Synthesis,Crystal Structures of Two Lanthanides Complexes with 2,6-Difluorobenzoic Acid
PENG Xiong-XinWANG Wen-MinZHONG Yu-Fei XIAO WeiBAO Guang-MingYUAN Hou-Qun*,
(1School of Science,Jiangxi Agricultural University,Nanchang 330045,China)
(2Assets and Laboratory management,Jiangxi Agricultural University,Nanchang 330045,China)
(3School of Animal Science and Technology,Jiangxi Agricultural University,Nanchang 330045,China)
Abstract:Two complexes[Tm(dfba)2(phen)(μ2-dfba)]2(1)and[Yb(dfba)2(phen)(μ2-dfba)]2(2)(dfba-=2,6-difluorobenzoate and phen=1,10-phenanthroline)were synthesized by the reaction of 2,6-difluorobenzote and phenanthroline with Tm3+and Yb3+ions,respectively.The complexes 1 and 2 were characterized by elemental analysis,FT-IR spectra,thermal gravimetric analysis(TGA)and their crystal structures were determined by single X-ray diffraction.The crystal structures analysis indicated that the complexes 1 and 2 are isostructural.Each Ln3+is coordinated with two dfba-ligands and one phen molecule to form a[Ln(dfba)2(phen)]+unit,and the neighboring two[Ln(dfba)2(phen)]+units are bridged by two different dfba-ligands to form a binuclear molecule,[Ln(dfba)2(phen)(μ2-dfba)]2(Ln=Tm,Yb).CCDC:1960656,1;1960657,2.
Keywords:2,6-difluorobenzoicacid;1,10-phenanthroline;Tm3+;Yb3+;crystal structure
0 Introduction
The lanthanide complexes have attracted much attention because of their flexible coordination geometry and high coordination numbers as well as multifarious useful properties,such as fluorescence[1-2],catalysis[3-4],magnetism[5-6]and gas sorption[7-8].It was found that the spatial structures and properties of the lanthanide complexes are mainly dominated by the ligands and the central metal ions in the past decades.For example,suitable ligands could improve the stability of the lanthanides complexes,and enhance fluorescent[9]and/or magnetic[10]properties.Therefore,selection of organic ligands plays a vital role in the designing of lanthanide complexes.Carboxylates and N-donor organic ligands have been widely used.For N-donor ligands,1,10-phenanthroline(phen)is very useful because of its strong coordination abilities and π-π stacking interactions[11-12].For carboxylates,polytopic aromatic acids are always used to construct the lanthanide complexes due to their versatile coordination modes.On the other hand,benzoic acid or its substituted derivatives which is the “parent” acid,has been explored rather rare in literature.However,some fantastic structures ofpolynuclear clusters with benzoic acids have been reported[13-14].Moreover,it was found that the fluorine substituted organic ligands showed more stable to oxidation and enhanced thermal stability[15].Many lanthanide complexes with fluorine substituted benzoic acid have been investigated,such as 2-fluorobenzoate[16-19],2,3,4,5-tetrafluorobenzoate[20-21],2,3,5,6-tetrafluorobenzoate[22],2,3,4,5,6-pentafluorobenzoate[23].It is noted that only few reports about lanthanide complexes with difluorosubstituted benzoic acid,such as 2,3-difluorobenzoate[24],2,4-difluorobenzoate[25-26],2,5-difluorobenzoate[27],have been studied,however,only one complex with 2,6-difluorobenzoate,[Pr(2,6-dfba)3(H2O)]n[28],was found in the current version of the Cambridge Structural Database(CSD).Therefore,we are interested in Ln-2,6-dfba systems to explore new functional lanthanide complexes.In this work,the crystal structures of two new binuclear lanthanide complexes,[Ln(dfba)2(phen)(μ2-dfba)]2(Ln=Tm,Yb,dfba-=2,6-difluorobenzoate)are reported,and their infrared spectra and thermogravimetric analysis are discussed.
1 Experimental
1.1 Materials and measurements
2,6-Difluorobenzoic acid,phen,NaHCO3,Tm(NO3)3·6H2O,and Yb(NO3)3·6H2O were purchased from commercial sources and used directly.Thermogravimetric analysis(TGA)was recorded on a PerkinElmer TGA4000 at heating rate of 10℃·min-1in N2atmosphere.Fourier transform infrared spectra(FT-IR)were recorded on a Perkin Elmer spectrum-Ⅱwithin a wavenumber range of 4 000~400 cm-1.
