Stretch-Induced Luminescent Changes of a Self-Healing Elastic Polymer Functionalized with Platinum Complex
QI Xiao-WeiLIU JianYANG Qian-YingZHANG Hua-Hong HAN Li-ZhiZHANG Xiao-Peng*,SHI Zai-Feng*,
(1College of Chemistry and Chemical Engineering,Key Laboratory of Water Pollution Treatment&Resource Reuse of Hainan Province,Hainan Normal University,Haikou 571158,China)
(2College of Chemical Engineering,Jiangsu Key Lab of Biomass-Based Green Fuels and Chemicals,Nanjing Forestry University,Nanjing 210037,China)
Abstract:An elastic orange-red film(PDMS-PtL1)has been prepared by grafting formyl-containing Pt(Ⅱ)complex Pt(N^C^N)Cl(N^C^N=1,3-dipyridyl benzene)onto polydimethylsiloxane (PDMS)backbone.The film was highly stretchable(up to 1500%strain)and shows room-temperature self-healing property.Interestingly,stretch-induced luminescent change of the film was observed,where an emission tentatively originating from3π-π*of separated platinum moieties was detected after stretching.Luminescence quenching is avoided in this elastic film,and the switch of emission state can be realized by changing external forces.CCDC:1966072,L2.
Keywords:platinum complex;functional film;stretch;self-healing;luminescent change
0 Introduction
Metallophilic Pt…Pt interaction,an attractive driven force capable of promoting supramolecular organizations with distinct morphologies and colors,has received intense attention for decades[1-3].Through exploiting Pt…Pt interactions,various supramolecular architectures from fiber and rod to metallogel on the nanoscale or sub-microscale can be induced,which give rise to a bathochromic shift of luminescence to near infrared (NIR)region and an improved conductivity[4].Accordingly,these assembled systems with appealing metallophilic Pt…Pt interactions are promising candidates for optoelectronic and bioimaging applications[5-6].Furthermore, metallophilic Pt…Pt interactions are sensitive to tiny changes of external stimuli,such as temperature,solvents,volatile organic compounds and compression,scratching,grinding or stretching forces[7-12].In common, effective intramolecular or intermolecular Pt…Pt interactions can be induced under external stimuli,and the emission state would derived from the metal-metal-to-ligand charge transfer transition (MMLCT),then the luminescence and color would have a significant red-shift that can be observed distinctly by naked eyes[13-14].Therefore,luminescent Pt(Ⅱ) complexes with metallophilic Pt…Pt interaction can be as an alternative ofactive component in environment-responsive materials.
Those square-planar Pt(Ⅱ)complexes endowing with stimuli-responsive ability have also been blended into or grafted onto a series of polymers to improve the feasibility in the application[15-16].The recovery rate of vapochromic and mechanochromic films obtained by blending square-planar platinum complexes into polymethacrylates matrices are depended on Tg(glass transition temperature),and the response can be facilely and quickly erased just through heating above their Tg[17].In addition,when incorporating into different polymers,microenvironment around Pt(Ⅱ)complexes has been influenced,therefore functional films based on Pt(Ⅱ)complexes would show different emission colors from yellow to red and distinct environmentresponsive behaviors, even completely opposite responsive phenomena have been observed[18-20].Upon heating or exposing to organic vapor,the response can be erased fast and the film can be recovered to the original state without detectable decomposition[17].Anchoring stimuli-responsive Pt(Ⅱ) complexesto polymersopensup a new approach to develop functional sensing materials.
