3COO)2·4H2O (1.0 mmol,0.245 g)and 1,3-bi(4-pyridyl)propane (bpp)(0.25 mmol,0.050 g)were dissolved in 17 mL H2O,and NaOH(2.0 mmol,0.080 g)was added to the solution.Then the mixture was placed in a Parr Teflon-lined stainless steel vessel(25 mL)and heated to 180℃for 72 h,and then cooled to room temperature over 72 h.Colorless crystals of 1 were obtained and collected by filtration,washed with water and ethanol,then dried in air (Yield:38%based on H3BOABA).Anal.Calcd.for C22H23Mn4O22(%):C,30.75;H,2.70.Found(%):C,30.88;H,3.04.IR(KBr,cm-1):3 498,3 418,3 076,2 932,1 602,1433,1409,1323,1267,1176,1079,771,727,639.
1.3 Synthesis of{(H3O)4[Mn7(BOABA)4(OH)6(H2O)2]}n(2)
The synthesis of 2 was similar with that of 1 except that MnSO4·H2O(1.0 mmol,0.245 g)was used instead of Mn(CH3COO)2·4H2O and the temperature of hydrothermal reaction was 160℃.Colorless crystals of 2 were obtained and collected by filtration,washed with water and ethanol,then dried in air(Yield:27%based on H3BOABA).Anal.Calcd.for C44H50Mn7O44(%):C,31.69;H,3.02.Found(%):C,32.16;H,2.65.IR(KBr,cm-1):3 496,3 418,3 088,2 922,1 599,1 565,1 465,1 433,1 409,1 323,1 279,1 172,1 079,959,847,781,727,639.
1.4 Synthesis of{(H3O)4[Mn7(BOABA)4(OH)4(Cl)2(H2O)2]·2H2O}n(3)
The synthesis of 3 was similar with that of 1 except that MnCl2·4H2O(1.0 mmol,0.199 g)was used instead of Mn(CH3COO)2·4H2O and the temperature of hydrothermal reaction was 140℃.Colorless crystals of 3 were obtained and collected by filtration,washed with water and ethanol,then dried in air(Yield:52%based on H3BOABA).Anal.Calcd.for C44H52Cl2Mn7O44(%):C,30.36;H,3.01.Found(%):C,30.78;H,2.73.IR (KBr,cm-1):3 490,3 098,2 922,1 610,1 565,1 465,1 433,1 409,1 323,1 279,1 172,1 079,969,847,786,727,639.
1.5 Single crystal structure determination
The diffraction data for 1~3 were collected on a Bruker Smart APEXⅡarea-detector diffractometer with Mo Kα radiation(λ=0.071 073 nm).Data intensity was corrected by Lorentz-polarization factors and empirical absorption.The structures were solved by direct methods and expanded with difference Fourier techniques.All non-hydrogen atoms were refined anisotropically.All hydrogen atoms were generated geometrically.All calculations were performed using SHELXS-97[21]and SHELXL-97[22]program packages.The crystal data and structure refinement parameters for 1~3 are summarized in Table 1,and the selected bond lengths and bond angles are listed in Table 2.
CCDC:851892,1;837178,2;837177,3.

