Effect of Liquid-phase Oxidation Impurities on Solubility of Water in Hydrocarbon Fuels
A.A.Boriaev
(Saint Petersburg State University of Architecture and Civil Engineering, 4 Vtoraya Krasnoarmeyskaya St., Saint Petersburg, 199005, Russia)
Introduction
Appearance of free water in a hydrocarbon fuel during operation affects many performance properties: it reduces the corrosion resistance of structural materials, increases static, and promotes biochemical decomposition, fuel oxidation, release of hydrogen (which is an explosion hazard) in tribochemical reactions, etc. Emulsified water lowers an important performance characteristic—low-temperature pump ability—due to the deposition of ice crystals on fuel system pipeline filters at freezing temperatures.
Dehydration is important for improving the quality of hydrocarbon fuels; this process requires modern, high-performance technologies[1-3].Kobyzev S. V.[1]proposes an expanded multi-element mathematical model of hydrocarbon fuel dehydration as a part of pre-launch preparation. The paper discloses the following models based on the proposed integrated mathematical model: the model of mass transfer into an isolated gas bubble, the model of mass transfer from the fuel liquid phase to the disperse gas phase within the preparation unit volume, the model of mass transfer on the liquid surface in the preparation unit, and the model of mass transfer on the liquid surface accounting for surface bubbling.
Aleksandrov A. A. et al.[2]consider characteristics of hydrocarbon fuel dehydration processes based on fuel sparging and the cyclic technology of discharge and super saturation using dry nitrogen. The specific consumption of nitrogen and time for fuel dehydration operations was compared and gave recommendations on their application in fuel storage and preparation systems and at launch sites.
Goncharov R. A. et al.[3]present results of theoretical studies of heat and mass transfer processes in devices for cooling and dehydration of hydrocarbon fuels at launch and technical areas of launch sites.
The causes of reduced low-temperature pump ability and emulsion water in fuel cannot be eliminated without knowing the natural laws and specifics of how water dissolves in hydrocarbon fuels in the presence of oxidation products, which generally have a complex chemical composition that partially changes during storage. Of great importance references[4-11]studied the oxidation mechanism, which leads to a wide variety of chemical substances (oxidation products), as well as the effect of the hydrocarbon fuel oxidation factor on water solubility, which ultimately leads to various concentrations of chemical compounds that are products of oxidation reactions.
Ma X. et al.[4]described the study of a novel oxidative desulfurization (ODS) method for liquid hydrocarbon fuels. The ODS method was applied to a model jet fuel and a real jet fuel (JP-8) in a batch system at ambient conditions. The remarkable advantages of the new ODS method are that ODS can be performed in the presence of O2at ambient conditions without using peroxides and aqueous solvent and thus without involving a biphasic oil-aqueous-solution system.
Fernández-Tarrazo E et al.[5]explored the applicability of one-step irreversible Arrhenius kinetics of first-order reactions to a numerical description of the combustion of partially premixed hydrocarbons. Computations of planar premixed flames were used to select three model parameters: heat of reactionq, activation temperatureTa, and preexponential factorB.
You X. et al.[6]proposed a detailed kinetic model for the combustion of normal alkanes up to n-dodecane above 850 K. The model was validated against experimental data. Combined with the base C1-C4model, the simplified model predicted fuel pyrolysis rate and product distribution, laminar flame speeds, and ignition delays as closely as the detailed reaction model.
Murugan P. et al.[7]investigated low-temperature oxidation (LTO) of Fosterton crude oil mixed with its reservoir sand in a tubular reactor. The general model for an nthorder reaction was used to obtain the kinetic parameters of the coke oxidation reaction. The activation energy, frequency factor and order of the reactions were determined using the model.
Al-Hamamre Z. et al.[8]stated that a very high temperature fuel-air mixture was necessary for the thermal partial oxidation of hydrocarbon fuels in order to have a high reaction temperature, which accelerated the reaction kinetics. For diesel fuel and due to the ignition delay time behavior, varying oxidation behavior may occur at various preheating temperatures.
Li D. et al.[9]described enhanced autoxidation experiments of hydrocarbon fuels, which were performed simultaneously. The thermal-oxidation stabilities for these fuels under various conditions were compared using ultraviolet-visible spectrometry and infrared spectra.
