2-甲基-5-(2-吡啶基)-1,3,4-噁二唑 Cuギ,Cdギ配合物的合成,晶體結(jié)構(gòu)和表征
卜德艷 郭艷紅 鄭 濤 孫愛晶 王作祥
(東南大學(xué)化學(xué)化工學(xué)院,南京 211189)
0 Introduction
1,3,4-Oxadiazole and its derivatives are important compounds,which have gained considerable attentions in recent years.1,3,4-Oxadiazole compounds play a significant role in medicine and pesticides.Some 1,3,4-oxadiazole derivatives have important physiological activities[1-5],such as analgesic[6],antibacterial[7],anticancer[8],anti-inflammatory[9]and insecticides[10].The 1,3,4-oxadiazole ring can be introduced into organic compounds to synthesize special materials,such as electron transporting materials[11],corrosion inhibitors ofmetals[12-14]and stabilizers for polymer[15].
1,3,4-Oxadiazole rings haveπ-electron conjugated planar structures.The nitrogen and oxygen atoms on the oxadiazole rings have strong electron donation capacities,which can coordinate to transition metal ions and form stable complexes[16-19].Somemetal complexes of 1,3,4-oxadiazole derivatives exhibit good biological activities[20-22].Khler and Rentschler reported the first 1,3,4-oxadiazole based dinuclear ironギcomplex showing spin crossover behavior in 2016[23].Although somemetal complexeswith substituted l,3,4-oxadiazole have been reported,the metal complexes with 2-methyl-5-(2-pyridyl)-1,3,4-oxadiazole have not been reported so far.Here we reported two metal complexes[Cu2L2(μ-Cl)2Cl2](1)and[CdL2(NO3)2](2),where L=2-methyl-5-(2-pyridyl)-1,3,4-oxadiazole.The crystal structure,spectral properties and thermal stability are studied.
1 Experimental
1.1 M aterials and measurements
All chemicals were analytical grade and used without further purification.The C,H,N elemental analyses were performed on a Perkin-Elmer240.Melting points were determined on an X4 digital microscopic melting point apparatus and uncorrected.The UV-Vis spectra were recorded on an UV-6000PC UV-Vis absorption spectrophotometer.The IR spectra were recorded from 400 to 4 000 cm-1using KBr pellets on an IR prestige-21 Shimadzu infrared spectrophotometer.Fluorescence spectral data were obtained on a F-2700FL spectrophotometer.1H NMR spectra were recorded on a Bruker Avance300 spectrometer at ambient temperature in CDCl3using TMS as an internal standard.Single crystal data were collected on a Bruker SMART APEXⅡCCD single crystalX-raydiffractometer.Thermogravimetricanalysis(TGA)was performed on a NETZSCH STA49F3 thermal analyzer in nitrogen atmosphere at the heating rate of 10 K·min-1.
1.2 Synthesis of 2-methyl-5-(2-pyridyl)-1,3,4-oxadiazole(L)[24]
The 2,5-disubstituted 1,3,4-oxadiazole are prepared from diacylhydrazines using p-toluenesulfonyl chloride or benzenesulfonyl chloride as dehydrating reagents.N-acetyl-N′-(2-picolinoyl)hydrazine (5.37 g,30 mmol),p-toluenesulfonyl chloride(8.6 g,45 mmol)and triethylamine(12.5mL,60 mmol)were added to acetonitrile(150 mL).Themixture was stirred for 5 h at room temperature,and then refluxed for 10 h at 90℃.Then the solvent was evaporated.The residue was dissolved in 10%NaOH solution (100 mL)and extracted with CHCl3(3×80 mL).The organic phase was dried over MgSO4and concentrated.Recrystallization from ethyl acetate gave a colorless crystals(4.13 g,Yield:85.6%),m.p.96~97 ℃.Anal.Calcd.For C8H7N3O(%):C 59.62,H 4.38,N 26.07;Found(%):C 59.45,H 4.27,N 25.93.1H NMR(CDCl3):δ2.704(s,3H),7.470~8.792(m,4H).13CNMR(CDCl3):δ164.729,164.023,150.127,143.462,137.307,125.754,122.869,11.168.IR (KBr,cm-1):3 062,3 000,2 930,1 578,1 553,1 454,1 358,1 254,1 152,1 103,1 047,1 034,971,955,798,749,709.UV-Vis(λmax/nm):240,276.
