由吡啶-三羧酸配體構筑的鋅Ⅱ和錳Ⅱ配合物的合成、晶體結構、熒光及磁性質
黎 彧 潘光敏 鄒訓重 馮安生 游 遨*, 邱文達*,
(1廣東輕工職業技術學院,廣東省特種建筑材料及其綠色制備工程技術研究中心/佛山市特種功能性建筑材料及其綠色制備技術工程中心,廣州510300)
(2廣東工業大學輕工化工學院,廣州510006)
0 Introduction
Recently,construction of coordination polymers received considerable attention not only as a result of their intriguing varieties of molecular architectures and topologies but also for their applications in catalysis,magnetism,luminescence,and gas storage[1-5].Although chemists and materials scientists have devoted much effort to rational design and syntheses coordination polymers,it is difficult to predict the structures of coordination polymers,because a lot of factors influence the construction of complexes,such as the structural features of organic ligands,the coordination requirementsofmetalions,solvent systems,temperatures,and pH values[6-12].
In this regard,various types of aromatic polycarboxylic acids have been proved to be versatile and efficientcandidates for constructing diverse coordination polymers due to their rich coordination chemistry,tunable degree of deprotonation,and ability to act as H-bond acceptors and donors[1,2,8,10,12-16].
As a combination of the aforementioned aspects and our previous research work,we have selected a novelpyridine-tricarboxylate ligand,4-(6-carboxypyridin-3-yl)-isophthalic acid (H3L,Scheme 1)and explored it for the construction of novel coordination polymers.The H3L block possesses the following features:(1)it can twist and rotate freely to generate different angles between the phenyl-pyridine planes via the C-C bond to furnish a subtle conformational adaptation;(2)it has seven potential coordination sites(six carboxylate O donors and one N donor),which can lead to diverse coordination patterns and high dimensionalities,especially when acting as a multiply bridging spacer;(3)apart from a limited number of coordination compounds derived from H3L[17-18],this acid block remains poorly used for the generation of coordination polymers.Given these features,the main objectiveofthe presentstudy consisted in the exploration of H3L as a novel pyridine-tricarboxylate block forthe assembly ofdiverse metal-organic networks.
Herein, we report the syntheses, crystal structures,luminescent and magnetic properties of ZnⅡand MnⅡcoordination compounds constructed from pyridine-tricarboxylate ligands.
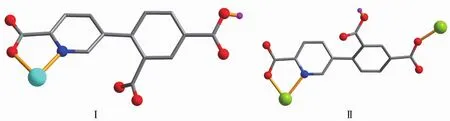
Scheme 1 Coordination modes of HL2-ligands in compounds 1 and 2
1 Experimental
1.1 Reagents and physical measurements
All chemicals and solvents were of AR grade and used without further purification.Carbon,hydrogen and nitrogen were determined using an Elementar Vario EL elementalanalyzer.IR spectra were recorded using KBr pellets and a Bruker EQUINOX 55 spectrometer.Thermogravimetric analysis(TGA)data were collected on a LINSEIS STA PT1600 thermal analyzer with a heating rate of 10℃.min-1.Excitation and emission spectra were recorded on an Edinburgh FLS920 fluorescenc e spectrometer using the solid samples at room temperature.Magnetic susceptibility data were collected in the 2~300 K temperature range on a Quantum Design SQUID Magnetometer MPMS XL-7 with a field of 0.1 T.A correction was made for the diamagnetic contribution prior to data analysis.
1.2 Synthesis of[Zn(H2biim)2(H2O)2][Zn(HL) (Hbiim)(H2O)]2.8H2O(1)
A mixture of ZnCl2(0.027 g,0.20 mmol),H3L(0.057 g,0.20 mmol),H2biim (0.027 g,0.20 mmol),NaOH (0.016 g,0.40 mmol),and H2O (10 mL)was stirred at room temperature for 15 min,and then sealed in a 25 mL Teflon-lined stainless steel vessel,and heated at 160℃for 3 days,followed by cooling to room temperature at a rate of 10℃.h-1.Colorless block-shaped crystals of 1 were isolated manually,and washed with distilled water.Yield:44% (based on H3L).Anal.Calcd.for C52H60Zn3N18O24(%):C 41.16,H 3.99,N 16.62;Found(%):C 41.37,H 4.02,N 16.49.IR(KBr,cm-1):3 443w,2 980w,1 688m,1 653s,1 622 m,1 602m,1 577s,1 485w,1 455w,1 419m,1 368w,1 311w,1 245w,1 169w,1 122w,1 046w,1 016w,944 w,908w,857w,822w,776w,699w,674w,587w,556w.
1.3 Synthesis of[Mn(μ-HL)(phen)(H2O)]n(2)
A mixture of MnCl2.4H2O (0.040 g,0.20 mmol),H3L(0.057 g,0.20 mmol),phen(0.040 g,0.20 mmol),NaOH (0.016 g,0.40 mmol),and H2O (10 mL)was stirred at room temperature for 15 min,and then sealed in a 25 mL Teflon-lined stainless steel vessel,and heated at 160℃for 3 days,followed by cooling to room temperature at a rate of 10℃.h-1.Yellow block-shaped crystals of 2 were isolated manually,and washed with distilled water.Yield:60%(based on H3L).Anal.Calcd.for C26H17MnN3O7(%):C 58.00,H 3.18,N 7.81;Found(%):C 57.76,H 3.16,N 7.85.IR(KBr,cm-1):3 418w,3 066w,1 699m,1 613s,1 597s,1 510w,1 424 m,1 383s,1 296w,1 245w,1 163w,1 143w,1 087w,1 041w,1 005w,929w,898w,847m,817w,776w,724 w,704w,684w,658w,531w.