In recentyears,self-healing metallopolymers functionalized with metalcomplexes have been reported and show great potentials in electronic skins and artificial muscles[21-24].An elastic polymer has been obtained through anchoring a carboxyl-containing cyclometalated Pt(Ⅱ)complex into a polydimethylsiloxane(PDMS)backbone,and good stretchability as well as interesting self-healing behaviors have been exhibited by combination of dynamic Pt…Pt and π-π interactions[25].As inspired by the above stretchable elastic polymer,we have synthesized two metallopolymers(PDMS-PtL1 and PDMS-PtL2)by grafting formylcontaining Pt(Ⅱ)complexes Pt(N^C^N)Cl(N^C^N=1,3-dipyridyl benzene)onto PDMS matrix (Fig.1).The PDMS-PtL1 film exhibits excellent stretch performance that the film can be stretched to over 15 times its original length.In addition,interesting stretch-induced luminescent change of the film has been observed,and room-temperature self-healing property is demonstrated.
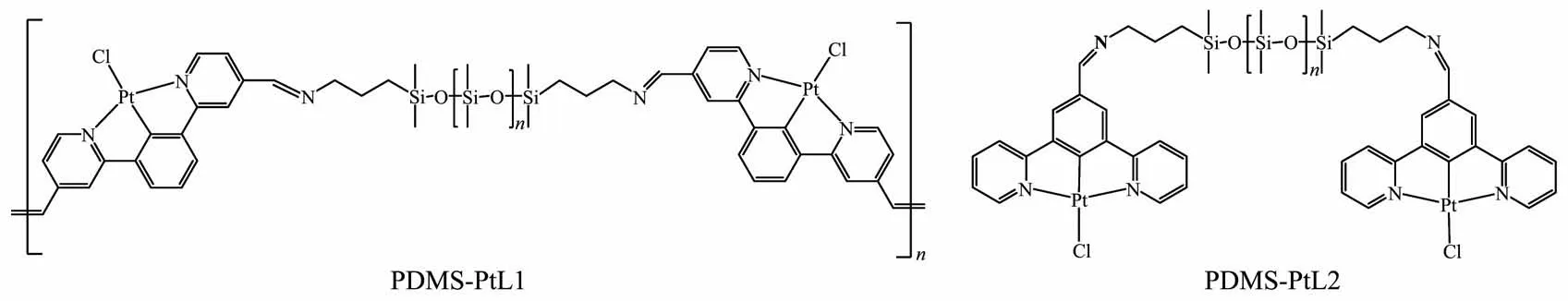
Fig.1 Chemical structures of metallopolymers PDMS-PtL1 and PDMS-PtL2
1 Experimental
1.1 Materials and general methods
All reagents were purchased from commercial suppliers and used as received withoutfurther purification.Mass spectra were acquired on an LCQ Fleet ESI Mass Spectrometer.The NMR spectra were obtained on Bruker DRX-400 spectrometer.Chemical shifts are referenced to TMS.Coupling constants are given in hertz.Photoluminescence (PL)spectra were measured by Hitachi F-7000 PL spectrophotometer(λex=350 nm).Elemental analysis was performed on Vario ELⅢ (CHN mode and O mode).The XPS spectra were measured by Thermo Scientific KAlpha+.Mechanical tensile-stress experiments were performed using an Instron 5944 Microtester.Samples were tested at room temperature (20℃).For selfhealing tests,the film was cut into two pieces and then put together.Films with the same size (30 mm×5 mm×1 mm)were then healed at different temperatures for different times.The healed films were then tested following the same procedure to obtain the stress-strain curves.Single-crystal X-ray diffraction measurement for L2 was carried out on a Bruker SMART APEX CCD based on diffractometer operating at room temperature.Intensities were collected with graphite monochromatized Mo Kα radiation (λ=0.071 073 nm)operating at 50 kV and 30 mA,using ω/2θ scan mode.The data reduction was made with the Bruker SAINT package[26].Absorption corrections were performed using the SADABS program[27].The structures were solved by direct methods and refined on F2by full-matrix least-squares using SHELXL-97 with anisotropic displacement parameters for all nonhydrogen atoms in the structure.Hydrogen atoms bonded to the carbon atoms were placed in calculated positions and refined as riding mode,with CH of 0.093 nm(methane)or 0.096 nm(methyl)and Uiso(H)=1.2Ueq(Cmethane)or Uiso(H)=1.5Ueq(Cmethyl).The reflections measured,unique reflections,reflections with F2>2σ(F2)were 4 950,2 634 and 1 359,respectively.All computations were carried out using the SHELXTL-97 program package[28].
