Isotopeeffectand Coriolis coup ling effect for the Li+H(D)Cl→LiCl+H(D)reaction*
Hongsheng Zhai(翟紅生),Guanglei Liang(梁廣雷),JunxiaDing(丁俊霞),and Yufang Liu(劉玉芳),?
1College ofPhysicsand Materials Science,Henan NormalUniversity,Xinxiang 453007,China
2SpectralMeasurementand Application of Infrared MaterialKey Laboratory ofHenan Province,Academy ofPhysicsand Materials Science,Henan NormalUniversity,Xinxiang 453007,China
3State Key Laboratory ofMolecularReaction Dynamics,Dalian Institute ofChemicalPhysics,Chinese Academy ofSciences,Dalian 116023,China
(Received 14 January 2019;revisedmanuscript received 12March 2019;published online4April2019)
Keywords:isotopeeffect,Coriolis coupling,rate constant,quantum wave packet
1.Introduction
As a prototype for the three-atom reactions between alkali metals and hydrogen halides,the reaction Li+HCl has been the subject of various theoretical and experimental investigations.[1–14]On aspects of experiments,Helbing and Rothe[1]measured the velocity dependence of the total cross section integral cross sections for the scattering of lithium by a series ofmolecules(include the HCl and DClmolecules).In Becker’s[2]group,themolecularbeam methodwasadopted to study the reaction of Li+HCl,the results showed that the majority of theenergy in this reaction goes to the translational energy of the product,and only a little energy is transferred to the vibration energy of the product.This reaction’s barrier is lower than 2.00 kcal/mol,and there is a van derWaalswell at the entrance.After that,Plane and Saltman[3]measured the reaction rate constants by the technique of time-resolved laser induced f luorescence spectroscopy of Libetween 700 K and 1000 K.The height of this reaction’s barrier is about 1.96±1.4 kcal/mol.
On the theoreticalside,in 1979,the f irstpotentialenergy surface(PES)was constructed by Shapiro and Zeiri[4]using asem i-empiricaldiatom ic-in-molecules(DIM)approach.The transition state derived from the semi-empirical calculations is located at the entrance channeland has collinear geometry.Its transition state energy barrier is 11.36 kcal/mol.Garcia et al.[5]presented a dynam ical investigation of the Li+HCl reaction carried outon the DIM potential energy of Zeiriand Shapiro.[6]The threshold energy estimated from thesecalculationsconf lictswith the largevalueof the reactive crosssection measured using a crossedmolecular beamsapparatus.Garcia and Lagana[7]reported the conf iguration interaction(CI)computation of thepotentialenergy atseveralgeometriesof the Li-HCl system and the computations exhibita bent structure for the transition state.Subsequently,Palm ieri etal.constructed a LiClH PES f itted by thebond order(BO)[8]polynomialand the new BO PES bymodifying the ab initio points.[9]Based on the new BO PES,[10]the rate constants and cross sections were calculated,and the rate constantsobtained from the new BOPESwere comparedwith theexperimental resultsof timeresolved laser induced f luorescencespectroscopy.Thedynamicscalculationsindicate that thehigherquality ab initio PESis needed.[11–15]In recentyears,Chen etal.[16]and Lin etal.[17]obtained high-precision global PES crucial for the LiHClsystem ground state,respectively.And Lin’s group reported the integralcrosssections,rate constants,and theirdependenceon the initial rotational states based on their new PES.The rate constantsare in agreementwith the experimental results.
Corioliscoupling effectscan inf luencedynamicalproperties of polyatom icmolecular reactions,and have been a subjectofexperimentaland theoretical investigations.[18–26]Coriolis coupled(CC)item is implemented by the time-dependent wave packetmethod with a split-operator scheme.[24,29]In order to analyze the Coriolis coupling effect,the centrifugal sudden(CS)approximation that ignores Coriolis coupling is usually employed in the dynam ics calculations.[22,23]For this work,both CC and CS calculations for the title reaction are implemented.[17]The restof this paper isarranged as follows.Section 2 presents the new ab initio PES and themethod of dynam ics calculation.Section 3 analyses the effectof the isotopeand Coriolis coupling on the title reaction,and Section 4 provides the conclusions.
2.Thecharacteristicsof thePESand calculation m ethod
The lowest 1A′adiabatic potential energy surface for the Li+H(D)Cl reaction[17]is constructed with the threedimensional cubic spine interpolation method by f itting the multi-reference conf iguration interaction calculations on a largebasisset.Asdescribed in the literature,[17]the title reaction isexotherm ic.There are two very shallow van derWaals wells(-0.645 kcal/mol and 0.68 kcal/mol)at the reactant channel,one low energy barrier(2.99 kcal/mol)at the location(RHCl=2.68 Bohr,RLiCl=4.29 Bohr,∠HLiCl=58.2°),and onewell(-8.35 kcal/mol)for the complex structure near the product channel(RHCl=4.02 Bohr,RLiCl=3.956 Bohr,∠HLiCl=57.27°).The reaction is likely dominated by a directmechanism.
The quantum dynam icscalculation is carried outwith the time-dependentwave packetmethod developed by Zhang et al.[28,29]The Ham iltonian of the LiHCl reactive system can be expressed as
where R is thedistancebetween the Liatom and thecenter-ofmassof HCl,r is the HClbond length,μRis the reducedmass of Liwith respect to HCl,μris the reduced mass of HCl,J is the totalangularmomentum,j is the rotationalangularmomentum of HCl,V(?R,?r)is the interaction potential,and h(r)is the diatom ic reference Ham iltoni an