1.4 Synthesis of{[Mn(μ-HL)(2,2′-bipy)(H2O)]. 0.5 (2,2′-bipy)}n(3)
The preparation of 3 was similar to that of 2 except 2,2′-bipy was used instead of phen.After cooling the reaction mixture to room temperature,yellow block-shaped crystals of3 were isolated manually,washed with distilled water,and dried.Yield:40%(based on H3L).Anal.Calcd.for C29H21Mn N4O7(%):C,58.79;H,3.57;N,9.46.Found(%):C,58.91;H,3.56;N,9.52.IR(KBr,cm-1):3 427w,3 060w,1 702w,1 607s,1 544m,1 471w,1 439w,1 382s,1 298 w,1 250w,1 161w,1 130w,1 082w,1 040w,1 009w,930w,893w,841w,815w,773m,757w,741w,710w,678w,657w,620w,558w,526w.
Thecompoundsareinsolublein waterand common organic solvents,such as methanol,ethanol,acetone and DMF.
1.5 Structure determination
Three single crystals with dimensions of 0.25 mmX0.23 mmX0.22 mm(1),0.25 mmX0.22 mmX0.21 mm(2),and 0.27 mmX0.24 mmX0.23 mm(3)were collected at 293(2)K on a Bruker SMART APEXⅡCCD diffractometer with Mo Kα radiation(λ=0.071 073 nm).The structures were solved by direct