Table 1 Crystal data and structure refinement for 1~3
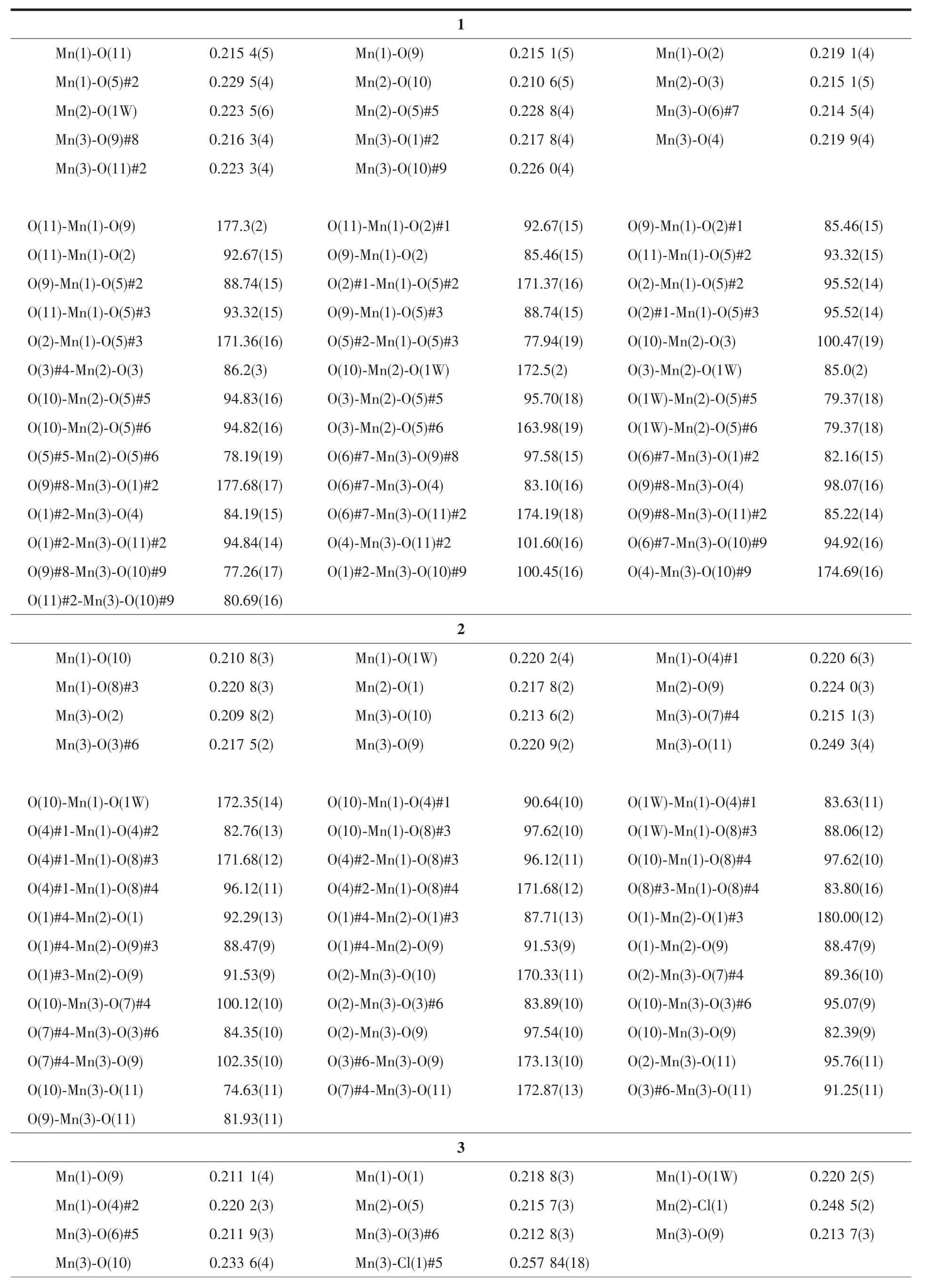
Table 2 Selected bond lengths(nm)and bond angles(°)for 1~3
Continued Table 1
2 Results and discussion
3,5-Bis-oxyacetate-benzoic acid and a combination of different Mn髤 salts such as Mn(CH3COO)2·4H2O,MnSO4·H2O and MnCl2·4H2O with 1,3-bi(4-pyridyl)propane(bpp)give rise to three CPs under hydrothermal conditions.The N-donor bpp auxiliary compound plays an important role in controlling the structures of the CPs and acts as structure-directing reagent in the process[14].The oxidation levels of the Mn髤ions were assigned by analysis of the Mn-O bond distances and bond valence sum (BVS).The bond valence sums(BVS)for the Mn metal centers were calculated according the literature[23]and the average BVS are 2.015(1.961~2.006)for 1,1.985(1.961~2.006)for 2 and 2.103(2.037~2.213)for 3.
2.1 Structure of{(H3O)[Mn4(BOABA)2(OH)3(H2O)]·H2O}n(1)
CP 1 crystallizes in the orthorhombic Pbcm space group.Besides the guest solvents and counter ion,the asymmetric unit contains two Mn髤ions,one BOABA3-ligand,one and a half μ3-OH-groups,a half coordination water molecule.The Mn髤ions are all six-coordinated in a distorted octahedral coordination environment(Fig.1).Mn(1)is coordinated to four carboxylate O atoms from four BOABA3-ligands and two μ3-OH-groups.Mn(2)is coordinated by four carboxylate O atoms from four BOABA3-ligands,one μ3-OH-group and one coordination water molecule.Mn(3)is coordinated by three carboxylate O atoms from three BOABA3-ligands and three μ3-OH-groups.Four Mn髤ions are connected through ten carboxylic groups and three μ3-OH-groups to form a tetra-nuclear[Mn4(μ3-OH)3(COO)10] SBU.The SBU polyhedrons share vertexes(O11)with each other generating a 1D inorganic chain along the b axis (Fig.2a),and the chains are further connected by the BOABA3-ligands to construct a 3D framework(Fig.2b).