with V(r)being the diatom ic reference potential.The initial wavepacketisstructuredwith thebody-f ixed(BF)[21]translationalvibration rotationalbasis.

The element of the centrifugal term or the CCmatrix is expressed as withλ±JK=[J(J+1)-K(K±1)]1/2.In the CS approximation,[22]theoff-diagonal termsareneglected.Then theabove equation can be simplif ied as

The initial Gaussian wave packet can be w ritten as the product of the motion wave packet and the initial vibration Eigen-function.The time-dependent Schr¨odinger equation(iˉh?ψ/?t=Hψ)is used in the reactant Jacobi coordinates by the split-operatorschemeas follows:

where the reference Ham iltonian H0is def ined as
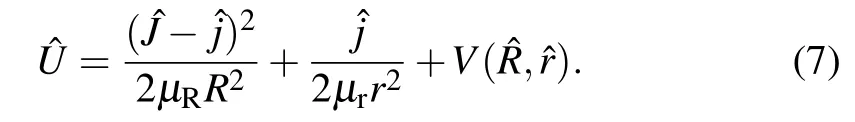

and theeffectivepotentialoperator U isdef ined as The reaction probability,the integral reaction cross sections,and the temperature dependent rate constantare expressed as
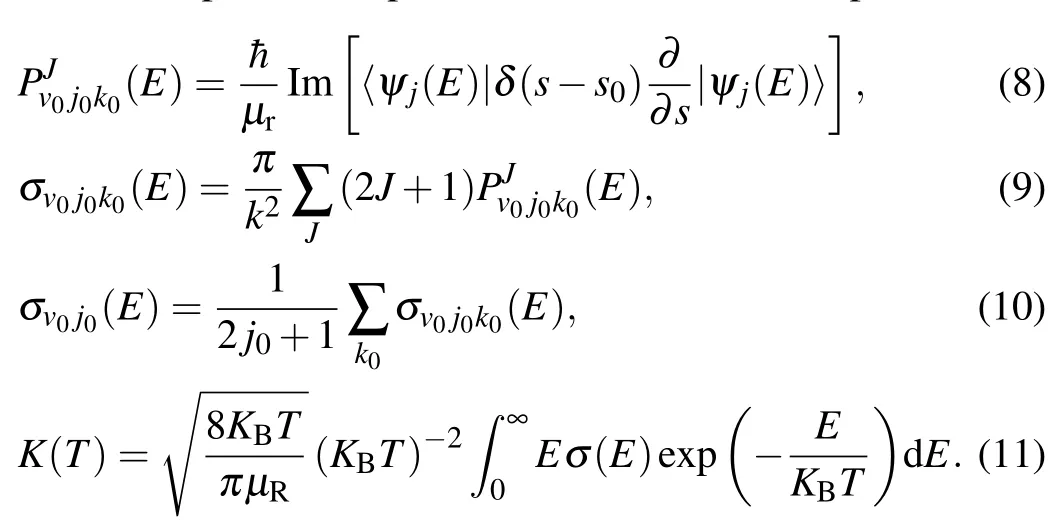
Here,ψ(E)is the f inalwave function,KBis the Boltzmann constant,E is the collision energy,andσ(E)is the f itted integral crosssection.
3.Resultsand discussion
Table1 shows thedynam icalparametersused in thequantum calculations for thiswork.Inorder toobtainbetterconvergence results,f irstly,the number of base functions is selected.383 cell points are divided by 2.2–20.0 a0at the R coordinate,199 points split the 1.2–16.0 a0at the r coordinate,and jmax=110.Then the related parameters in the initialwave packetaredetermined.The initialwavepacketwith aw idth of 0.32a0,an average translational energy of 0.14 eV,and propagation time 45000 is long enough.Finally,the absorption potential(3.0/0.03)is introduced to prevent the wave packet from bouncing at the boundary.
Figure 1 compares the dependence of the reaction probabilitieswith different J(0,30,50,70)on the collision energy of the Li+HCl(v=0,j=0)reaction between the calculationsof Lin etal.[17]and thiswork.Although our calculation resultsare slightly higher than thoseof Lin’sgroup at low collision energy and small J,the two reaction probabilitiesobviously agreewellwith each other.

