高等植物中LINC復(fù)合體的研究進(jìn)展
盧 汀
(攀枝花學(xué)院生物與化學(xué)工程學(xué)院,中國四川攀枝花617000)
Many important functions are carried out by nuclear envelope(NE),including protecting and enclosing chromatin,facilitating material nucleo-cytoplasm transport,taking part in cell signal transduction,and bridging nucleoskeleton and cytoskeleton physically and structurally.All these functions are essential in various organisms from single cells to complex metazoans,and are achieved by a series of inner nuclear membrane(INM)and outer nuclear membrane(ONM)proteins.The NE communication is bridged by proteins forming a link across periplasmic space between INM and ONM.These proteins have multiple functions including anchoring genetic materials and nucleoskeleton,moving nucleus during cell movement and acting in signalling pathways.There exist significant differences among these proteins even though they are conserved in eukaryotes[1].Recent progresses in plant NE proteins that form the bridge between INM and ONM are reviewed in this article.
1 LINC complexes to bridge the nuclear periplasm
Many connections between cytoplasm and nucleoplasm with structural proteins are viewed under the microscope[2].Nuclear membranes are separated by a space of around 50 nm width.The INM-ONM protein interactions happen in this space.The bridging LINC(linker of nucleoskeleton and cytoskeleton)complex exists in this space[3].Among eukaryotes the LINC complex compositions are conserved.The LINC complex supports and shapes the nuclear envelope and positions the nuclear pore complex(NPC)(Fig.1).Two series of protein families are involved in forming LINC complex:the Sad1/UNC84(SUN)domain proteins on INM and Klarsicht/Anc/Syne-1 homology(KASH)domain proteins on ONM[3].SUN proteins interact with KASH proteins at their C-terminal SUN domains and KASH domains respectively.There are two types of exclusively expressed SUN domain proteins in opisthokonts whilst KASH proteins are far more diverse.
2 Plant SUN domain proteins
SUN domain proteins came from the description of UNC84 in Caenorhabditis elegans and Sad1(a spindle pole body component)in Schizosaccharomyces pombe[4~5].Both UNC84 and Sad1 contain SUN-domain at C-terminus.Two exclusively expressed homologs,which are typeⅡintegral membrane proteins,were discovered in opisthokonts.The N-terminus of SUN domain proteins is anchored in nucleoplasm interacting with type-B lamins while the C-terminus of SUN domain proteins is anchored in periplasm interacting with KASH domain proteins.The C-terminus is highly conserved in SUN domain proteins,but the N-terminus is not.For example,in HsSUN3,HsSUN4 and HsSUN5,the lamin binding domain is absent[6~8].Trimmers are formed by proteins with a classical C-terminal SUN domain,such as the crystal structure of human SUN2[1,9].KASH domains are bound with these trimmers and strengthened by a disulphide bridge over conserved cysteine.SUN trimmers on INM and KASH domains nearby the ONM form the majority of the LINC bridge[10].SUN domain proteins are involved in mul-tiple functions.The yeast SUN domain proteins Mps3(Saccharomyces cerevisiae)and Sad1(S.pombe)are positioned at the INM and spindle pole bodies(SPBs)[11~12].Particularly,ScMps3 and SpSad1 are positioned to the half bridge[5,13].Three SUN domain proteins SUN3,SUN4 and SUN5 are expressed in mammals as well as the inclusively expressed SUN1 and SUN2[14].In some species,different types of SUN domain proteins are expressed in different stages of development:for instance,C.elegans SUN1 is expressed in early embryo,UNC84 is found in adult somatic cells[6~7].