1.2 Synthesis of[Tm(dfba)2(phen)(μ2-dfba)]2(1)
A H2O/EtOH solution (12 mL,1∶5,V/V)of 2,6-difluorobenzoic acid(50 mg,0.31 mmol)and NaHCO3(25 mg,0.28 mmol)were added into Tm(NO3)3·6H2O(50 mg,0.12 mmol)which was dissolved in 8 mL EtOH.Then 5 mL EtOH dissolving phen(20 mg,0.10 mmol)was added into the above solution.The resulted solution was kept stirring for 2 hours,and then stood at room temperature.The colorless columnar crystals were obtained after one week.Yield:35 mg(42.66%).Anal.Calcd.for C66H34F12N4O12Tm2(%):C 48.31,H 2.09,N 3.41;Found(%):C 48.55,H 2.13,N 3.24.FTIR(KBr,cm-1):3 067(m),2 276(w),1 932(w),1 670(m),1 624(m),1 558(w),1 522(s),1 462(s),1 419(s),1 350(m),1 272(m),1 235(s),1 142(s),1 106(s),1 057(w),1 008(s),864(s),849(s),807(s),774(s),748(w),728(s),714(w),642(s),595(m),584(w),518(m),492(w),420(s).
1.3 Synthesis of[Yb(dfba)2(phen)(μ2-dfba)]2(2)
The complex 2 was synthesized by the similar method for complex 1 except using Yb(NO3)3·6H2O(50 mg,0.11 mmol)to replace Tm(NO3)3·6H2O.Yield:40 mg (48.51%).Anal.Calcd.for C66H34F12N4O12Yb2(%):C 48.07,H 2.08,N 3.40;Found(%):C 48.34,H 2.15,N 3.25.FT-IR (KBr,cm-1):3 066(m),2 276(w),1 934(w),1 670(m),1 624(m),1 558(w),1 522(s),1 462(s),1 419(s),1 350(m),1 272(m),1 235(s),1 141(s),1 106(s),1 057(w),1 008(s),864(s),849(s),807(s),775(s),748(w),728(s),716(w),642(s),595(m),584(w),518(m),492(w),420(s).
1.4 Crystal structure determination
The crystals with suitable size(0.30 mm×0.10 mm×0.10 mm for complex 1,0.18 mm×0.08 mm×0.08 mm for complex 2)were selected and detected on Agilent Gemini CCD-Diffractometer (Xcalibur,Eos,Gemini)with monochromatic Mo Kα radiation(λ=0.071 073 nm)and an EOS detector.The crystallographic data were collected at room temperature.The CrysAlisPro was used for data collection and processing.Empirical absorption correction using spherical harmonics,implemented in SCALE3 ABSPACK scaling algorithm,was applied to all data.The structures of complex 1 and 2 were solved by Patterson methods and refined on F2by full-matrix least-squares techniques using SHELXTL software[29-30].All non-hydrogen atoms were refined anisotropically.All H atoms were fixed in calculated positions and refined isotropically.The crystallographic data of 1 and 2 are displayed in Table 1 and selected bond lengths and angles are listed in Table 2.
CCDC:1960656,1;1960657,2.