Thomas S. et al.[10]described pyrolysis and fuel-rich oxidation experiments in an isothermal laminar-flow reactor, using the model fuel catechol (ortho-dihydroxybenzene), a phenol-type compound representative of structural entities in coal, wood and biomass, to better understand the effects of oxygen on the formation and destruction of polycyclic aromatic hydrocarbons (PAH) during the burning of complex solid fuels. The PAH products, ranging in size from two to nine fused aromatic rings, had been analyzed by gas chromatography with flame-ionization and mass spectrometric detection, and by high-pressure liquid chromatography with diode-array ultraviolet-visible absorbance detection.
Shakeri A. et al.[11]presented a new approach to reduce large detailed or skeletal mechanisms of hydrocarbon fuel oxidation to a low-cost skeletal mechanism. The method involved an integrated procedure including a Sensitivity Analysis (SA) and a Gradual Evaluation of Ignition Error (GEIE).
According to the literature review, the composition of hydrocarbon fuel oxidation products depends on oxidation reaction conditions, and is characterized by various reaction mechanisms and the variety of generated chemical compounds, including significant concentrations of oxidation products, which can be considered surfactants based on their structures.
The authors found no published data on the effect of the hydrocarbon fuel oxidation factor and, correspondingly, the effect of impurities generated from the liquid-phase oxidation reaction on the solubility of water in hydrocarbon fuels. As shown below, it is impossible to create an effective procedure for dehydrating hydrocarbon fuels without assessing the impact of oxidation factor, since during our experiments we found a significant difference in the nature of equilibrium water solubility curves for various batches of the same hydrocarbon fuel.
Studying the solubility of water in hydrocarbon fuels is associated with an important performance characteristic: low-temperature pump ability, which causes various emergencies due to fuel supply failure[12].
The conducted experiments determined the reasons for significant differences in the equilibrium solubility of water as a function of temperature for various batches of the same hydrocarbon fuel. The main reason is the different oxidation factors of product samples, resulting from the accumulation of oxidation products, which are natural surfactants, in hydrocarbon fuels. Surfactants are organic substances containing a hydrocarbon radical and one or several active polar groups. The surfactant hydrocarbon portion may consist of paraffinic, isoparaffinic, naphthenic aromatic, and other hydrocarbons of various structure. For the most common active groups are oxygen-containing (ether, carboxyl, hydroxyl, etc.) and nitrogen-containing (nitro-, amino-, amido-, etc.) groups.
1 Experimental-theoretical studies
It is known that water dissolved in a hydrocarbon liquid follows Henry′s law like a dissolved gas:
(1)
wherePiH2Ois the partial pressure of water vapor in the space above the fuel surface;PSH2Ois the saturated water vapor pressure; ψ is the relative humidity;Climis the limit or maximum possible value of water dissolution at a given temperature[12].
The value ofClim=f(T) is similar to the Henry′s law constant for gases, which is a physical constant for individual liquid/gas systems, i.e. it is constant for hydrocarbon liquid/water systems.
The limit solubilityClimdetermines the equilibrium solubility of water in the fuel and is a function of temperature. As for most liquid/gas systems, water solubility decreases as temperature decrease and, in accordance with thermodynamic laws of supersaturated solutions, free water forms in the product mass in the form of micro-droplets, followed by crystallization at temperatures below freezing. Ice crystals block up filters in fuel lines and stop fuel supply. This, in its turn, leads to emergencies. It should be noted that the equilibrium temperature corresponding toClimis referred to as the “cloud point”, since, during experimental determination of the solubility curve (to perform analysis), a slight decrease in temperature relative to the equilibrium temperature leads to clouding of the product due to formation of a new, finely dispersed phase (free water or ice). After experimental determination of the functionClim=f(T), it is easy to establish permissible limits for the dissolved water content in hydrocarbon fuels that exclude the possibility of ice crystal formation at specified low-temperature operating conditions.
In the course of our experiments to determine the equilibrium functionClim=f(T) for hydrocarbon fuels, we found a significant difference in the nature of equilibrium water solubility curves for various batches of the same hydrocarbon fuel. Figure 1 shows experimental curves for the naphthene-base hydrocarbon fuel (Figure 1(a)) and T-6 hydrocarbon fuel (Figure 1(b)).
The results made it more difficult to determine reasonable limits for the permissible water concentration in hydrocarbon fuels to prevent fuel system failure due to the formation of ice crystals when fuel temperature decreases. For example, at a residual water concentration in fuel ofCw=0.001%, ice crystals may form on curve 1 is possible only at temperatures below 40 ℃, on curves 2 and 3 — at temperatures below 14 ℃, and on curve 4 — at temperatures below 6 ℃ (Figure 1(a)). The same tendency is also observed for T-6 hydrocarbon fuel (Figure 1(b)).