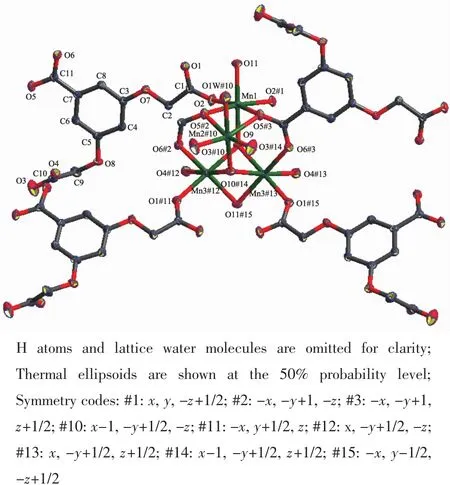
Fig.1 Coordination environment of the Mn髤ions in 1
Topologically,each organic BOABA3-ligand links five tetra-nuclear[Mn4(μ3-OH)3(COO)10]SBUs and thus can be simplified as 5-connected node(Fig.3a).Each tetra-nuclear[Mn4(μ3-OH)3(COO)10]SBU links ten organic BOABAL3-ligands and two[Mn4(μ3-OH)3(COO)10]SBUs and can be considered as 12-connected node(Fig.3b).The resulting network can be rationalized as a binodal(5,12)-connected net with a Schlfli symbol of(32.47.5)2(38.423.514.621)(Fig.3c).
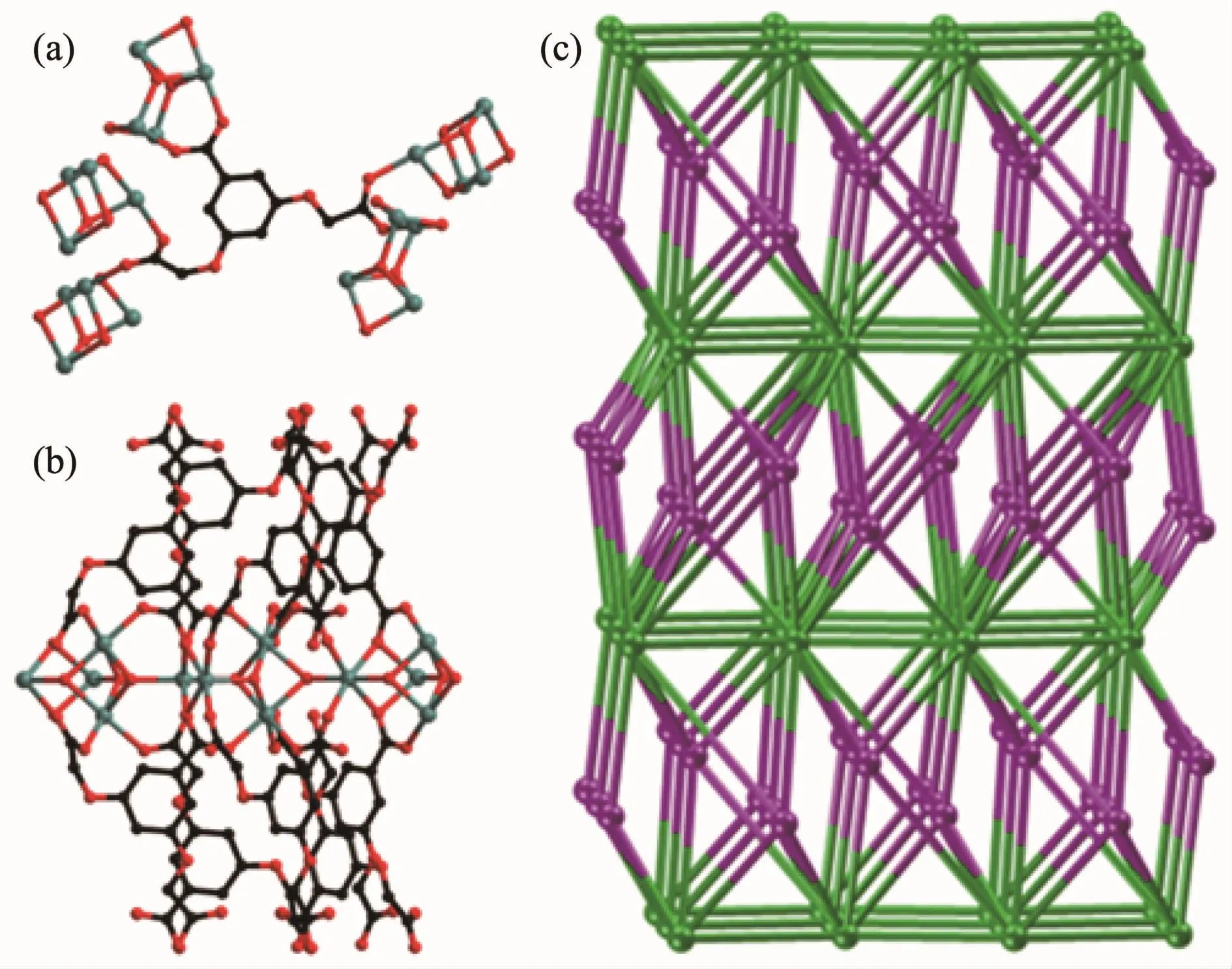
Fig.3 Five-connected node(a),12-connected node(b)and schematic representation of the(5,12)-connected topology(c)in 1
2.2 Structures of{(H3O)4[Mn7(BOABA)4(OH)6(H2O)2]}n(2)and{(H3O)4[Mn7(BOABA)4(OH)4(Cl)2(H2O)2]·2H2O}n(3)
Single-crystal X-ray diffraction analysis revealed that 2 and 3 are isostructural,and the two μ3-OH-groups in 2 was replaced by two μ3-Cl-ions in 3.Herein 2 was representatively described in detail.CP 2 crystallizes in the orthorhombic Cmca space group.Besides the guest solvents and counter ion,the asymmetric unit contains one and three quarters Mn髤ion,one BOABA3-ligand,one μ3-OH-group,a half μ2-OH-groups,and a half coordination water molecule.The Mn髤ions are all six-coordinated in a distorted octahedral coordination environment(Fig.4).Mn(1)is coordinated by six carboxylate