methods and refined by full matrix least-square on F2using the SHELXTL-2014 program[19].All non-hydrogen atoms were refined anisotropically.All the hydrogen atoms were positioned geometrically and refined using a riding model.In compound 3,N atom of the lattice 2,2′-bipy is disordered over two sites,which are N4 and C27,with occupancy of 0.5.A summary of the crystallographic data and structure refinements for 1 and 2 is given in Table 1.The selected bond lengths and angles for compounds 1 and 2 are listed in Table 2.Hydrogen bond parameters of compounds 1 and 2 are given in Table 3 and 4.
CCDC:1917771,1;1917772,2;1917773,3.
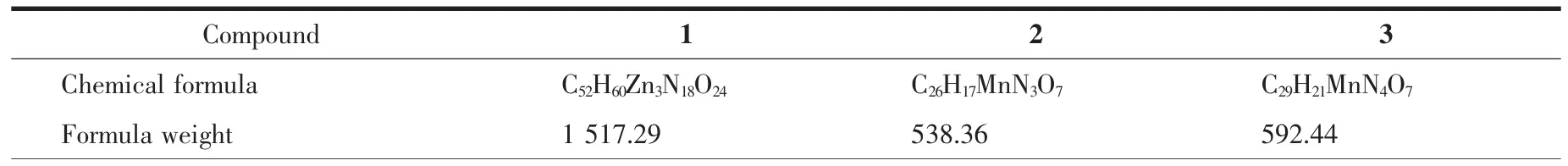
Table 1 Crystal data for compounds 1~3
Continued Table 1
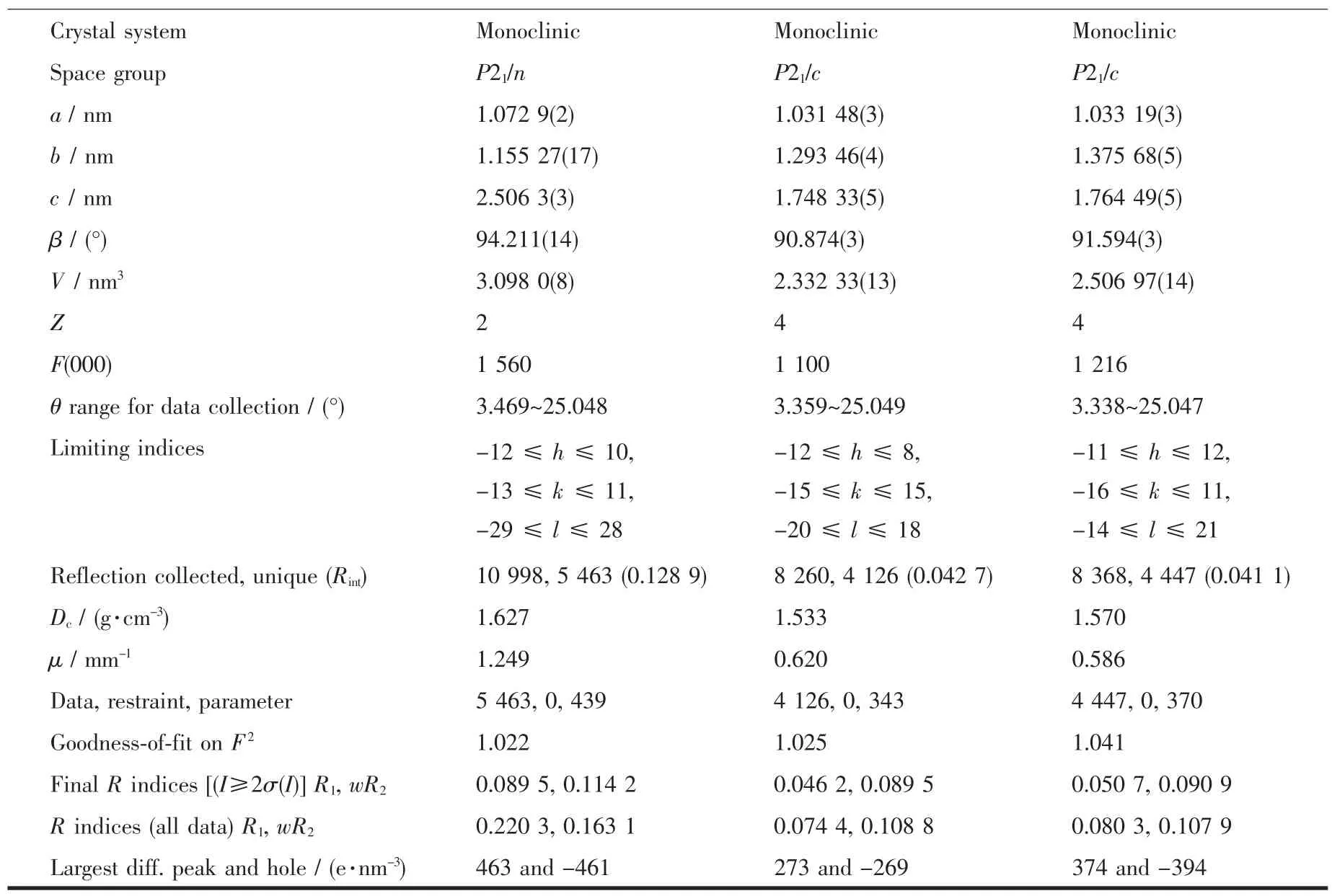
Crystal system Monoclinic Monoclinic Monoclinic Space group P21/n P21/c P21/c a/nm 1.072 9(2) 1.031 48(3) 1.033 19(3)b/nm 1.155 27(17) 1.293 46(4) 1.375 68(5)c/nm 2.506 3(3) 1.748 33(5) 1.764 49(5)β /(°) 94.211(14) 90.874(3) 