1.3 Synthesis of com plex 1
0.170 Grams of CuCl2·2H2O(1mmol)was added to a warm solution of L (0.322 g,2 mmol)in 30 mL acetonitrile.The solution turned green and a precipitation formed.The solution gradually became clear after 4 mL of distilled water was added,filtered,and the filtrate was left to stand at room temperature for slowly evaporating.Several days later green crystals were collected (0.236 g,Yield:48.0%),and a single crystal suitable for X-ray was picked.Anal.Calcd.For C16H14Cl4Cu2N6O2(%):C 32.50,H 2.39,N 14.22;Found(%):C 32.38,H 2.33,N14.07.IR(KBr,cm-1):3 074,3 019,2 965,1 600,1 575,1 560,1 488,1 411,1 248,1 161,1 097,1 057,1 046,1 027,940,922,788,757,740,707.UV-Vis(λmax/nm):240,275.
1.4 Synthesis of com plex 2
0.231 g of Cd(NO3)2·4H2O(0.75mmol)was added to a warm solution of L(0.242 g,1.5 mmol)in 20 mL acetonitrile.A colorless mixture was formed and filtered.The filtratewas left to stand at room temperature for slowly evaporating.Several days later colorless crystalswereobtained(0.199 g,Yield:42.0%).A single crystal suitable for X-ray was picked.Anal.Calcd.for C16H14CdN8O8(%):C 34.39,H 2.53,N 20.05;Found(%):C 34.27,H 2.44,N 19.87.IR(KBr,cm-1):3 072,2 940,1 583,1 567,1 555,1 492,1 433,1 421,1 398,1 378,1 289,1 160,1 135,1 098,1 047,1 034,1 011,948,920,818,803,754,735,707.UV-Vis(λ/nm):240,276.
1.5 Crystal structure determ ination
Well-shaped single crystals of L,1 and 2 were picked for X-ray diffraction studies.The data were collected at 296(2)K on a Bruker SMART APEXⅡCCD X-ray diffractometer with a detector distance of 5 cm and frame exposure time of 10s using graphitemonochromated Mo Kα (λ=0.071 073 nm)radiation.The structures were solved by direct methods and refined on F2by full-matrix least squares procedures using SHELXTL software[25].All non-hydrogen atoms were anisotropically refined,and all hydrogen atoms on carbon atoms were generated geometrically and allowed to ride on the parent atoms,but not refined.Crystal data and structure refinement for L,1 and 2 are listed in Table 1.Selected bond lengths and angles for L,1 and 2 are listed in Table 2.The molecular graphics were prepared by using the DIAMOND 3.1 program.
CCDC:1851091,L;1851090,1;1851089,2.