CCDC:1966072,L2.
1.2 Synthesis
Our synthetic route to metallopolymers PDMSPtL1 and PDMS-PtL2 is outlined in the Scheme 1.Ligands L1 and L2 were prepared through Suzuki coupling and Stille coupling reactions,respectively.The followed condensation between ligands L1,L2 and NH2-PDMS-NH2gave PDMS-L1 and PDMS-L2 according to the reported method[29-30].At last,PDMSPtL1 and PDMS-PtL2 were obtained through the coordination reaction of PtCl2(DMSO)2and PDMS-L1,PDMS-L2.Ligands L1 and L2 were characterized by ESI and NMR.In addition,the structure of L2 was confirmed by X-ray single crystal diffraction.
1.2.1 Synthesis of 2,2′-(1,3-phenylene)diisonicotinaldehyde(L1)
A mixture of 1,3-phenylenediboronic acid (3 mmol),2-bromoisonicotinaldehyde (7 mmol),cesium carbonate(9 mmol)and palladium tetrakis(triphenylphosphine)(80 mg)were refluxed in 1,4-dioxane/H2O(5∶1,V/V)solvents for 24 h under argon atmosphere.The solvents were evaporated and the solid residue wasextracted with dichloromethane.The organic layers were combined and washed twice with water and once with brine,dried over anhydrous Na2SO4.Evaporation of the solvent gave the crude product which was purified by flash column chromatography(VPE∶VEA=10∶1,PE=petroleum ether,EA=ethyl acetate)to yield L1 as a white solid (622 mg,72%).MS(ESI)(m/z):[M-H]+Calcd.for C18H13N2O2,289.1;Found,289.0.1H NMR (400 MHz,CDCl3):δ 10.19 (s,2H),8.99(d,J=4.8 Hz,2H),8.80(d,J=2.0 Hz,1H),8.27(t,J=1.2 Hz,2H),8.18(dd,J1=8.0 Hz,J2=2.0 Hz,2H),7.69(dd,J1=4.8 Hz,J2=1.6 Hz,2H),7.67(t,J=8.0 Hz,1H).13C NMR(100 MHz,CDCl3):δ 191.6,158.6,151.2,142.6,139.0,129.7,128.3,125.7,120.9,119.1.Elemental Anal.Calcd.for C18H12N2O2(%):C,74.99;H,4.20;N,9.72;O,11.10.Found(%):C,74.97;H,4.22;N,9.69;O,11.12.