91.594(3)V/nm3 3.098 0(8) 2.332 33(13) 2.506 97(14)Z 2 4 4 F(000) 1 560 1 100 1 216 θ range for data collection/(°) 3.469~25.048 3.359~25.049 3.338~25.047 Limiting indices -12≤h≤10,-13≤k≤11,-29≤l≤28-11≤h≤12,-16≤k≤11,-14≤l≤21 Reflection collected,unique(Rint) 10 998,5 463(0.128 9) 8 260,4 126(0.042 7) 8 368,4 447(0.041 1)Dc/(g.cm-3) 1.627 1.533 1.570 μ/mm-1 1.249 0.620 0.586 Data,restraint,parameter 5 463,0,439 4 126,0,343 4 447,0,370 Goodness-of-fit on F2 1.022 1.025 1.041 Final R indices[(I≥2σ(I)]R1,wR2 0.089 5,0.114 2 0.046 2,0.089 5 0.050 7,0.090 9 R indices(all data)R1,wR2 0.220 3,0.163 1 0.074 4,0.108 8 0.080 3,0.107 9 Largest diff.peak and hole/(e.nm-3) 463 and-461 273 and-269 374 and-394-12≤h≤8,-15≤k≤15,-20≤l≤18
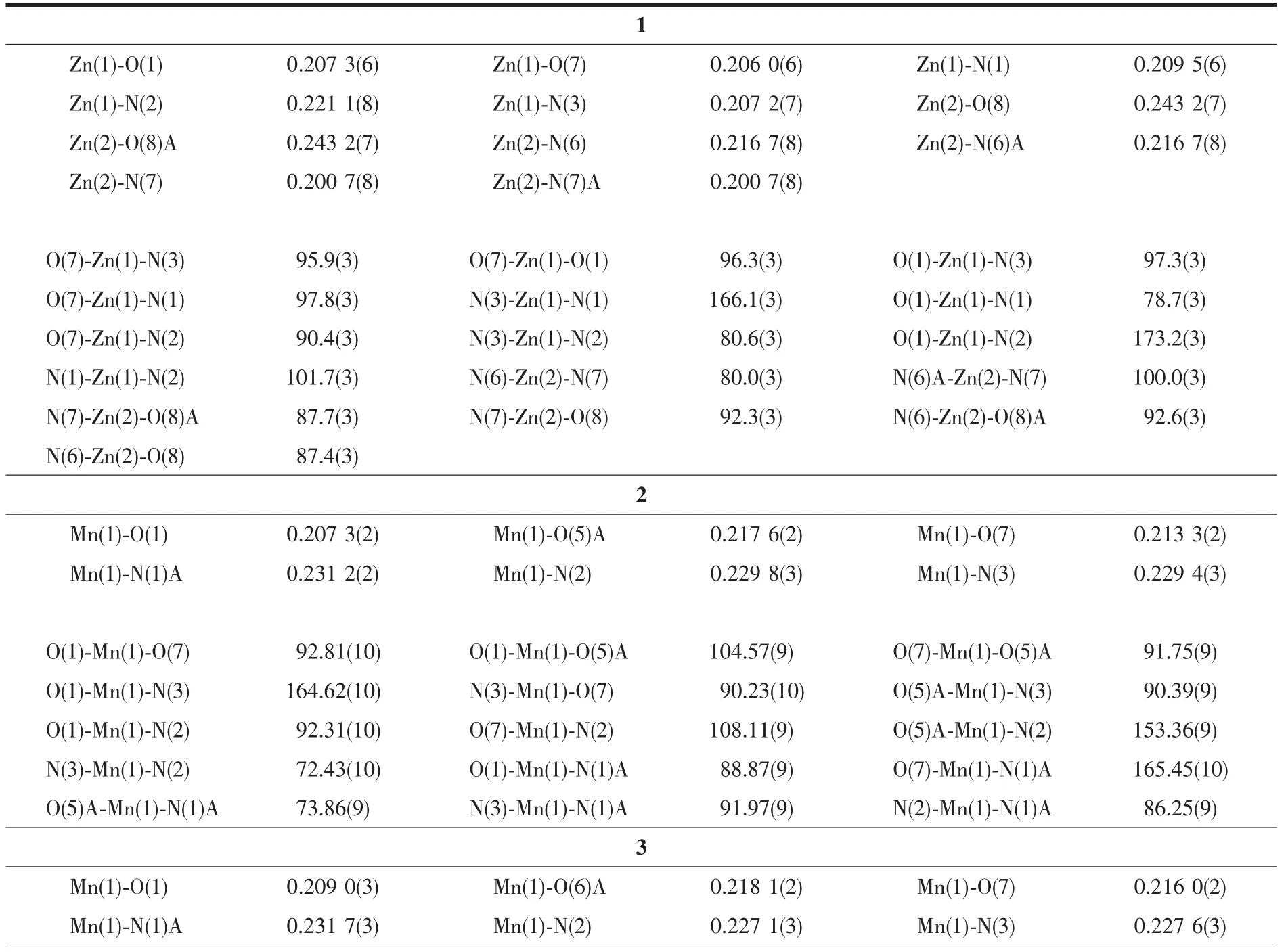
Table 2 Selected bond distances(nm)and bond angles(°)for compounds 1~3
Continued Table 2
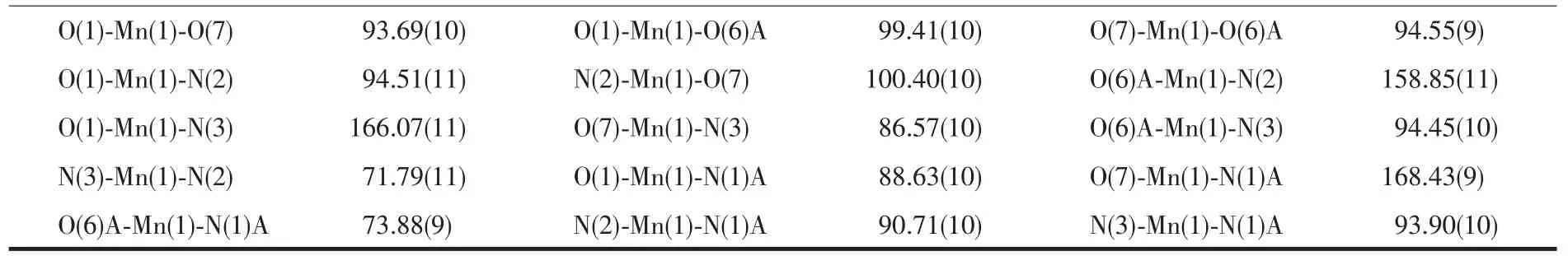
Symmetry codes:A:-x+1,-y+1,-z+2 for 1;A:-x,y+1/2,-z+1/2 for 2;A:-x+1,y-1/2,-z+1/2 for 3.