O atoms from four different BOABA3-ligands,one μ3-OH group and one water molecule.Mn(2)is coordinated by four carboxylate O atoms from four different BOABA3-ligands and two μ3-OH groups.Mn(3)is coordinated by three carboxylate O atoms from three BOABA3-ligands,one μ2-OH group and two μ3-OH groups.Seven Mn髤 ions are connected through sixteen carboxylic groups and six μ-OH-groups to form a hepta-nuclear[Mn7(OH)6(COO)16]SBU.The adjacent[Mn7(OH)6(COO)16]SBUs are linked by four carboxylate groups of four BOABA3-ligands to form 1D inorganic chain along the c axis(Fig.5a)and the chains are further connected by the BOABA3-ligands to construct a 2D framework being parallel to ac plane (Fig.5b).The distance of the adjacent layers is 0.802 5 nm(Fig.5c).
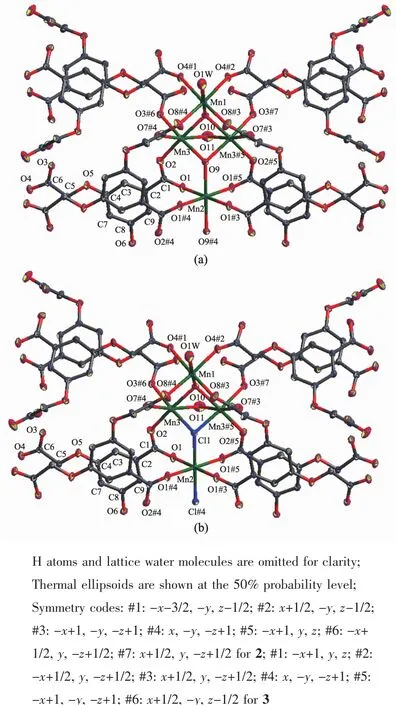
Fig.4 Coordination environments of the Mn髤ions in 2(a)and 3(b)
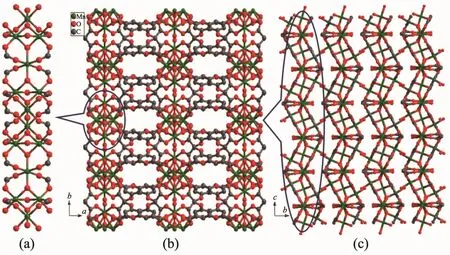
Fig.5 One dimensional inorganic metal chain along the c axis(a),2D layer parallel to ab plane(b)and view of crystalline framework along a axis in 2
Each BOABA3-ligand as a 3-connected node is linked to three[Mn7(OH)6(COO)16]SBUs(Fig.6a)and each[Mn7(OH)6(COO)16]SBU as a 12-connected node is surrounded by twelve BOABA3-ligands(Fig.6b).Thus,the overall 2D structure can be rationalized as a(3,12)-connected topology network with a Schlfli symbol of(43)4(430.624.812)(Fig.6c).