Table 1.Parameters for the calculations in thiswork(allquantitiesare given in unitsof a.u.,unlessotherw ise indicated).

Fig.1.Dependenceof the reaction probabilitieswith different J(0,30,50,70)on the collision energy of the Li+HCl(v=0,j=0)reaction.The resultsof Lin etal.[17](solid line)and thiswork(dashed line)are shown.
In Fig.2,the reaction probabilities of the Li+H(D)Cl(v=0,j=0)reaction at J=45,50,and 55with NK=4 and 5 are shown,where NKis the number of K states used in CC calculation.Limited by the computational resourcesand time,convergent NKis required in precise calculations.As shown in Fig.2,there are little differences in probabilities between NK=4and NK=5athigher totalangularmomentum(J=45,50,55).To save computation time and resourceswithoutsacrif icing calculation accuracy,NK=4 is used.On this basis,the Coriolis coupling effectof the title reaction is calculated.
Figure3 shows the CC and CSprobabilities for the initial quantum numbers v=0,j=0 at J=0,20,40,60,65,70,and 75 of the Li+HCl reaction.It can be found that for small J and low collision energy,the differencesbetween the CC and CS probabilitiesare very little.Only at the high collision energy,the CC resultsare lower than the CSones.The remarkable differences are observed with increasing J.At J=60,the CC result ishigher than the CSoneat the collision energy from 0.25 eV to 0.35 eV,but lower than the CSoneat thehigh collision energy.As J keeps increasing,the CC calculation resultsareenhanced,and exceed theCSonesatthewhole collision energy region.From the above discussion,we f ind that the centrifugal potentialbarrier playsan important role in the Li+HCl reaction.The differences between the CS and CC resultsbecomeapparentas the collision energy increases.

Fig.2.NK-dependent reaction probabilities of the Li+H(D)Cl(v=0,j=0)reaction at J=45,50,and 55 for the CC calculation.

Fig.3.Dependence of the reaction probabilities on the collision energy of the Li+HCl(v=0,j=0)reaction at J=0,20,40,60,65,70,and 75.
Figure4 presents the reaction probabilitiesversus the collision energy of Li+DCl(v=0,j=0)reaction at J=0,20,40,60,and 80.There is a clear distinction between Li+HCl and Li+DCl reactions.In Fig.4,the Li+DCl reaction probabilitiesaresignif icantly depressed.At J=0,the reaction probability hasadecreaseat0.2–0.35 eV and then returns to asignif icant risewith the increaseof thecollisionenergy.Different from the Li+HCl reaction,the Coriolis coupling effectplays amore import role in the Li+DCl reaction.With the increase of J,thediscrepancy between CC and CSbecomes larger than that in the Li+HCl reaction.And the reaction threshold in the CS calculations is lower than that in CC.The shifting of the reaction thresholds is entirely due to the centrifugal barrier.According to the literature[16,17],the Li+HCl reaction is dominated by a direct reactionmechanism,and the H atom is too light to be impacted away.However,when the H atom is replaced by a D atom,themolecular rotation ismore likely to participate in the collision process.Here,the isotope effect hasa great inf luence on the title reaction.On the one hand,it leads to reduction of the reaction probabilities.On the other hand,itcauses theCoriolis coupling effect to be remarkable.
Figure5 shows the reaction probabilitiesof Li+H(D)Cl reaction at j=0,v=0–2.The probabilities for v=1 and 2 are higher than that for v=0,especially at the low collision energy.Both the probabilities for v=1 and 2 become close to one.This illustrates that Li+H(D)Cl→LiCl+H(D)is the predom inant channel.As the vibrational quantum number v increases,the reaction probability increases.With the increase of the diatom vibration excitation energy,the reactantiseasier to cross the barrier,and the reaction productive rate increases accordingly.