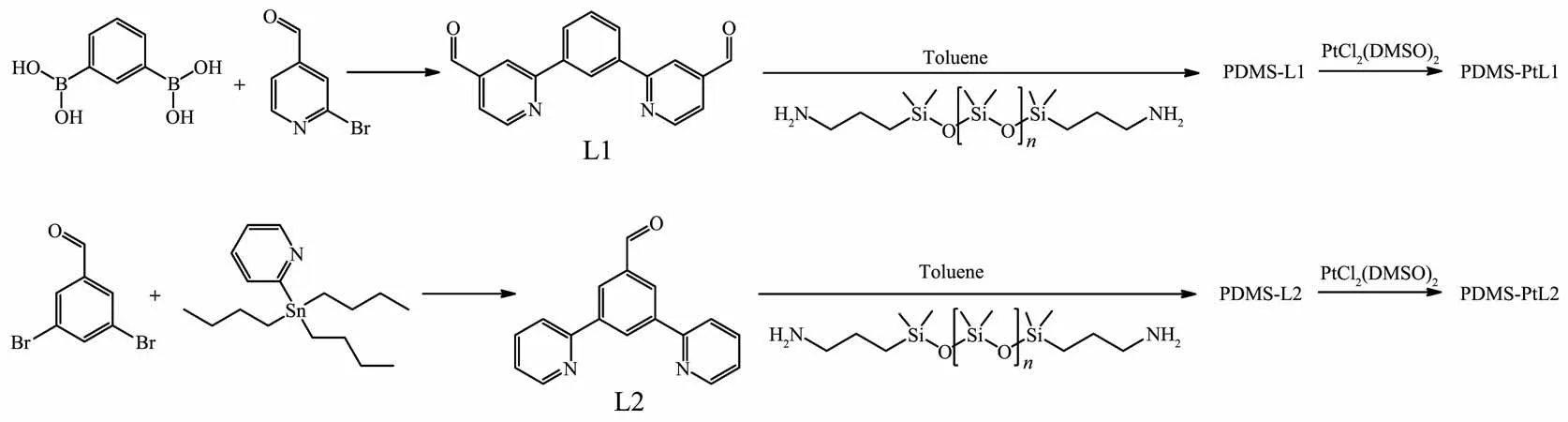
Scheme 1 Synthesis route of PDMS-PtL1 and PDMS-PtL2
1.2.2 Synthesis of PDMS-L1
Under stirring and argon atmosphere,L1(288 mg,1 mmol)was added to a redistilled toluene(100 mL)containing NH2-PDMS-NH2(4.5 g,Mn=5 000).The catalyst TsOH·H2O (3.6 mg,0.02 mmol)in 20 mL toluene was added dropwise during 10 min.The mixture was refluxed at 110℃for 36 h,and in the reaction process,the generated H2O was removed by A Dean-Stark trap.After reaction,the solution was concentrated to ca.25 mL under reduced pressure,followed pouring 50 mL anhydrous methanol into it for purification.White precipitate-like viscous liquid arised after standing for 0.5 h,which can be used to coordinate with PtCl2(DMSO)2.Atomic fraction according to XPS:C1s(283.0 eV,51.63%),N1s(397.1 eV,0.68%),O1s (530.5 eV,24.60%),Si2p (100.5 eV,23.09%).
1.2.3 Synthesis of PDMS-PtL1
A mixture ofthe viscousliquid PDMS-L1 obtained above and PtCl2(DMSO)2(422 mg,1 mmol)was stirred at 80℃for 24 h in mixed solvents of tetrahydrofuran (20 mL)and water (10 mL)under argon atmosphere.The solvents were removed under reduced pressure.The resulting pale yellow metallopolymer PDMS-PtL1 was peeled into a PTFE mold and volatilized at room temperature for 4 h followed by drying at 85℃under reduced pressure for 24 h.At last,orange-red transparent films have been obtaind.Atomic fraction according to XPS:C1s(283.0 eV,51.56%),N1s(397.4 eV,0.79%),O1s(530.6 eV,24.15%),Si2p (100.6 eV,22.90%),Pt4f(71.3 eV,74.4 eV,0.26%),Cl2p(196.9 eV,0.34%).