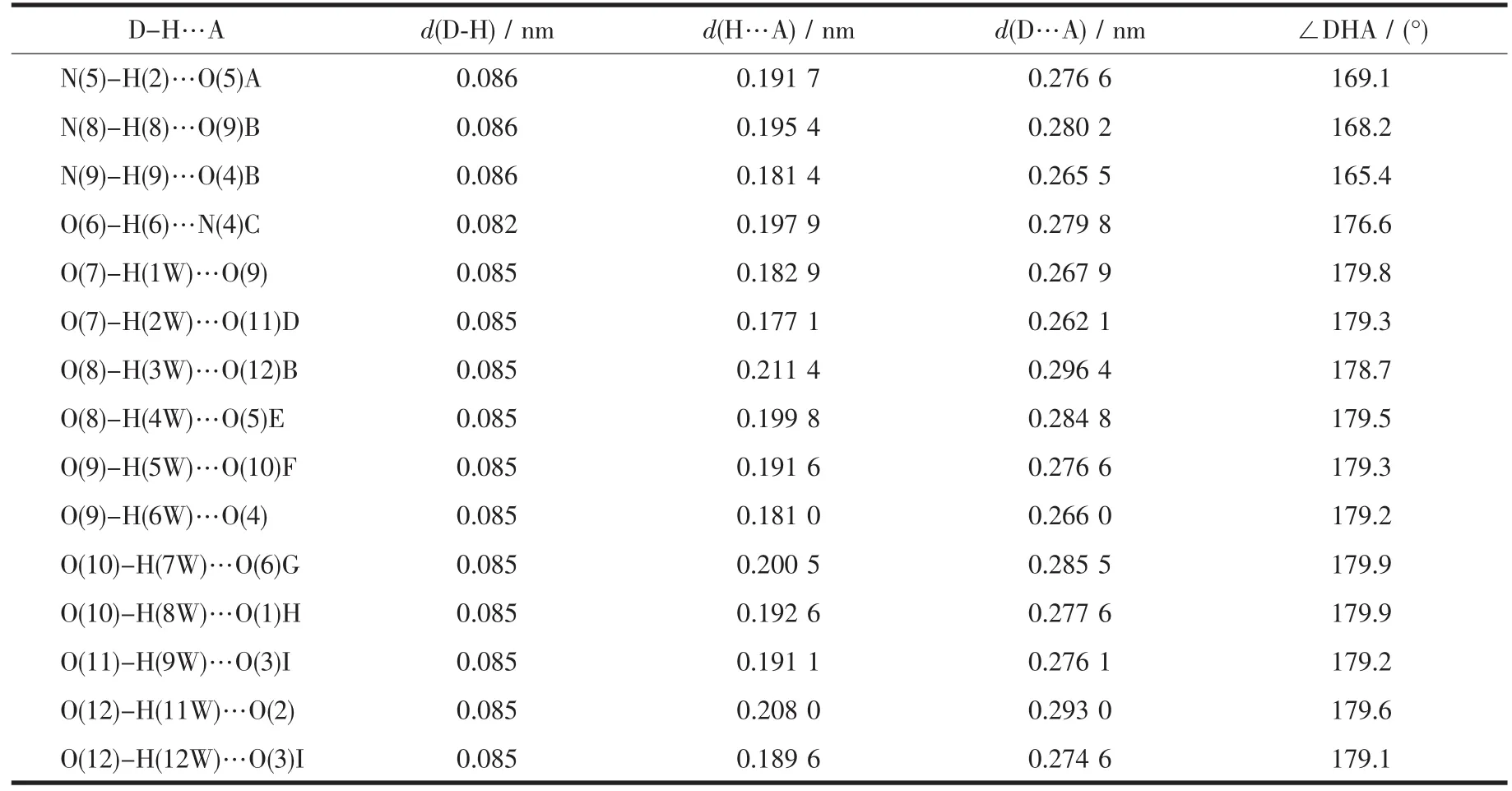
Table 3 Hydrogen bond parameters of compound 1
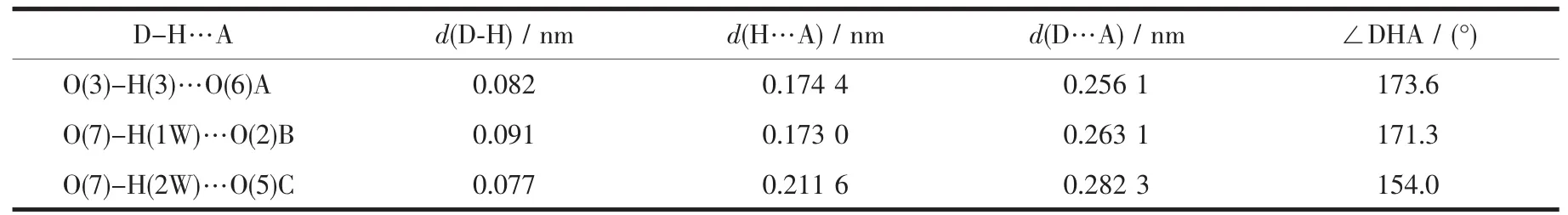
Table 4 Hydrogen bond parameters of compound 2
2 Results and discussion
2.1 Description of the structure
2.1.1 [Zn(H2biim)2(H2O)2][Zn(HL)(Hbiim)(H2O)]2.8H2O(1)
The structure of 1 is composed of a [Zn(H2biim)2(H2O)2]2+cation,two[Zn(HL)(Hbiim)(H2O)]-anions,and eight lattice water molecules(Fig.1).In the cation,the Zn2 atom adopts an octahedral {ZnN4O2}geometry filled by four N atoms from two H2biim moieties and two O atoms from two water ligands.In the anion,the Zn1 center is five-coordinated and reveals a quadrangular pyramidal{ZnN3O2}environment,which is completed by one O and one N atom from the HL2-block,two N donors from the H2biim moiety,and one O atom from the water ligand.The lengths of the Zn-O and Zn-N bonds are 0.206 0(6)~0.243 2(7)nm and 0.200 7(8)~0.221 1(8)nm,respectively; these are within the normal values for related ZnⅡderivatives[10,12].In 1,the HL2-block acts as a terminal ligand(modeⅠ,Scheme 1).The dihedral angle of two aromatic rings in the HL2-ligand is 30.54°.The cations and anions are multiply interconnected by the O-H…O/N or N-H…O hydrogen bonds to assemble a 3D H-bonded architecture(Fig.2 and Table 3).