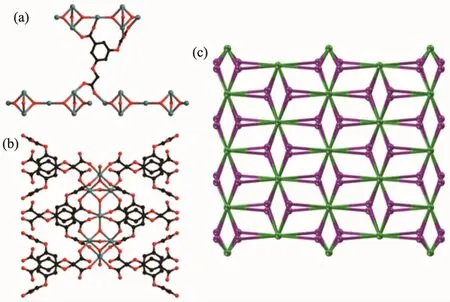
Fig.6 Three-Connected node(a),12-connected node(b)and schematic representation of the(3,12)-connected topology(c)in 2
2.3 PXRD patterns and thermal properties
X-ray powder diffraction(XRPD)patterns of 1~3 were recorded at room temperature.As shown in Fig.7,the peak positions of simulated and experimental patterns are in good agreement with each other,indicating the phase purity of the products.The differences in intensity may be due to the preferred orientation of the crystalline powder samples.
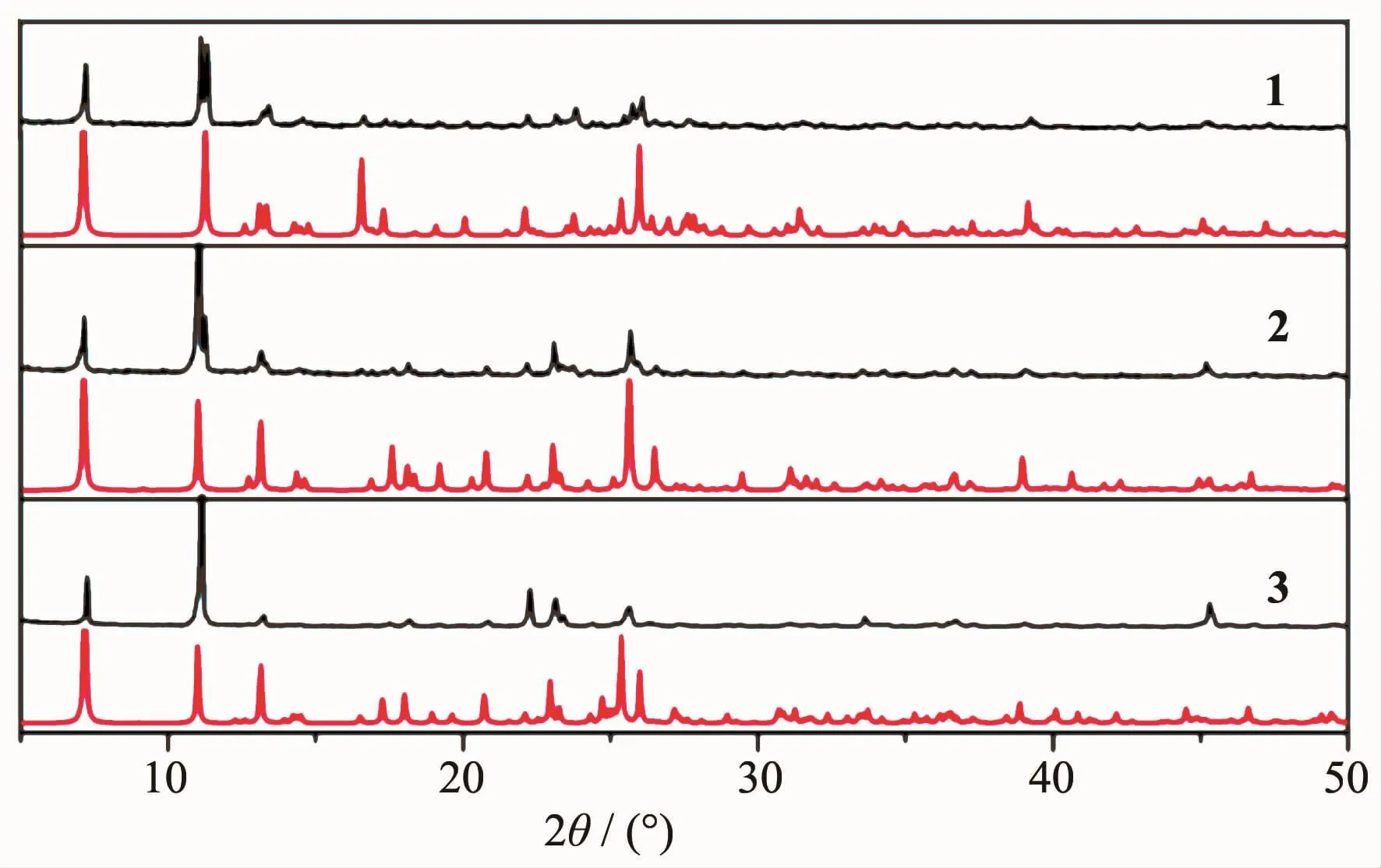
Fig.7 Experimental(black)and simulated(red)XRPD patterns of 1~3
Thermogravimetric analyses (TGA)were carried out under air atmosphere to examine the thermal stability of the CPs and the results are shown in Fig.8.The CPs are air-stable and can retain their crystalline integrity at ambient conditions.These CPs exhibit the similar thermal