Table 1 Crystal data and structure refinement for L,1 and 2
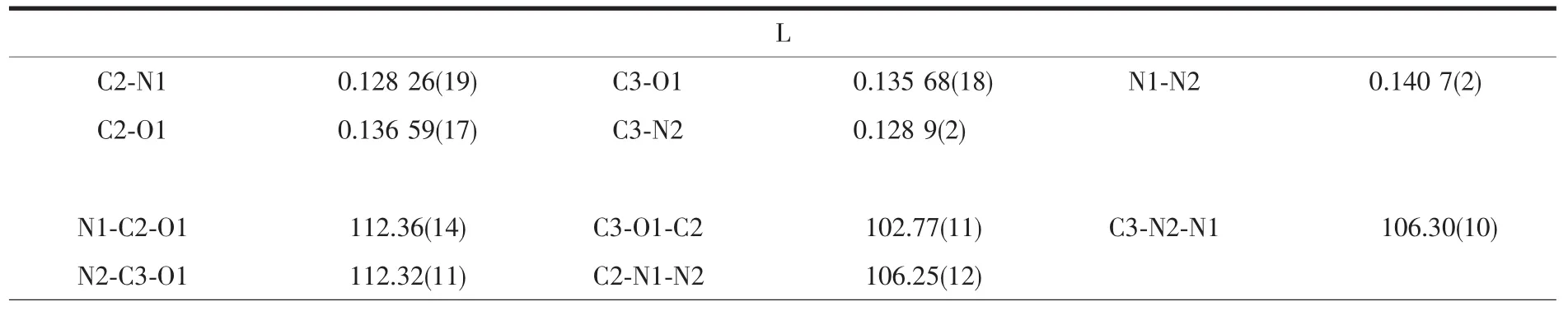
Table 2 Selected bond lengths(nm)and bond angles(°)for L,1 and 2
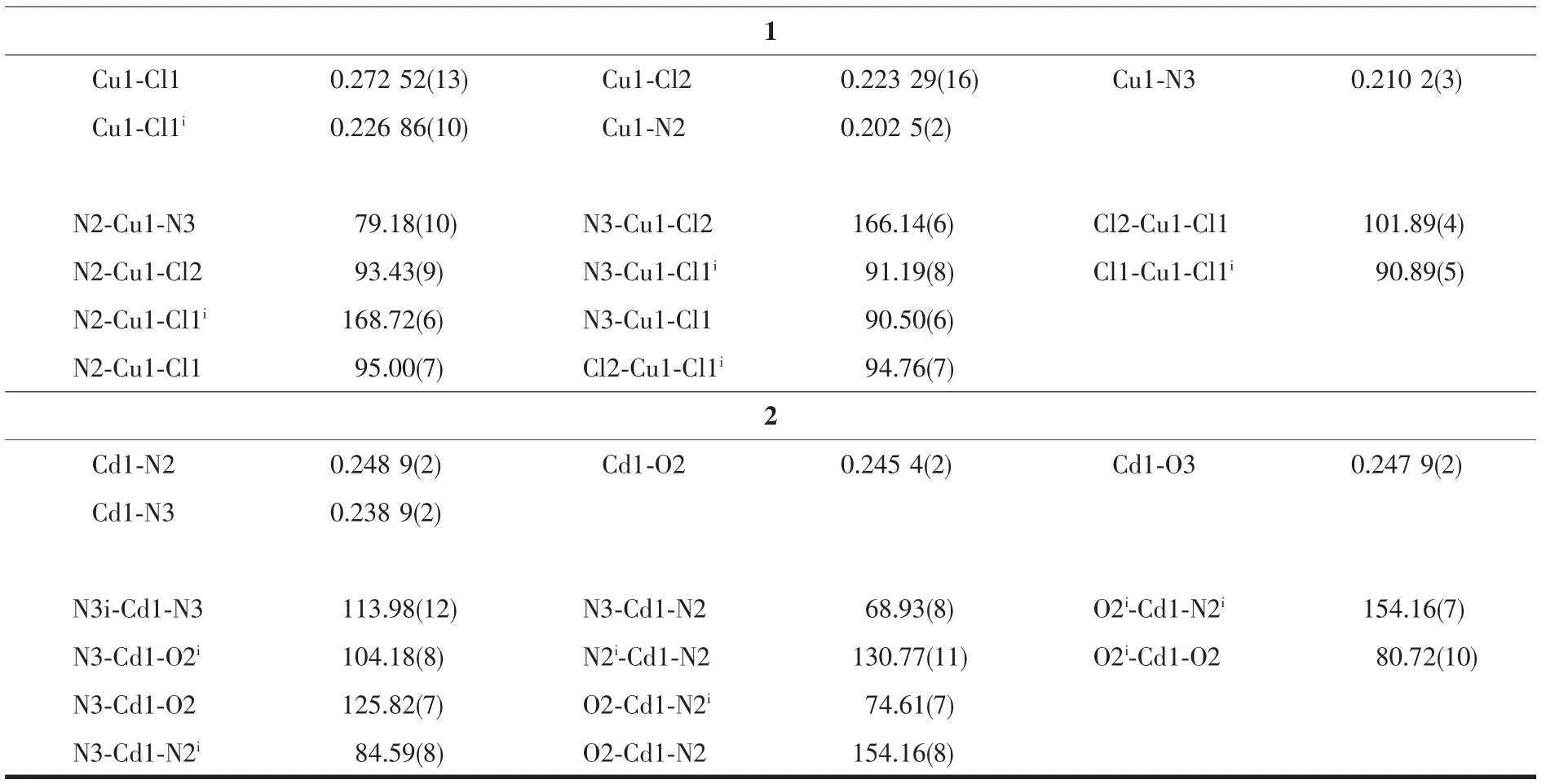
Continued Table 1
2 Results and discussion
2.1 Crystal structural analyses
2.1.1 Crystal structure of L
X-ray diffraction structure analysis reveals that L crystallizes in monoclinic system with space group P21/c.The oxadiazole ring and pyridyl ring of L are close to coplanar.The dihedral angle between them is 10.66°.In the crystals there is a non-classical hydrogen bond interactions of C1-H1A…N2i,and a weak face-to-faceπ…πinteraction exists between the 1,3,4-oxadiazole ring and pyridyl ring (Table 3 and Fig.1).All these interactions link the Lmolecules into a two-dimensional network structure.
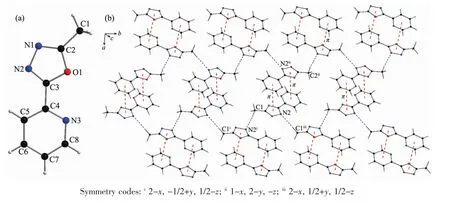
Fig.1 (a)Structure of Lwith atomic labeling;(b)2D structure of L linked by hydrogen bond andπ…πinteractions

Table 3 Hydrogen bond andπ…πinteractions for L
2.1.2 Crystal structure of 1