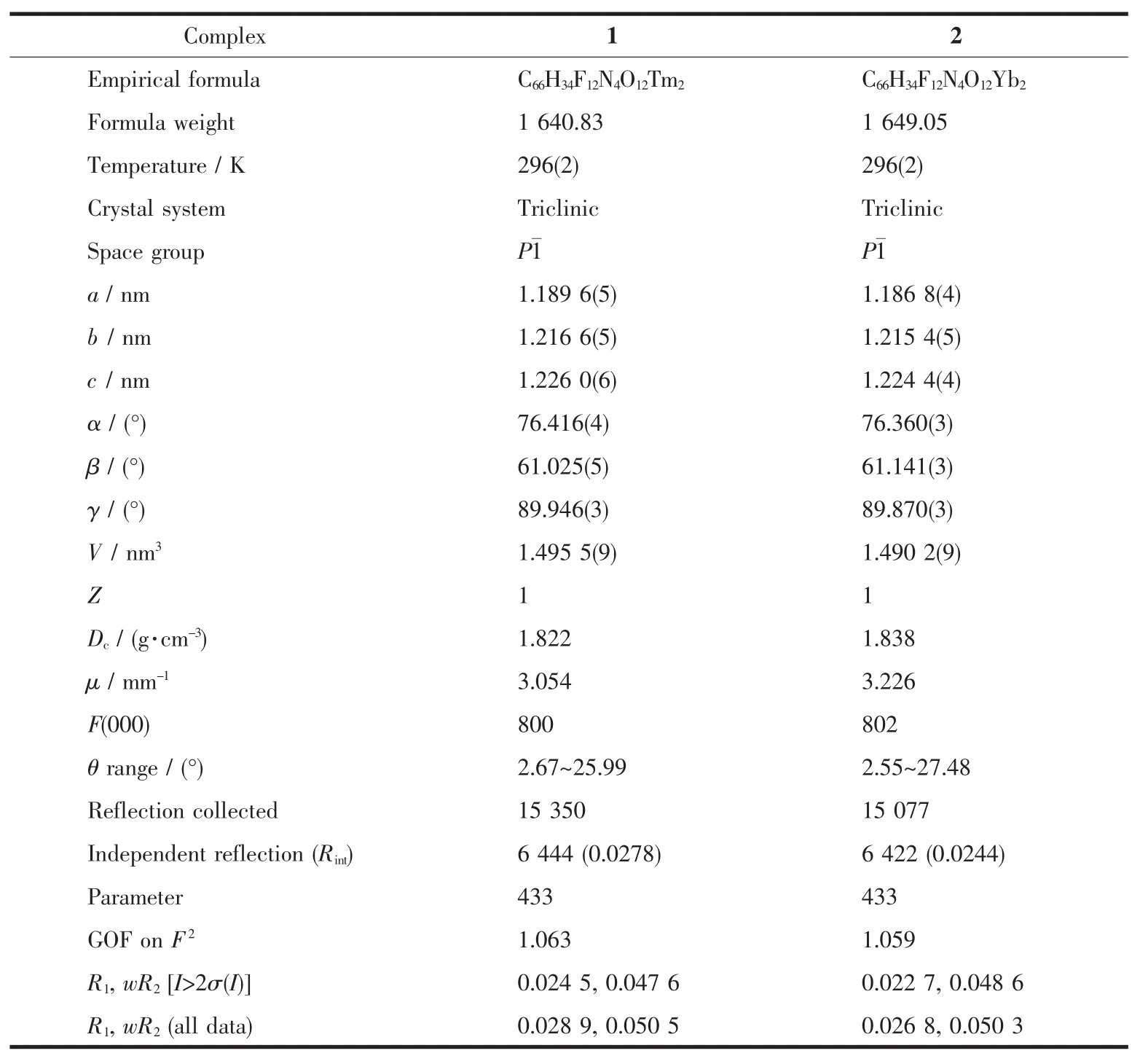
Table 1 Crystallographic data for complexes 1 and 2
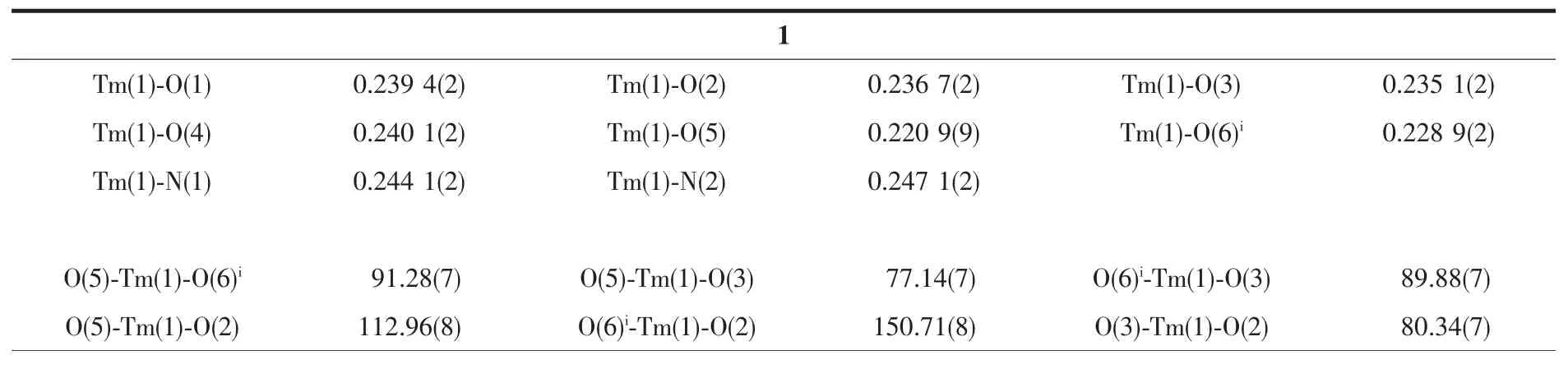
Table 2 Selected bond lengths(nm)and bond angles(°)for complexes 1 and 2
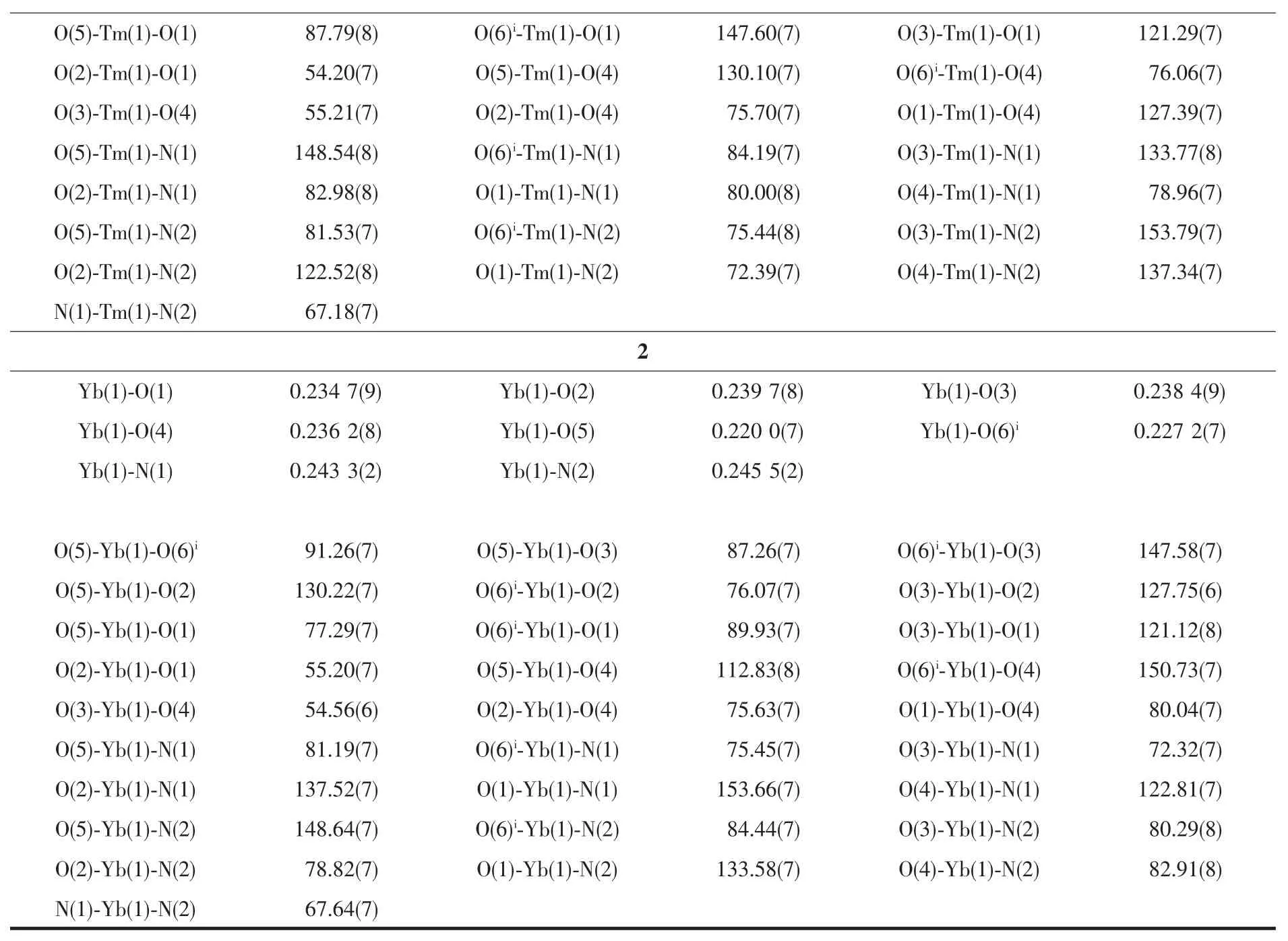
Continued Table 2
2 Results and discussion
2.1 IR spectra
As shown in the experimental section,the IR spectra of complexes 1 and 2 were almost same.The weak band at 3 067 cm-1are attributed to the C-H stretching of benzene ring.The strong vibrations at 1 624,1 418 cm-1in complexes are attributed to the asymmetric and symmetric stretching vibrations of the carboxylate groups,indicating that dfba-exist in the complexes.The strong band at 1 235 cm-1is assigned to C-F stretching vibration.The stretching vibration of C-N are observed at 1 558 cm-1,showing that phen is included in the complexes.The bending vibrations of C-H of benzene ring were observed in a