behavior,so 1 was discussed as an example.The weight loss of 12.70% takes place betweenroom temperatureand358℃,which corresponds to the loss of the guest solvent molecules,counter ion,coordinated water molecules and OH-ions (Calcd.12.34%).Upon further temperature increases,the compound started to decompose slowly.The final residue may be Mn2O3(Obsd.37.37%,Calcd.36.76%).
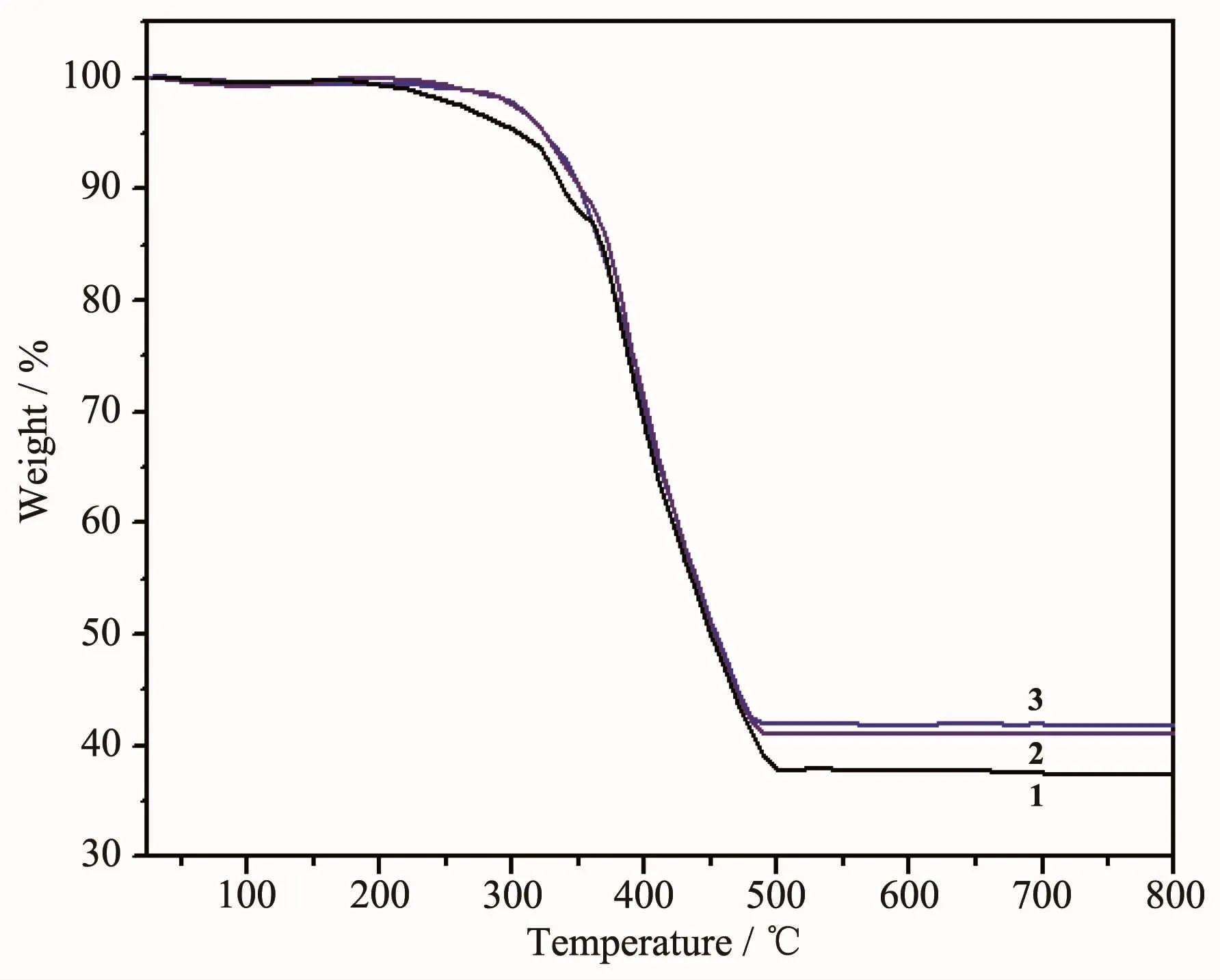
Fig.8 TG curves of 1~3
2.4 Electrochemical properties
The electrochemical properties of 1~3 in 0.1 mol·L-1KCl aqueous solution were investigated.As shown in Fig.9,the cyclic voltammetric behavior exhibits two pairs of redox peaks in the potential range from-0.2 to 1.2 V.The significant pair of redox peaks(Ⅰ-Ⅰ′)potentials(E1/2=(Epc+Epa)/2)are 0.835 V for 1,0.794 V for 2 and 0.811 V for 3,which might be ascribed to the oxidation of Mn髤/Mn髥[24-26].The pair of redox peaks(Ⅱ-Ⅱ′)potentials are-0.042 V for 1,-0.012 V