Complex 1 is a double-bridged binuclear Cuギcomplex (Fig.2)crystallizing in triclinic system with space group.Two copperギions are bridged by two Cl atoms (Cl1 and Cl1i).The N2,N3,Cl1iand Cl2 atoms are close to coplanar around the Cu1ギion.The Cl1 atom sits at the side of the planemaking the center Cu1ギ ion form a distorted tetragonal geometry[CuCl3N2].The bond lengths of Cu1-N2,Cu1-N3,Cu1-Cl1 and Cu1-Cl2 are 0.202 5(2),0.210 2(3),0.272 52(13)and 0.223 29(16)nm,respectively.The bond lengths of Cu-N are within the normal ranges observed for distorted tetragonal Cuギcomplex or trigonal bipyramid Cuギcomplexes[26].
In complex 1,there are three non-classical intermolecular hydrogen bonding interactions:C5-H5…Cl1i,C7-H7…Cl2iiiand C8-H8…Cl1ii.All these intermolecular hydrogen bonding interactions link complex 1 molecules into a two-dimensional network structure (Table 4 and Fig.2).A face-to-faceπ…π interaction exists between the 1,3,4-oxadiazole and pyridyl rings with a centroid-centroid distance of 0.387 4(4)nm(Table 4).Another face-to-faceπ…π interaction is found between the pyridyl rings with a centroid-centroid distance of0.362 9(4)nm.Complex 1 molecules are linked into a layered three-dimensional networks through theseπ…πinteractions(Fig.2).