This fundamental difference in results requires an explanation to prevent emergencies in equipment operation and use of hydrocarbon fuels.
We have previously ascertained that the hydrocarbon group composition has significant influence on water solubility in hydrocarbon fuels. The fractional composition of fuels and the content of mechanical impurities have a particular influence as well. However, their influence has a definite value which corresponds to the standards for production, while experiments have shown that permissible variations of these parameters in the standards could not have a significant effect on water solubility.
Considering differences in storage conditions for study samples of hydrocarbon fuels from various batches, it was assumed that various oxidation factors of fuel samples may affect water solubility, despite the fact that analysis of the main parameters of oxidation factor using current standardized methods (presence of soluble gums, acidity—determination according to state standards (GOST) or technical specifications for fuels) showed there are practically no liquid-phase oxidation products. We assumed that surfactants accumulating in the fuel during its oxidation play the determining role in changes of the equilibrium water solubility in hydrocarbon fuels.
Thus, the foregoing defines the need to carry out a set of experiments to confirm this assumption.
This paper presents the results of experimental studies of the oxidation factor′s effect and the effect of artificially introduced surfactants on water solubility in naphthene-base fuel.
According to standards, oxidation parameters of naphthene-base fuel (acidity and gums) must not exceed 0.5 and 2.0 mg per 100 mL, respectively. Experiments have shown that water solubility in hydrocarbon fuels can vary considerably (Figure 1). However, water solubility for products with zero parameters of acidity and gums varies over a wide range as well[13-14], which indicates insufficient sensitivity of the methods used to determine these parameters at low oxidation factors of hydrocarbon fuels[15-17].
2 Experimental research
To assess the effect of oxidation products on water solubility in hydrocarbon fuels, stripped, silica-gel-filtered naphthene-base fuel was selected from a certain batch, and samples with various oxidation factors were prepared under the same conditions (samples were prepared with air sparging through the product layer att=100 ℃). After oxidation, various physical and chemical parameters were determined for each fuel sample that reacted with a defined quantity of oxygen. Surface tension was determined in accordance with GOST R 50003-92 (ISO 304-85). Electrical and physical parameters (dielectric permittivity and dielectric loss tangent) were measured using an AC bridge with an automatic balancer and a transducer (three-electrode, contact, temperature-controlled sensor)[18].
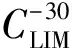
The data presented in Figure 2 show that an increase in fuel oxidation factor leads to changes in all parameters except surface tension at the product/air interface. The horizontal axis, showing the total quantity of reacted oxygen, can be divided into three characteristic regions: the first region is the interval of 0-55cm3/L, the second region is the interval of 55-88cm3/L and the third region is the interval of 88-165cm3/L. Gums and acidity (GOST parameters for fuel) begin to grow only in the third region, not reacting to oxidation prior to that. In the first region, the surface tension σ at the product/air interface and the dielectric loss tangent tan δ change most significantly. Their change indicates the appearance of oxygen compounds in the fuel. A sharp decrease in surface tension σ is evidence that these compounds are surfactants. Based upon the nature of changes in parameters (sharp inflections in the second region), surfactants are in the molecular, unassociated state (true surfactant solution in the hydrocarbon liquid up to an oxidation factor of 55cm3/L).
Upon further oxidation in the second region, the surfactant concentration reaches critical micelle concentration (CMC), and the fuel can be considered a colloidal surfactant solution in the hydrocarbon liquid with all properties inherent to them, in particular the capacity for solubilization, i.e. for increase in solubility of any substance due to its introduction into micelles. In this case, the solubilization of water molecules into surfactant micelles is observed.
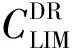
Changes in electrical and physical parameters are notable as well. The tanδincreases more than three-fold upon increase in the oxidation factor in the first region. The value then drops sharply back to baseline and then increases slowly.
According to the Debye theory, there is a relationship between tanδand the concentration of polar molecules (here, oxy groups):
(2)
whereκis the Boltzmann constant;εis dielectric permittivity;ωis angular frequency of measurement, andτis relaxation time.
Therefore:
tanδ=Bμ2C
(3)
whereBis a constant multiplier.