for 2 and-0.015 V for 3,which may be corresponded to the one-electron processes of Mn髥/Mn髧[26].The values of peak-to-peak separation between the corresponding anodic and cathodic peaks(ΔEp)are-0.339,-0.184 and-0.205 V (Ⅰ-Ⅰ′),and-0.237,-0.078 and-0.089 V (Ⅱ-Ⅱ′),respectively.So the redox processes are apparently irreversible.
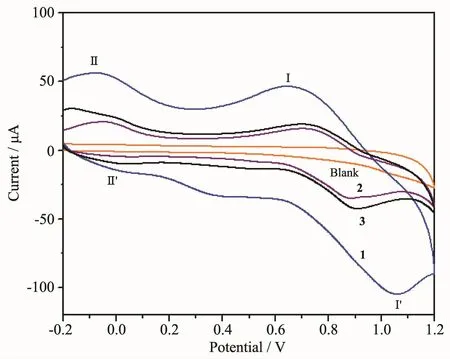
Fig.9 Cyclic voltammograms for 1~3 in 0.1 mol·L-1KCl aqueous solution
3 Conclusions
Three polynuclear manganese髤 coordination polymers were successfully synthesized by hydrothermal reactions of different manganese髤salts and a tri-carboxylate ligand 3,5-bis-oxyacetate-benzoic acid with 1,3-bi(4-pyridyl)propane as structure-directing reagent.CP 1 features a binodal(5,12)-connected 3D topology network based on tetra-nuclearMn髤clusters.CPs 2 and 3 are isostructural and present binodal(3,12)-connected 2D topology network based on hepta-nuclear Mn髤clusters.