2.1.3 Crystal structure of 2
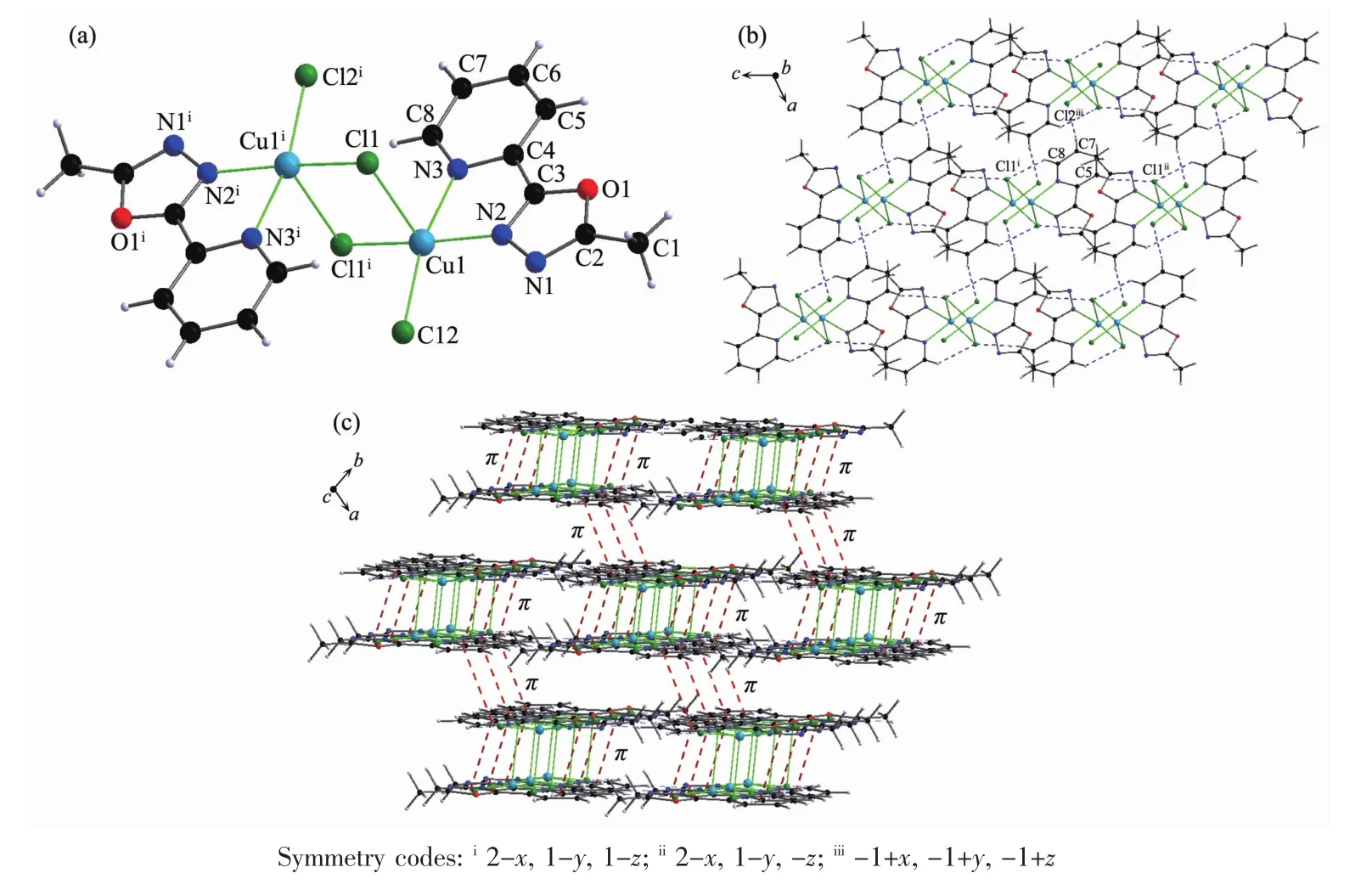
Fig.2 (a)Structure of 1 with atomic labeling;(b)2D structure of 1 through the hydrogen bond interactions;(c)3D structure of 1 linked by hydrogen bond andπ…πinteractions
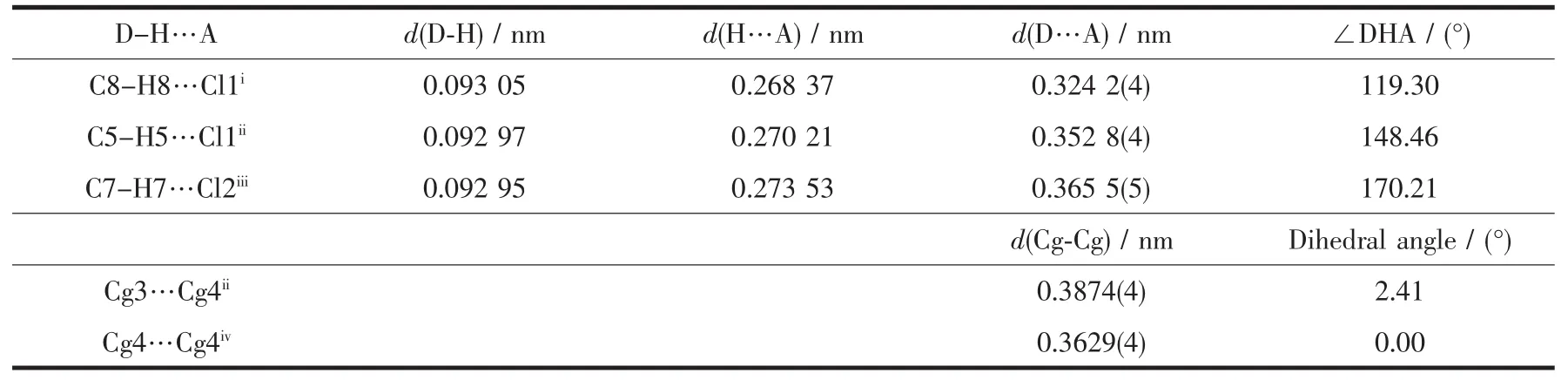
Table 4 Hydrogen bond andπ…πinteractions for 1
Complex 2 crystallizes in monoclinic system with space group C2/c. The center Cd1ギ ion are coordinated by two molecules of L and two nitrate ions.Four coordinated nitrogen atoms (N2,N2i,N3,N3i)and two oxygen atoms(O2,O2i)make the center Cd1ギ ion form a distorted octahedral geometry[CdN4O2](Fig.3).The bond lengths of Cd1-N2,Cd1-N3 and Cd1-O2 are 0.248 9(2),0.238 9(2)and 0.245 4(2),respectively.The Cd-N bond lengths are within the normal ranges observed for the 1,3,4-oxadiazole based Cdギcomplex[17].
In complex 2,there are three non-classical intermolecular hydrogen bonding interactions:C1-H1C…O2ii,C5-H5…O2iiiand C7-H7…O3iv(Table 5 and Fig.3).In addition,the pyridyl rings involve a face-tofaceπ…πinteractionwith a centroid-centroid distance of 0.391 01(19)nm.All these interactions link the complex 2 molecules into a three-dimensional network structure.