1.2.4 Synthesis of 3,5-di(pyridin-2-yl)benzaldehyde(L2)
The procedure for the preparation of L2 was similar to a reported method of synthesizing 1,3-di(2-pyridyl)benzene[31].Under an argon atmosphere a mixture of 3,5-dibromobenzaldehyde(1.05 g,4 mmol),2-(tributylstannyl)pyridine (4.42 g,12 mmol)and Pd(PPh3)2Cl2(254 mg,0.36 mmol)and LiCl(1.70 g,40 mmol)in redistilled toluene was refluxed for 72 h.After the reaction and cooling to room temperature,a saturated KF solution (15 mL)was added,and the resultant mixture was stirred for 30 min.The solvent was evaporated under reduced pressure.The residue was extracted with dichloromethane (3×150 mL),and the combined organic solution was washed twice with water and once with brine,dried over anhydrous Na2SO4.Evaporation of the solvent gave the crude product which was purified by flash column chromatography(VPE:VEA=10:1)to yield L2 as a white solid(670 mg,65%).MS(ESI)(m/z):[M-H]+Calcd.for C17H13N2O,261.1;Found,261.1.1H NMR(400 MHz,CDCl3):δ 10.19(s,1H),8.96(t,J=2.0 Hz,1H),8.74(m,2H),8.56(d,J=2.0 Hz,2H),7.90(dt,J1=8.0 Hz,J2=2.0 Hz,2H),7.80 (td,J1=8.0 Hz,J2=2.0 Hz,2H),7.29 8.74 (m,2H).13C NMR(100 MHz,CDCl3):δ 192.1,155.7,149.8,140.8,137.4,137.0,131.0,128.3,122.9,120.7.Elemental Anal.Calcd.for C17H12N2O(%):C,78.44;H,4.65;N,10.76;O,6.15.Found(%):C,78.42;H,4.68;N,10.74;O,6.16.The structure of L2 also have been confirmed by the single-crystal X-ray diffraction.As shown in Fig.2,The bond lengths range from 0.121 0(3)to 0.149 4(3)nm.The nitrogen atoms of lateral pyridines point towards the opposite position with a trans-geometry.Both of lateral pyridines are almost coplanar with the central benzene ring,and the dihedral angles are 11.084(77)°and 10.815(79)°.
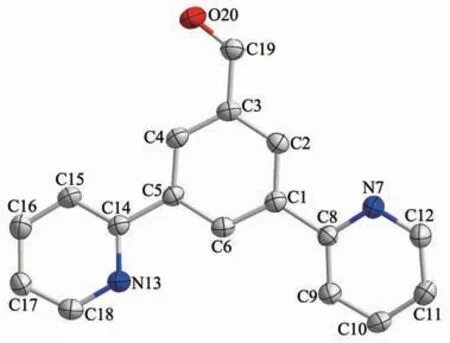
Fig.2 X-ray crystal structure of L2 with 30%ellipsoidal probability
1.2.5 Synthesis of PDMS-L2
The preparation of viscous liquid PDMS-L2 was similar to that of PDMS-L1.Atomic fraction according toXPS:C1s(283.0eV,51.72%),N1s(397.0 eV,0.82%),O1s(530.5 eV,24.70%),Si2p(100.5 eV,22.76%).
1.2.5 Synthesis of PDMS-PtL2
The metallopolymer PDMS-PtL2 was also prepared through the reaction of PDMS-L2 with PtCl2(DMSO)2.However,after peeling into a PTFE mold and volatilizing,only brown mucus has been obtained.Atomic fraction according to XPS:C1s(283.0 eV,51.49%),N1s(397.5 eV,0.87%),O1s(530.6 eV,24.36%),Si2p (100.5 eV,22.62%),Pt4f(71.1 eV,74.3 eV,0.29%),Cl2p(196.8 eV,0.37%).
2 Results and discussion
2.1 Mechanical properties
As shown in Fig.3,when the metallopolymers PDMS-PtL1 and PDMS-PtL2 were poured into PTFE molds for film formation,only orange-red transparent film of PDMS-PtL1 has been obtained,while brown viscous liquid was exhibited for PDMS-PtL2.In the condensation reaction between aldehyde groups and amino groups of PDMS-PtL1,di-aldehyde functionlized Pt(Ⅱ)complex can be served as a cross-linking agent to bridge long chains of PDMS polymers to improve molecular weight and promote film formation.However,just one aldehyde group in L2 reacted with one terminal aldehyde group of linear PDMS polymers,therefore molecualr weight can not be enhanced and the film formation failed in PDMS-PtL2.In the following discusssion,we will focus on the investigation of metallopolymer PDMS-PtL1.