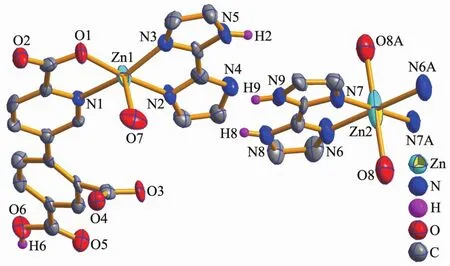
Fig.1 Drawing of the asymmetric unit of compound 1 with 50%probability thermal ellipsoids
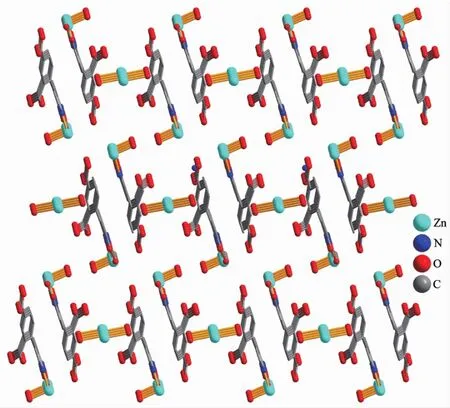
Fig.2 View of a 3D H-bonded architecture in compound 1 along b axis
2.1.2 [Mn(μ-HL)(phen)(H2O)]n(2)and{[Mn(μ-HL)
(2,2′-bipy)(H2O)].0.5(2,2′-bipy)}n(3)
Compounds 2 and 3 feature essentially similar 1D chain structures (Table 1).A main difference concerns the type of supporting ligand:phen in 2 and 2,2′-bipy in 3.Given similarity of structural and topologicalcharacteristics,the structure of2 is described in detail as an example.The asymmetric unit of 2 bears one Mn1 center,one μ-HL2-linker,one chelating phen and one terminal water ligand(Fig.3).The Mn1 center is six-coordinated and reveals a distorted octahedral {MnN3O3}geometry.It is completed by two carboxylate O and one N donors from two μ-HL2-moieties,a pair of phen N donors,and an H2O ligand.The Mn-O(0.207 3(2)~0.217 6(2)nm)and Mn-N(0.229 4(3)~0.231 2(2)nm)bonds are within standard values[1,8,10].The HL2-block behaves as a tridentate μ-linker(Scheme 1,mode Ⅱ)that interconnects the adjacent Mn1 atoms to form a zigzag 1D metal-organic chain with a Mn1…Mn1 separation of 1.259 5(3)nm(Fig.4).Furthermore,such 1D chains are further extended by H-bonds into a 2D H-bonded network(Fig.5 and Table 4).