range of 864~728 cm-1.Moreover,the bands at 518,420 cm-1are assigned to the vibrations ofLn-O and Ln-N,respectively,confirming that dfba-and phen are coordinated to lanthanide ions.
2.2 Crystal structures
Since complexes 1 and 2 are isostructural,herein only the structure of complex 1 is discussed in detail.As show in Fig.1,the asymmetric unit of complex 1 is composed of one Tm3+ion,three dfba-anions and one phen molecule.Each Tm3+ion is eight-coordinated,with six oxygen atoms from three dfba-ligands,and two nitrogen atoms from one phen molecule,forming a distorted two-cap-triprism coordination geometry.O3,O5,O6,O1,N1,O2 atoms are located at the vertex,and N2,O4 atoms are located above the face of the rectangles(Fig.2).The dfba-ligands have two different coordination modes.The carboxylate groups of O1-C1-O2 and O3-C8-O4 are in a chelating mode,whereas the carboxylate group of O5-C15-O6 adopts bridging mode.The two adjacent Tm3+ions are bridged by two dfba-ligands with the Tm…Tm distance of 0.509 7 nm to form a dimeric unit.Each phen molecule coordinates to one Tm3+ion using two N atoms.The distances of Tm-O are in a range of 0.220 9(9)~0.240 1(2)nm,in which the distance of Tm1-O4 is 0.02 nm longer than that of Tm1-O5.The distances of Tm1-N1 and Tm1-N2 are 0.244 1(2)and 0.247 1(2)nm,respectively.The O-Tm-O bond angles range from 54.20(7)°to 150.71(8)°.The phenanthroline molecule is a little distorted,in which the dihedral angle between N1C22C23C24C25C26 ring and C25C26C27 C28C29C30 ring is 1.969(179)°,and it is 1.437(173)°between C25C26C27C28C29C30 ring and N2C27C28 C31C32C33 ring.The carboxylate groups are almost twisted out of the benzene ring planes,in which the dihedral angles between the carboxylate groups and their benzene rings are 46.499(213)°,53.673(199)°,and 52.961(278)°,respectively.