The orange-red film PDMS-PtL1 exhibited excellent stretchable and mechanical strength.The film could be stretched to over 15 times its original length(from 3 to 45 mm)(Fig.4).The stretchability of elastic polymers depends tightly on the stain rate.When accelerating the stretching rate,the stress of materials increased gradually,accordingly the strain declined significantly (Fig.5).The elongation of orange-red film decreased sharply to (510±50)%as the stretching rate was increased to 50 mm·min-1.Under a higher stretching rate,cracked dynamic Pt…Pt interactions in the internal film do not have sufficient time to form again,and the strain has a significant decrease[25].
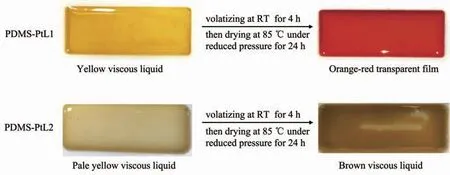
Fig.3 Film formation processes of PDMS-PtL1 and PDMS-PtL2
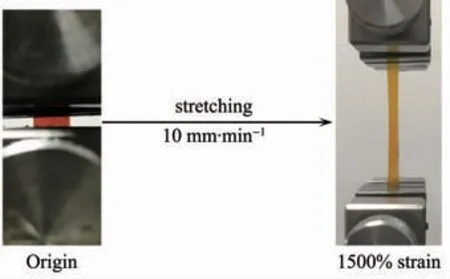
Fig.4 Stretching photograph of a PDMS-PtL1 film from 3 to 45 mm
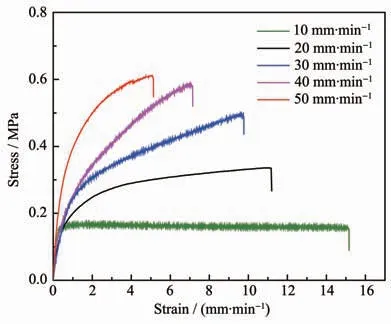
Fig.5 Stress-strain curves of PDMS-PtL1 films with different stretching rates,for samples with gauge length of 3 mm
The Young′s modulus of PDMS-PtL1 polymer film was calculated to be (1.2±0.2)MPa from the initial slope of stress-strain curves(stretching rate=20 mm·min-1),and the toughness was calculated to be(3.18±0.06)MJ·m-3by manually integrating the area under the stress-strain curve,while the stress and strain were (0.34±0.03)MPa and (1 100±80)%,respectively.The mechanical behaviors of PDMS-PtL1 are consistent with the previous studies of elastic polymer incorporating cyclometalated Pt(Ⅱ)complex[25].In virtue of intermolecular Pt…Pt and/or π-π interactions,the film will be more tenacious.
2.2 Self-healing properties
Self-healing properties of PDMS-PtL1 have been explored (Fig.6).As the orange-red film was cut into two pieces and then put together,the wound of film could be healed completely after healing for 12 h under room temperature.When the stretching stress was applied,the recovery film could be facilely elongated to 2 times its orginal length.Subsequently,the external force was withdrawed,and the film could restore to the initiallength rapidly,implying a excellent elasticity of the film PDMS-PtL1.Finally,the cut-heal-stretch-springback process showed a good repeatability.Furthermore,the recycling and plasticity of PDMS-PtL1 has been examined at room temperature,and the film can be well moulded under 15 kPa pressure for 5 min(Fig.7).
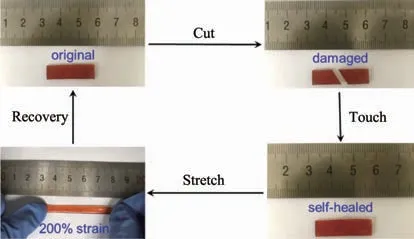
Fig.6 Photos of self-healing process of PDMS-PtL1 at RT

Fig.7 Recycling and plasticity of PDMS-PtL1
Commonly, the self-healing efficiency was calculated from the ratio of healed toughness and original toughness[32].As shown in Fig.8,in comparasion with the original film,the damaged film could achieve (18.9±5)% recovery of the toughness after healing at RT for 4 h.After healing for 24 h,the scratch totally disappeared and the healed film attained (91.8±3)%healing efficiency.In addition,after healing for 24 h at RT,the stress and strain were(0.33±0.03)MPa and(1 050±78)%,respectively,which were very close to the state of original film.It can be inferred thatthePDMS-PtL1 polymerfilm owns excellentself-healing capability justunderroom temperature,and healing effect increases with the prolongation of time.