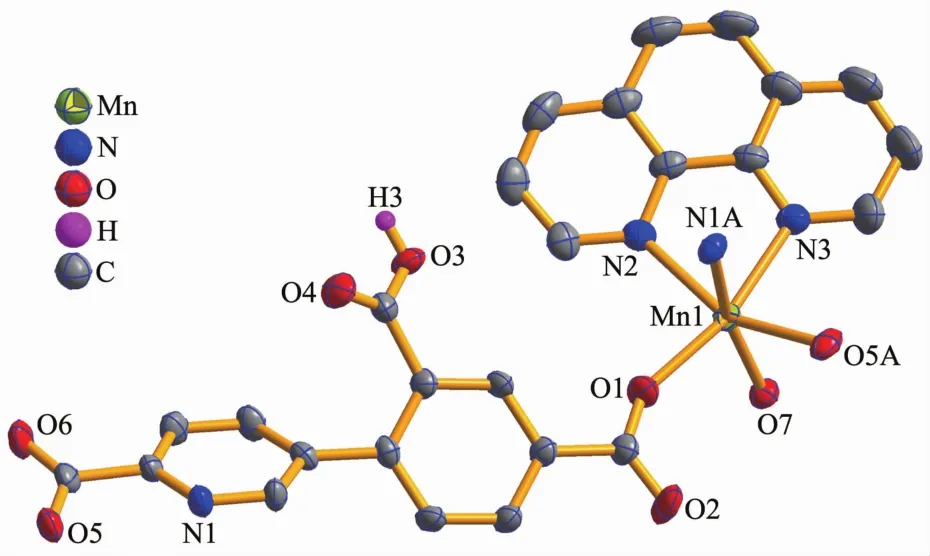
Fig.3 Drawing of the asymmetric unit of compound 2 with 50%probability thermal ellipsoids
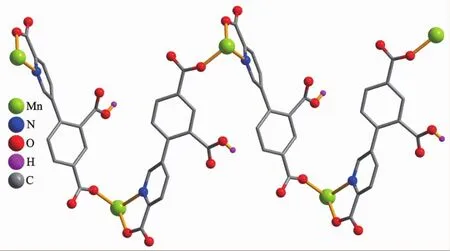
Fig.4 View of 1D metal-organic chain along c axis
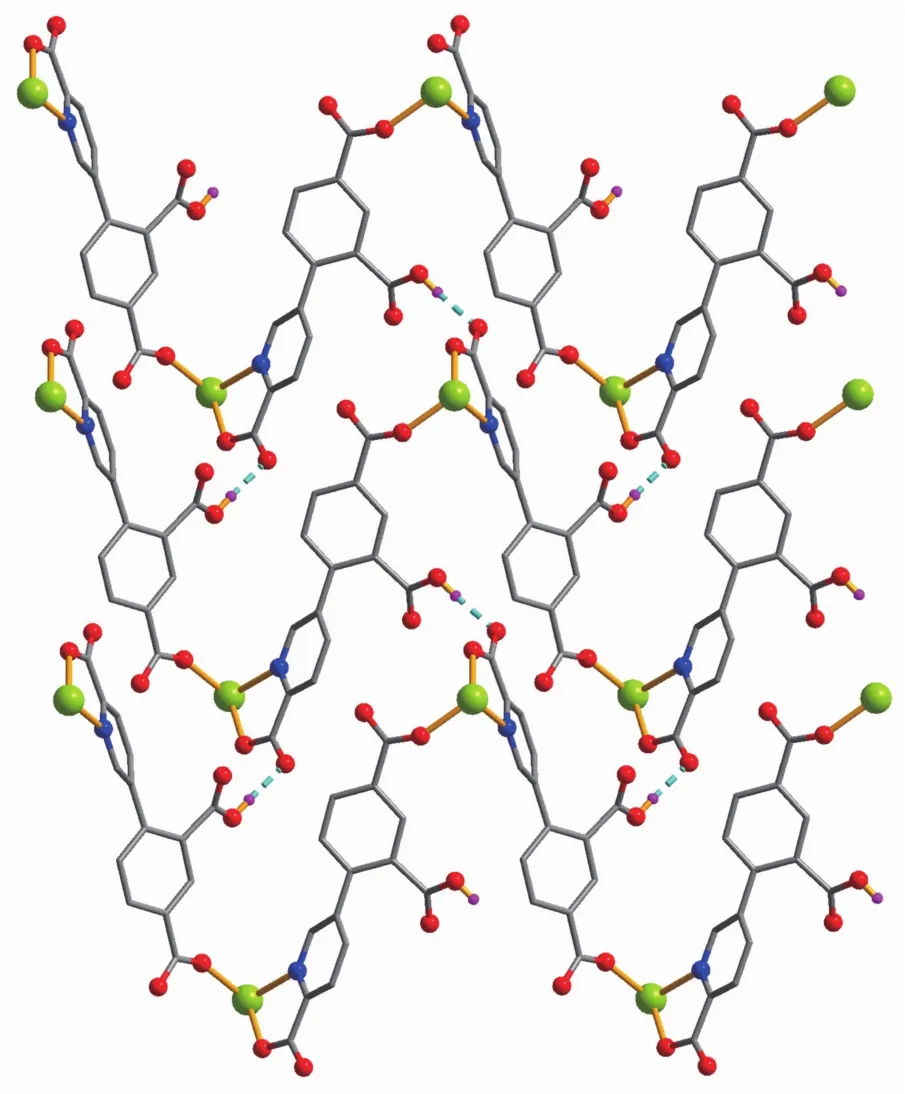
Fig.5 Perspective of 2D supramolecular network parallel to bc plane in 2
2.2 TGA analysis
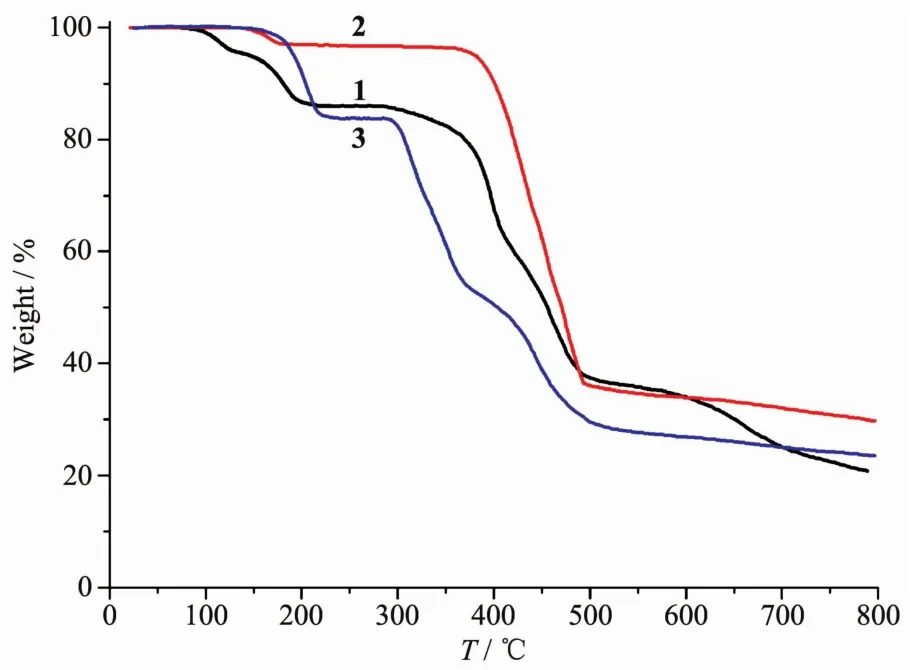
Fig.6 TGA curves of compounds 1~3
To determine the thermal stability of compounds 1~3,their thermal behaviors were investigated under nitrogen atmosphere by thermogravimetric analysis(TGA).As shown in Fig.6,TGA curve of compound 1 showed that there was a loss of eight lattice and four coordinated water molecules between 80 and 211℃(Obsd.14.1%,Calcd.14.2%);further heating above 286℃led to a decomposition of the dehydrated sample.Compound 2 lost its one water ligand in a range of 134~100℃(Obsd.3.3%,Calcd.3.3%),followed by the decomposition at 356℃.For 3,there is a thermal effect in the 140~227℃range,corresponding to a removal of a half of one 2,2′-bipy moiety and the H2O ligand(Obsd.16.3%,Calcd.16.2%);remaining samples are stable up to 290℃.