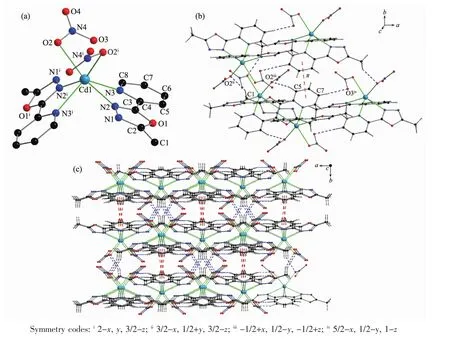
Fig.3 (a)Structure of 2 with atomic labeling;(b)Hydrogen bond andπ…πinteractions in 2;(c)3D structure of 2
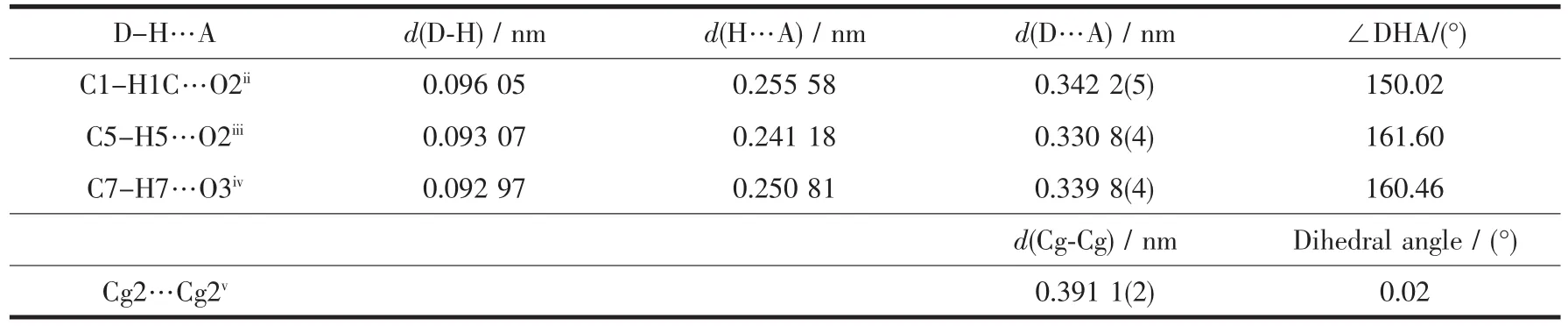
Table 5 Hydrogen bond andπ…πinteractions for 2
2.2 Spectral characterization
In the IR spectrum of L (Fig.4),the bands of 1 578,1 553 and 1 454 cm-1are attributed to the aromatic rings stretching vibrations of pyridine and 1,3,4-oxadiazole.As the nitrogen atoms coordinate to the central Cuギ or Cdギ ions in complexes 1 and 2,the corresponding absorption bands are blue-shifted.In the spectra of 1 and 2,absorption bands at 1 600 cm-1(or 1 583 cm-1),1 575 cm-1(or 1 567 cm-1)and 1 488 cm-1(or 1 492 cm-1)are observed,which are assigned to the aromatic rings stretching vibrations.This indicates that the Cu1ギ and Cd1ギ ions are coordinated by nitrogen atoms from oxadiazole and pyridine rings.In complex 2,the bands of 1 289 cm-1are attributed to NO3-.
The UV-Vis spectra of L is given in Fig.5(a).Maximum absorption peak appears at 240 and 276 nm,which are attributed to the π-π*and n-π*transitions,respectively.The fluorescence property of L has been examined in ethanol solution at room temperature.The fluorescence spectrum is shown in Fig.5(b),which shows L is fluorescent.Maximum emission peak and excitation wavelength is 403 and 250 nm,respectively,which is attributed to π-π*electronic transition[27].
In the UV-Vis spectra of 1 and 2,themaximum absorption peak of 1 appears at 240.04 nm and 275.09 nm,and those for 2 appear at 240.13 nm and 276.05 nm.This is attributed to π-π*and n-π*transitions.The fluorescence properties of complexes 1 and 2 have been examined in methanol solution at room temperature,but no fluorescence properties were observed.