Fig.8 Self-healing efficiencies of PDMS-PtL1 films at a stretching rate of 10 mm·min-1(left)and typical stress-strain curves after healing for described times(right)at RT
2.3 Stretch-induced luminescent change
As exhibited in Fig.9,when excited at 350 nm,only one peak at 628 nm was observed in the luminescent spectrum of DMS-PtL1,and the red luminescence is ascribed to excimer emission due to the existence of effective Pt…Pt and/or π-π interactions[33-35].Interestingly,when the film is stretched to 2 and 3 times its original length,a new peak at 483 nm could be detected,and the intensity of high energy emission increased slightly with the length of elongation,which can be attributed to the3π-π*state from separated molecules according to the emission spectrum of PDMS-PtL1 in dichloromethane solution[33-35].After settling for 24 h of stretched film,the PDMS-PtL1 polymer film could relax to original length, and stretch-induced separated platinum molecules would aggregate again to form excimer through Pt…Pt and/or π-π interactions.Correspondingly,the high-energy emission state at 483 nm disappeared,and only low-energy emission at 628 nm could be observed.Furthermore,in both the stretching and relaxing process,the emission intensity at 628 nm almostremained unchanged.So,in the tensile process,the emission of PDMS-PtL1 polymer film is closely dependent on the disconnection and formation of Pt…Pt and/or π-π interactions.
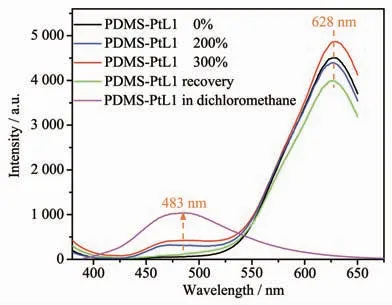
Fig.9 Stretch-induced luminescent change of PDMS-PtL1 films and emission spectrum of PDMS-PtL1 in dichloromethane solution
3 Conclusions
In summary,a stretchable (elongation 1500%)and room-temperatureself-healable metallopolymer PDMS-PtL1 hasbeen successfullyprepared.The orange-red film exhibited excellent mechanical strength with Young′s modulus and toughness being(1.2±0.2)MPa and (3.18±0.06)MJ·m-3,respectively.Dynamic Pt… Pt and/or π-π interactions tentatively account for good stretchable and self-healable properties of PDMS-PtL1.Furthermore,the film showed excimer emission at 628 nm,and a new peak at 483 nm could be detected as the film was stretched,which can be assigned to the3π-π*states of separated molecules.Once the film relaxed to its original length,the high-energy emission at 483 nm disappeared and only low energy-emission at 628 nm was observed.The interesting stretch-induced luminescent change of self-healing elastic polymers may be used in mechanical sensors and wearable electronics.
- 無機化學學報的其它文章
- Effect of Promoter on the Ni-Al Alloy&the Corresponding Raney-Ni Catalyst and Hydrogenation Performance of 1,4-Butylenediol
- Hierarchically Porous Nanosized Red Phosphorus with Enhanced Photo-Oxidation and Photo-Reduction Activities
- Tb(Ⅲ)-Based Metal-Organic Framework for Simultaneously Luminescent Detection of Cu2+and Fe3+Ions
- Solvent-Induced Syntheses,Crystal Structures and Magnetic Properties of Two Mononuclear Er(Ⅲ)Complexes
- Synthesis,Crystal Structures of Two Lanthanides Complexes with 2,6-Difluorobenzoic Acid
- Hydration Mechanism and Stability Regulation of Hemihydrate Calcium Sulfate Whiskers