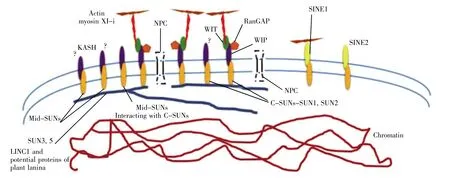
Fig.1 Plant NE:known and suggested proteins
Putative homologs of SpSad1 in plants were first suggested in Arabidopsis thaliana and Oryza sativa[15~16].Higher plant SUN domain proteins were firstly examined as AtSUN1 and AtSUN2[17].Proteins with a classical C-terminal SUN domain are presented across the plant kingdom:the monocots(ex.Zea mays),dicots,the club moss Selaginella moellendorffii,the moss Physcomitrella patens and algae[17~20].AtSUN1 and AtSUN2 share a higher level of homology(68%identity,1.00E-178)with each other than with ZmSUN1 and ZmSUN2.This indicates that separate gene duplication happens.Among SUN proteins,there are differences in function,binding and location.AtSUN1 and AtSUN2 are much smaller than mammalian counterparts and close to yeast Sad1 in size.A structural conserved phenomenon is a single coiled coil domain in between C-terminal SUN domain and N-terminal transmembrane domain[17,21].Apart from interacting with putative plant KASH proteins on ONM,it has been described that plant SUN proteins interact with NMCP/LINC family[22~24].The N-termini of plant SUN proteins can interact with LINC1 and position it at the nuclear periphery[22].The plant SUN-LINC linkages are the first evidence of SUN-nucleoskeletal anchorage with the absence of lamins.A study in higher plants revealed an extra group of proteins comprising a central SUN domain[20].Genomic studies of maize indicated mid-SUN proteins including ZmSUN3,ZmSUN4 and ZmSUN5.ZmSUN3 and ZmSUN4 are inclusively expressed whilst ZmSUN5 is pollen exclusive.These three proteins are located at the nuclear periphery[20].They were described in opisthokonts as well with osteopotentia(Opt)as a rough endoplasmic reticulum(rER)resident[25].Opt functions as an adaptor stabilizing the rER through interacting with cytoskeleton.The other yeast mid-SUN protein was SLP1[26].
3 Plant KASH domain proteins
KASH protein family contains members from Drosophila melanogaster(Klarsicht),C.elegans(ANC1),and mammals(Syne-1 and Syne-2)[27~29].KASH domain proteins are transmembrane proteins with a conserved C-terminal KASH domain anchored at ONM.Four criteria are used to define KASH domain proteins:ONM localization;interaction between KASH and SUN domain;ONM localization determined by SUN-KASH interaction;cytoplasmic N-terminus that interacts with actin and dynein[3,30].It is typical for KASH proteins to include a transmembrane domain and a short length of 9 to 35 amino acids which are usually PPPX at the periplasm end[14,29].The largest KASH domain protein is Msp300 with 1 300 kD molecular weight.In KASH proteins,an N-terminal binding domain is separated by a spectrin repeats or coiled coils from a transmembrane domain[31].KASH domain proteins form homodimers like SUN domain proteins[32~33].Binding of SUN-KASH proteins is formed by PPPT motif of the KASH proteins and hydrophobic pocket of SUN proteins.The penultimate proline which is widely conserved appears to be crucial for this binding[14].Metazoan nesprins have different sizes and interactions.The biggest is the 1 000 kD Nesprin1 Giant(Nesp1G).Nesp1G has an N-terminal actin binding domain comprising two calponin homology domains.Nesp2G has a relative molecular mass of 800 kD with a C-terminal actin binding domain as well[28,34~35].Nesprin3(100 kD)lacks actin binding domain but binds to plectin interacting with intermediate filaments(IF).Nesprin4(42 kD)interacts with cytoskeleton through kinesin.Some nesprins interact with emerin on INM[33,36~37].Klarsicht/Anc/Syne-1 homology domain proteins control the nuclear size in non-plant systems[38].