2.3 Luminescent properties
Solid-state emission spectra of H3L and zincⅡcompound 1 were measured at room temperature(Fig.7).The spectrum of H3L revealed a weak emission with a maximum at 375 nm (λex=322 nm).In comparison with H3L,the coordination compound 1 exhibited more extensive emission with a maximum at 371 nm (λex=318 nm).These emissions correspond to intraligand ππ*or n-π*transition of H3L[8,10,12].Enhancement of the luminescence in 1 vs H3L can be explained by the coordination of ligands to ZnⅡ;the coordination can augment a rigidity of ligands and reduce an energy loss due to radiationless decay[1,10,12].

Fig.7 Solid-state emission spectra of H3L and compound 1 at room temperature
2.4 Magnetic properties
Variable-temperature magnetic susceptibility measurements were performed on powder samples of 2 in a temperature range of 2~300 K(Fig.8).For the 1D MnⅡ polymer 2,the room temperature value of χMT,4.40 cm3.mol-1.K,is close to the value (4.38 cm3.mol-1.K)expected for a magnetically isolated highspin MnⅡion (S=5/2,g=2.0).If temperature was lowered,the χMT values decreased continuously to a minimum of 3.32 cm3.mol-1.K at 2.0 K.Between 2 and 300 K,the magnetic susceptibility could be fitted to the Curie-Weiss law with C=4.44 cm3.mol-1.K and θ=-2.73 K.These results also indicate an antiferromagnetic interaction between the adjacent MnⅡcenters.We attempted to fit the data for 2 by applying the following expression for a 1D MnⅡchain[20]:
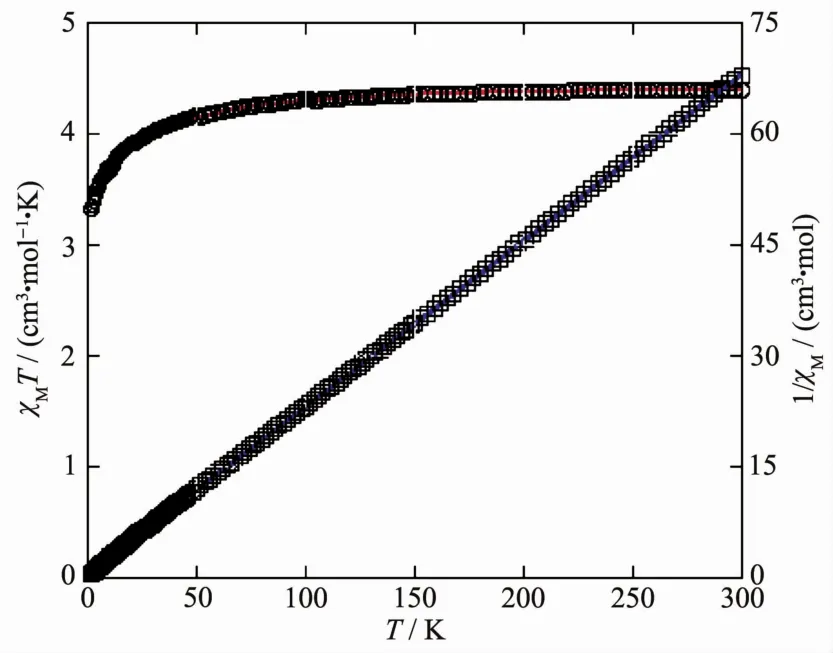
Fig.8 Temperature dependence of χMT(○)and 1/χM(□)for compound 2
χchain=[Ng2β2/(kT)](A+Bx2)(1+Cx+Dx3)-1
with A=2.9167,B=208.04,C=15.543,D=2 707.2,and x=|J|/(kT)
Between 50 and 300 K,the susceptibility for 2 was simulated using this rough model,and resulting in J=-0.46 cm-1,g=2.02,and R=2.17X10-5.The negative J parameter indicates a weak antiferromagnetic exchange coupling between the adjacent MnⅡcenters in 2,which is in agreement with a negative θ value.
3 Conclusions
In summary,we have successfully synthesized and characterized three new zinc and manganese coordination compounds by using one pyridinetricarboxylic acid as ligand under hydrothermal condition.Compound 1 shows a 0D structure composed of three monomers of ZnⅡunits.Compounds 2 and 3 feature 1D chains.Besides,the luminescent(for 1)and magnetic (for 2)properties were also investigated and discussed.The results show that such pyridinetricarboxylic acid can be used as a versatile multifunctional building block toward the generation of new coordination polymers.