Thus, the concentration of polar surfactant molecules in the first region increases (true solution) during fuel oxidation, and since the oxidation groups (-COOH,-O=O, -OH, etc.) have similar dipole momentμ, tanδincreases linearly in this region with surfactant concentration. Upon further oxidation, micelle structures begin to form at a certain point. Micelle formation leads to a sharp decrease in the dipole moment attributable to the monomer unitμ/n, wherenis the number of molecules in a micelle. A several-fold decrease in the dipole moment leads to a sharp drop in tanδ. Upon further increase in the number and size of micelles, the dipole moment remains practically unchanged, and the dielectric loss tangent begins to increase linearly again with surfactant concentration.
The deviation of the dielectric permittivity diagram from linearity is also explained by the decrease in dipole moment per surfactant moleculeμ/n.
Figures 3 and 4 present the infrared spectra of hydrocarbon fuel samples observed using a UV-1800 spectrometer.
Water dissolved in non-polar solvents has an asymmetrical oscillation frequency ofυ= 3705cm-1and a symmetrical oscillation frequency ofυ=3614cm-1.Infrared spectra were determined for fuel samples with equilibrium solubility of 0.0006-0.0015%(mass fraction), and the spectral bands corresponding to these oscillations are almost negligible (absorption increases somewhat atυ=3630cm-1).The most pronounced absorption band in the spectrum is atυ=3550cm-1, increasing with fuel oxidation factor and “limit dryness”.
Experimental data support the conclusion that the absorption band atυ=3550cm-1corresponds to bound water molecules in non-polar organic solvents.
The absorption band in the IR spectrum atυ=3550cm-1corresponding to water molecules bound to each other, and the relationship between its intensity and “limit dryness” confirms micelle formation concentration upon fuel oxidation. Most likely, this band corresponds to the bound (solubilized) water located inside inverse surfactant micelles-products of liquid-phase oxidation.
Apparently, the initial product contains a certain amount of neutral resins—substances of liquid or semi-liquid consistency with very weak surfactant properties. They have heterogeneous composition and are a mixture of various aromatic hydrocarbons with long chains, condensed aromatic and naphthenic aromatic compounds with short chains, phenolic and nitrogen bases, and other compounds.
Neutral resins readily enter into oxidation, bodying and condensation reactions, reacting to form asphaltenes, carbenes, and carboids. Asphaltenes are quite strong surfactants at the hydrocarbon/water interface. Due to their surfactant properties, resinous asphaltenes play an important role in the production, transport, and refining of oil, increasing its wettability. Naphthenic (carboxylic) acids, which are widespread oil-soluble surfactants, are also oxidation products. Colloidal surfactants are of particular interest. The main distinctive feature of these substances is their ability to form thermodynamically stable heterogeneous disperse systems (associative or micellar colloids). The main characteristics of colloidal surfactants are high surface activity, capacity for spontaneous micelle formation, and capacity of surfactant solutions to solubilize, i.e. to increase the solubility of a substance because its molecules penetrate into micelles.
3 Conclusions
Thus, we can conclude that if CMC is achieved upon further oxidation of hydrocarbon liquids, micelle formation processes occur spontaneously in the solution, and the true solution becomes a colloidal system (sol). The resulting micelles are structured with hydrocarbon radicals of molecules toward the outside and hydrophilic (polar) groups toward the inside. Water molecules are located inside micelles and held so securely that water molecules do not aggregate as temperature decreases. These processes explain the experimental data we obtained showing significant differences in the equilibrium solubility of water as a function of temperature for various batches of the same hydrocarbon fuel.
The conducted experiments determined the reasons for significant differences in the equilibrium solubility of water as a function of temperature for various batches of the same hydrocarbon fuel. The main reason is the different oxidation factors of product samples, resulting from the accumulation of oxidation products, which are natural surfactants, in hydrocarbon fuels. Surfactants are organic substances containing a hydrocarbon radical and one or several active polar groups. The surfactant hydrocarbon portion may consist of paraffinic, isoparaffinic, naphthenic aromatic, and other hydrocarbons of various structure. For the most common active groups are oxygen-containing (ether, carboxyl, hydroxyl, etc.) and nitrogen-containing (nitro-, amino-, amido-, etc.) groups. Thus, dehydration is important for improving the quality of hydrocarbon fuels; this process requires modern, high-performance technologies based on well-understood natural laws and specific mechanisms of how water dissolves in hydrocarbon fuels, one of which is the effect of liquid-phase oxidation impurities on water solubility.
:
[1] Kobyzev S V. Modelirovanie obezvozhivanija uglevodorodnogo gorjuchego s primeneniem azota pri vypolnenii tehnologicheskih operacij podgotovki raketnogo topliva na startovom komplekse[J]. Engineering Bulletin of the Bauman Moscow State Technical University, 2014(11):11-15.