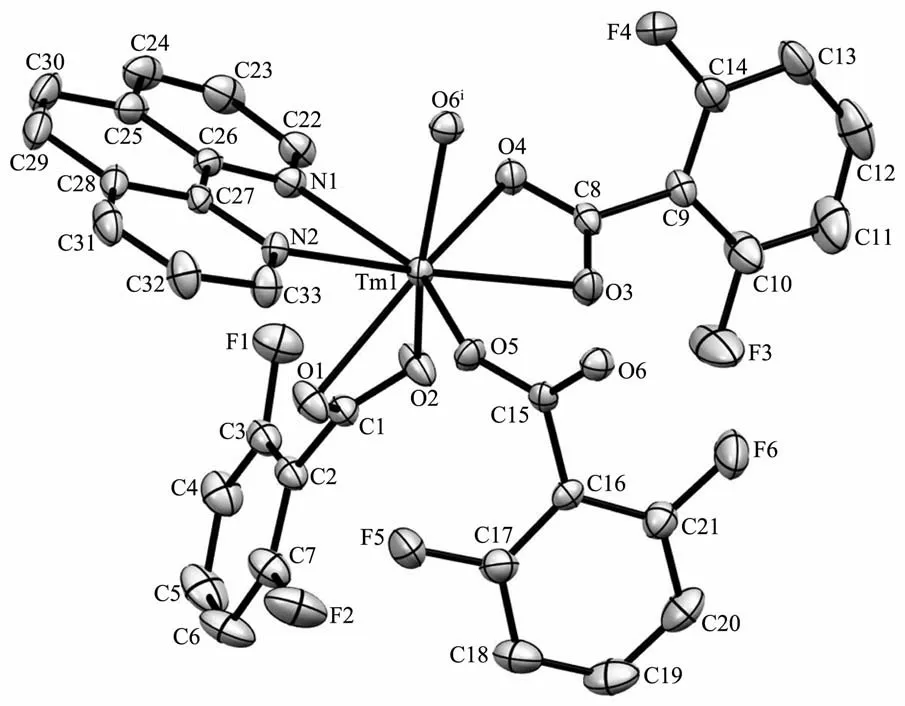
Fig.1 Asymmetric structure unit of complex 1 with 30%probability ellipsoids
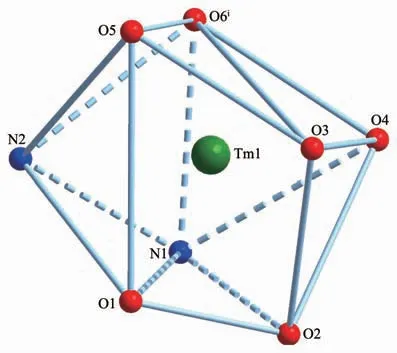
Fig.2 Perspective view of coordination geometry of Tm3+in complex 1
2.3 TGA
As shown in Fig.3,the thermal decomposition steps of two complexes appeared very similar,and complex 1 showed a little bit more stable than complex 2.There was no obvious weightlessness before 280℃for complex 1 and 270℃for complex 2,indicating that there is no guest molecules included in the complexes,which is consistent with the crystal structure analysis.For complex 1,the first weight loss was 35.11%in a range of 280~340 ℃,corresponding to the loss of two dfba-ligands (Calcd.38.30%).The second stage occurred in a range of 340~880 ℃,and the weight loss of 38.14%is attributed to the loss of one phen and 0.8 dfba-ligands(Calcd.39.48%).The remaining component continued to decompose and it did not end at the temperature limit.For complex 2,the first weight loss was 38.18%in a range of 270~325℃,corresponding to the loss of two dfba-ligands(Calcd.38.11%).The second decomposition stage with the weight loss of 33.28%corresponds to the loss of one phen and 0.5 dfba-ligands (Calcd.33.57%),which occurred in a range of 325~790 ℃.Unlike complex 1,complex 2 reached a platform after 790℃.
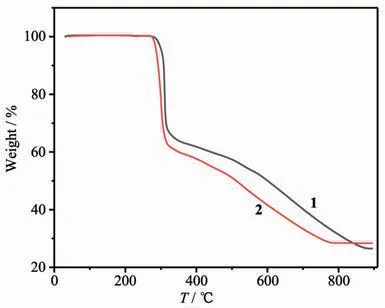
Fig.3 TG curves of complexes 1 and 2
- 無機化學學報的其它文章
- Effect of Promoter on the Ni-Al Alloy&the Corresponding Raney-Ni Catalyst and Hydrogenation Performance of 1,4-Butylenediol
- Hierarchically Porous Nanosized Red Phosphorus with Enhanced Photo-Oxidation and Photo-Reduction Activities
- Tb(Ⅲ)-Based Metal-Organic Framework for Simultaneously Luminescent Detection of Cu2+and Fe3+Ions
- Stretch-Induced Luminescent Changes of a Self-Healing Elastic Polymer Functionalized with Platinum Complex
- Solvent-Induced Syntheses,Crystal Structures and Magnetic Properties of Two Mononuclear Er(Ⅲ)Complexes
- Hydration Mechanism and Stability Regulation of Hemihydrate Calcium Sulfate Whiskers