Fig.5.Dependenceof the reaction probabilitieson the collision energy of the Li+H(D)Cl(j=0,v=0,1,2)reaction at J=0:(a)Li+HCl,(b)Li+DCl.
Figure 6 shows the reaction probabilities versus the collision energy of Li+H(D)Cl reaction at v=0,j=0–7.For the Li+HCl reaction,with higher j,the probabilities show the distinct increase at the low collision energy.When j is greater than 5,this advantage is disappearing.The Li+DCl reaction has the same dynam ical behavior at rotational quantum number j=0 and 1.Both probabilities decrease in the collision energy region of 0.2–0.4 eV.With the increase of j,the descending disappears and the probabilities rise in the whole collision energy region.
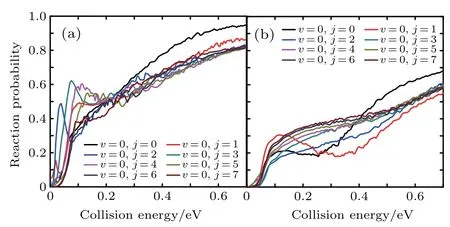
Fig.6.Dependenceof the reaction probabilitieson the collision energy of Li+H(D)Cl reaction at v=0,j=0–7:(a)Li+HCl,(b)Li+DCl.
Figure 7 shows the integral reaction cross section calculated by CS and CC for the Li+H(D)Cl reaction.As can be seen from Fig.7,the Li+HCl reaction integral reaction cross sections increasewith collision energy.However,for the isotope reaction Li+DCl,there is a signif icantdepression in the collision energy rangeof 0.2–0.4 eV,which is consistentwith the resultsof the reaction probability.As is shown above,the CS integral reaction cross section for the Li+HCl reaction is consistentwith the resultof CC in the low energy region.As the action on the high J of the Coriolis effect,the CC probability isbelow the CS result.For the Li+DCl reaction,due to the heavy quality factor,the Coriolis coupling effect ismore obvious.The different of the integral cross section between the CSand CS calculations isoutstanding in the collision region of 0.13–0.7 eV.And the CC result ishigher than the CS one.

Fig.7.Integral reaction section for reaction Li+H(D)Cl.
The rate constantsof the title reaction are given in Fig.8.Aswe can see from thegraph,the calculated resultsof CSand CC from thiswork arewithin theerrorboundsat the low temperature.In the experiment,the HClmolecules are normally in the ground state of vibration and the rotation stateof j=5 is themost distributed.[17]As the literaturementioned,[31,32]thehigh rotationalexcitation and vibration excitation can promote the reaction.The probabilities of different j in Fig.6 also conf irm this conclusion.

Fig.8.Rate constants.The experimentaldata[30](black cubes)and its error bounds(black lines),and the literature data of Lin[17](j=0,the orange dashed line)arealso shown.
4.Conclusion
The CC and CS reaction probabilities as well as the total cross sectionsare calculated.The differencesbetween the CS and CC results become apparent as the collision energy increases.The lower reaction thresholds of the CC calculations are themain cause of the enhancement of the CC integral cross sections.Therefore,the Coriolis coupling effect can effectively promote the title reaction.The comparison of the calculationsbetween the Li+HCland Li+DCl reactions reveals that the isotopeeffecthasagreat inf luence on the title reaction.On theonehand,it leads to reduction of theprobabilitiesof the Li+DCl reaction.On theotherhand,itcauses the Corioliscoupling effectof the Li+DCl reaction to be remarkable.The probability calculationsat v=0–2,j=0–7 indicate that the high rotationalexcitation and vibration excitation can promote the title reaction.The rate constantcalculationsmanifestthatthehigh rotationalexcitationsof the reactanthave the positive contribution to the results.
Acknow ledgment
The calculation in thiswork was supported by the High Performance Computing Centerof Henan NormalUniversity.
- Chinese Physics B的其它文章
- Computational study of inverse ferrite spinels
- Effectsof chem icalpressure on dilutedmagnetic sem iconductor(Ba,K)(Zn,M n)2As2*
- Particle–hole f luctuationsand possible superconductivity in dopedα-RuCl3*
- Surface stabilized cubic phaseof CsPb I3 and CsPbBr3 atroom tem perature*
- Raman scattering study ofmagnetic layered M PS3 crystals(M=M n,Fe,Ni)*
- Low tem perature Pmmm and C2/m phases in Sr2CuO3+δ high temperature superconductor*