It was a long time before the breakthrough took place in searching plant KASH domain proteins[39].NE-associated WPP(tryptophan-prolineproline)in Arabidopsis was identified in previous work[40].This family includes RanGAP1,WPP1 and WPP2,which are all characterized by WPP motif repeats in sequence[41~42].Interactions with WPP domain interacting proteins(WIPs)and the WPP interacting tail anchored proteins(WITs)were shown to be localized to the NE.WIPs and WITs provide WPP anchorage by oligomerising,for instance,the anchorage of RanGAP1 to NE[40].WIPs and WITs take part in generating RanGTP gradient.Both WIPs and WITs are membrane proteins with C-terminus anchored in the periplasm.AtWIPs were found to include a conserved X-VPT motif[39].This motif contains proline and is conserved in plant system[39].Confirmation of WIPs as plant KASH domain proteins is about interaction with SUN domain proteins.VVPT motif interaction with SUN domain proteins was demonstrated.Deletion or truncation of SUN domain damaged interaction of AtWIP1,AtWIP2 and AtWIP3 with AtSUN2[39].Fluorescence recovery after photobleaching(FRAP)experiment revealed that AtWIPs mobility increased after VVPT domain deletion[39].In a sun1-KO/sun2-KD mutant,transformed GFP-AtWIP1 fluorescence signal was found mainly in the cytoplasm,confirming the necessity of SUN domain proteins for nuclear positioning.Comparison of AtWIPs C-terminus to opisthokont KASH domains showed low similarity.AtWIPs are similar in size to D.discoideum Interaptin,KDP-1,and C.elegans ZYG-12B[43~45].RanGAP at the nuclear pore complex was the first plant LINC complex identified protein with bona fide function,which differs from that of mammals.In mammalian system,RanGAP was anchored by RanBP2 direct attachment.More functions about plant LINC system are studied.Kaku1 as an Arabidopsis myosin XI-i mutant was deployed to explore the attachment of cytoskeleton to NE[46].Plant nuclei response to blue light and fungal infection by moving in an actin-based system[47~48].It was showed that the LINC complex attached myosin XI-i interacts with actin in plant cytoplasm.A recent study of plant KASH proteins identified the SUN interacting NE(SINE)proteins.These proteins are also C-terminally anchored to nuclear periplasm with X-VPT motif.SINE interaction with SUN pro teins is regulated by VPT[49].SINE2 is shown to have the function of engaging in innate immunity response and SINE1 takes part in nuclei positioning.SINE1 has tissue specific expression pattern[49].Г-tubulin complex protein 3(GCP3)interacting proteins(GIPs)recruits γ-tubulin complexes(γ-TuCs)to interact with the microtubule cytoskeleton in interphase[50].
4 Plant LINC complexes
Sequence alignment suggests that crucial amino acids such as S641 are conserved in AtSUN1 and AtSUN2,hence,the existence of LINC complexes in plants is implied[51].In animals,Cter-SUN proteins are the INM components;in yeasts,mid-SUNs are considered as the counterparts.The Cter-SUNs interacting with mid-SUNs would imply mid-SUNs as the secondary components of LINC complexes similar to WITs.Plant LINC complexes could be highly diverse in composition with SUN 1~5 located at INM[51].LINC complexes are described involved in many cell functions including connecting nucleoskeleton and cytoskeleton,anchoring telomeres,interacting with the centrosome and nuclear migration[30].These functions,as mentioned above,are still in hypothesis for plant LINC complexes,however,the newly discovered plant SUN and KASH proteins will significantly improve the insight of plant LINC functions.The LINC complexes support and shape the nuclear envelope and position the nuclear pore complex(NPC).The LINC complex performs as crossing-NE multi-functional bridge,participating in establishing nuclear shape,size and movement,and linking nucleoskeleton and cytoskeleton,mitosis and meiosis[52].
5 Conclusion
LINC is a highly conserved complex for both nuclear shaping and movement.As more and more facts about SUN-KASH systems in plants have been discovered,studies on this aspect are becoming obviously important.The functions regarding C-SUNs and mid-SUNs are needed to be elucidated in the next phase of study,together with the identification of more and more KASH proteins in plants.SUN-KASH system is essential in plants for many vital functions including gene expression regulation and chromosome segregation in meiosis.