[1]Lin Z J,Lu J,Hong M C,et al.Chem.Soc.Rev.,2014,43:5867-5895
[2]Stock N,Biswas S.Chem.Rev.,2012,112:933-969
[3]Li J R,Sculley J,Zhou H C.Chem.Rev.,2012,112:869-893
[4]Moon H R,Lim D W,Suh M P.Chem.Soc.Rev.,2013,42:1807-1824
[5]Chaemchuen S,Kabir N A,Zhou K,et al.Chem.Soc.Rev.,2013,42:9304-9332
[6]O′keeffe M,Peskov M A,Ramsden S J,et al.Acc.Chem.Res.,2008,41:1782-1789
[7]Gao W Y,Chrzanowski M,Ma S.Chem.Soc.Rev.,2014,43:5841-5866
[8]Lu W,Wei Z,Gu Z,et al.Chem.Soc.Rev.,2014,43:5561-5593
[9]Deria P,Mondloch J E,Karagiaridi O,et al.Chem.Soc.Rev.,2014,43:5896-5912
[10]Evans J D,Sumby C J,Doonan C J.Chem.Soc.Rev.,2014,43:5933-5951
[11]Yang J,Xie G,Chen X,et al.CrystEngComm,2015,17:1326-1335
[12]Rao C N R,Natarajan S,Vaidhyanathan R.Angew.Chem.Int.Ed.,2004,43:1466-1496
[13]Kitagawa S,Kitaura R,Noro S.Angew.Chem.Int.Ed.,2004,43:2334-2375
[14]He Y H,Feng Y L,Lan Y Z,et al.Cryst.Growth Des.,2008,8:3586-3594
[15]Tong Y,Jiang X R,Feng Y L.Z.Anorg.Allg.Chem.,2013,639:600-605
[16]Jiang X R,Yuan H Y,Feng Y L.J.Solid State Chem.,2012,185:181-190
[17]Shao K Z,Zhao Y H,Lan Y Q,et al.CrystEngComm,2011,13:889-896
[18]Wang Y,Zhao F H,Che Y X,et al.Inorg.Chem.Comm.,2012,17:180-183
[19]Sun Y Y,Zhao S W,Ma H R,et al.J.Solid State Chem.,2016,238:284-290
[20]Chen Y Q,Tian Y.J.Solid State Chem.,2017,247:60-66
[21]Sheldrick G M.SHELXS 97,Program for the Solution of Crystal Structures,University of G觟ttingen,Germany,1997.
[22]Sheldrick G M.SHELXTL-97,Program for the Refinement of Crystal Structures,University of G觟ttingen,Germany,1997.
[23]Thorp H H.Inorg.Chem.,1992,31:1585-1588
[24]Chakraborty P,Majumder I,Banu K S,et al.Dalton Trans.,2016:742-752
[25]Banu K S,Chattopadhyay T,Banerjee A,et al.Dalton Trans.,2009:8755-8764
Three Manganese髤Coordination Polymers Composed of Polynuclear Cluster Blocks and 3,5-Bis-oxyacetate-benzoic Acid Linkers:Syntheses,Structures and Redox Properties
L譈 Ling-ZhiWANG Xiao-Juan JIANG Xian-Rong FENG Yun-Long*
(College of Chemistry and Life Sciences,Zhejiang Key Laboratory for Reactive Chemistry on Solid Surfaces,Zhejiang Normal University,Jinhua,Zhejiang 321004,China)
Three polynuclear manganese髤 coordination polymers(CPs),{(H3O)[Mn4(BOABA)2(OH)3(H2O)]·H2O}n(1),{(H3O)4[Mn7(BOABA)4(OH)6(H2O)2]}n(2)and{(H3O)4[Mn7(BOABA)4(OH)4(Cl)2(H2O)2]·2H2O}n(3)(H3BOABA=3,5-bis-oxyacetate-benzoic acid)have been synthesized underhydrothermalcondition and structurally characterized by single crystal X-ray diffraction analyses,XRPD,IR spectra and thermal analyses.CP 1 can be rationalized as a binodal(5,12)-connected 3D topology network based on tetranuclear[Mn4(μ3-OH)3(COO)10]clusters with point symbol of(32.47.5)2(38.423.514.621).CPs 2 and 3 are isostructural and present binodal(3,12)-connected 2D topology network based respectively on heptanuclear[Mn7(OH)6(COO)16]and[Mn7(OH)4Cl2(COO)16]clusters with point symbol of(43)4(430.624.812).The cyclic voltammograms of the CPs reveal that the processes of the redox are all irreversible.CCDC:851892,1;837178,2;837177,3.
coordination polymer;3,5-bis-oxyacetate-benzoic acid;crystal structure;redox property
O614.71+1
A
1001-4861(2017)12-2169-08
10.11862/CJIC.2017.280
2017-03-16。收修改稿日期:2017-10-13。
國家自然科學基金(No.21173197)資助項目。
*通信聯系人。 E-mail:sky37@zjnu.cn;會員登記號:S06N0984M1401。