[2] Aleksandrov A A, Zolin A V, Kobyzev S V, et al. Sravnitel′nyj analiz tehnologij obezvozhivanija raketnogo topliva s primeneniem azota dlja nazemnyh kompleksov kosmodromov [J]. Bulletin of the Bauman Moscow State Technical University, Mechanical Engineering Series, 2013 (1):12-22.
[3] Goncharov R A, Zolin A V, Kobyzev S V, et al. Modelirovanie teplomassoobmennyh processov podgotovki uglevodorodnogo gorjuchego pered zapravkoj v toplivnye baki rakety na startovom komplekse [C]∥7thInternational Aerospace Congress. Moscow:Horuzhevskij A I, 2012:242-243.
[4] Ma X, Zhou A, Song C. A novel method for oxidative desulfurization of liquid hydrocarbon fuels based on catalytic oxidation using molecular oxygen coupled with selective adsorption [J]. Catalysis Today, 2007, 123(1-4):276-284.
[5] Fernández-Tarrazo E, Sánchez A L,Lián A,et al. A simple one-step chemistry model for partially premixed hydrocarbon combustion[J]. Combustion and Flame, 2006,147 (1/2):32-38.
[6] You X, Egolfopoulos F N,Wang H. Detailed and simplified kinetic models of n-dodecane oxidation: the role of fuel cracking in aliphatic hydrocarbon combustion [J]. Proceedings of the Combustion Institute, 2009, 32(1):403-410.
[7] Murugan P, Mahinpey N, Mani T, et al. Effect of low-temperature oxidation on the pyrolysis and combustion of whole oil [J]. Energy, 2010 ,35 (5):2317-2322.
[8] Al-Hamamre Z, Trimis D. Investigation of the intermediate oxidation regime of diesel fuel [J]. Combustion and Flame, 2009, 156(9):1791-1798.
[9] Li D, Fang W, Xing Y, et al. Spectroscopic studies on thermal-oxidation stability of hydrocarbon fuels [J]. Fuel, 2008, 87 (15/16):3286-3291.
[10] Thomas S, Wornat M J. The effects of oxygen on the yields of polycyclic aromatic hydrocarbons formed during the pyrolysis and fuel-rich oxidation of catechol [J].Fuel, 2008, 87(8):768-781.
[11] Shakeri A,Mazaheri K, Owliya M. Using sensitivity analysis and gradual evaluation of ignition delay error to produce accurate low-cost skeletal mechanisms for oxidation of hydrocarbon fuels under high-temperature conditions [J]. Energy Fuels, 2017,31(10):11234-11252.
[12] Borjaev A A, Korichev A A. Himmotologija. Avtomobil′nye topliva i processy, protekajushhie v toplivnyh sistemah avtomobil′noj tehniki [M]. Saint Petersburg: Publishing House of the Saint Petersburg State University of Service and Economics, 2014:208.
[13] Kobyzev S V. Metodika rascheta koefficientov massootdachi pri osushke uglevodorodnogo raketnogo topliva [J]. Science and Education. Bauman Moscow State Technical University (electronic journal),2011(11). Available at: http:∥technomag.edu.ru/doc/245147.html (accessed: 24.09.2012).
[14] Sorenson K L. Comparative studies on oxygen mass transfer for the design and development of a single-use fermentor [D].http:∥digitalcommons.usu.edu/etd/738 (accessed: 23.11.2017).2010.
[15] Masood R M A, Rauh C, Delgado A. CFD simulation of bubble column flows: an explicit algebraic reynolds stress model approach [J]. International Journal of Multiphase Flow, 2014 (66):11-25.
[16] Gilbert D E, Wagoner D E, Smith F. Guidelines for Development of a Quality Assurance Program: Determination of Phosphorus in Gasoline, Vol. XII[R].Washington D C.: US EPA,2013: 70.
[17] Clark A Q, Smith A G, Threadgold S, et al. Dispersed water and particulates in jet fuel: size analysis under operational conditions and application to coaleser disarming [J]. Industrial & Engineering Chemical Research, 2011, 50(9): 5749-5765.
[18] Litvinenko A N, Shlejfer A A. Sposob podgotovki otverzhdennogo uglevodorodnogo topliva k primeneniju i ustanovka dlja ego osushhestvlenija:RU,2289064 [P]. 1990.