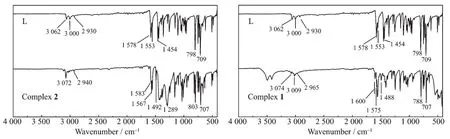
Fig.4 IR spectra of L,1 and 2
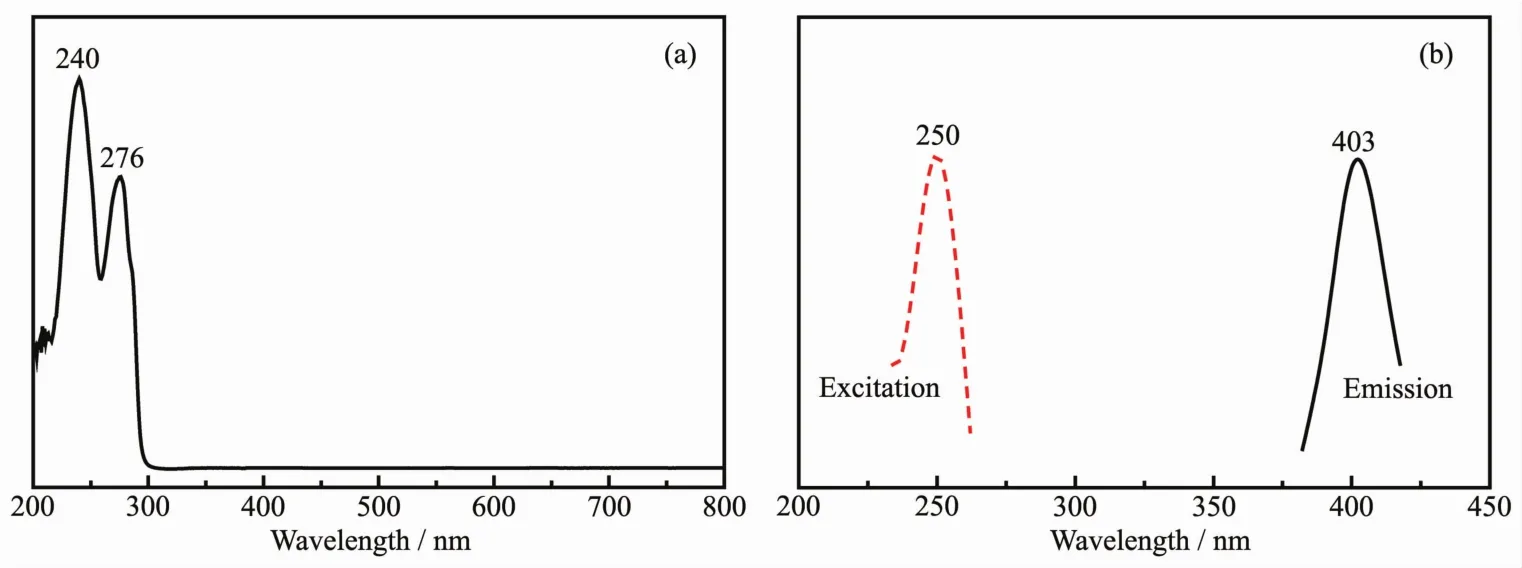
Fig.5 IR,UV-Vis and fluorescence spectra of L
2.3 Thermal gravimetric analysis
Thermal gravimetric analyses(TGA)of complexes 1 and 2 were accomplished in nitrogen atmosphere with the heating rate of 10 K·min-1.The TGA curves of complexes 1 and 2 are presented in Fig.6.When the temperature is below 95℃,complex 1 is stable.Complex 1 decomposed gradually with the temperature raised between 95 and 800℃.Total weight loss was close to 71.00%at 800℃.The final residue is CuO(Calcd.26.9%).Complex 2 is stable below 150℃.Complex 2 decomposed sharply with the temperature raised between 200 and 300℃.When the temperature reached to 650℃,totalweight loss is close to 80.97%(Calcd.77.02%).

Fig.6 TGA curves for complexes 1 and 2
3 Conclusions
Ligand L,complexes 1 and 2 were synthesized and characterized.The free ligand L exhibits fluorescence.Complex 1 is a double-bridged binuclear Cuギ complex.Two copperギ ions are bridged by two Cl atoms,and the central copperギions have distorted tetragonal geometries [CuCl3N2].In complex 2,the central Cdギion have a distorted octahedral geometry[CdN4O2].
Supporting information isavailable athttp://www.wjhxxb.cn

