Formation of Zirconium Chloride Guanidinate Complexes from the Reaction of their Amide Analog with CCl4
Bhavna Sharma XUE Zi-Ling
(Department of Chemistry,the University of Tennessee,Knoxville,Tennessee 37996,USA)
Abstract:Reaction of CCl4 with zirconium amide guanidinate Zr(NMe2)2[iPrNC(NMe2)NiPr]2(1)has been found to give ZrCl(NMe2)[iPrNC(NMe2)NiPr]2(2)as an intermediate and later ZrCl2[iPrNC(NMe2)NiPr]2(3).The reaction is likely radical in nature.Complex 2 has been independently prepared from the reaction of ZrCl(NMe2)3 with diisopropylcarbodiimide,iPr-N=C=N-iPr,and characterized by nuclear magnetic resonance(NMR)and elemental analysis.
Keywords:zirconium;chlorine;amide complex
0 Introduction
Earlier transition metal complexes with alkyl,amide,and hydride ligands[1-5]often contain polar bonds between metal atoms and anionic ligands.These ligands usually behave as nucleophiles similar to their main group counter parts such as LiR,RMgX (X=halide),LiNR2and NaBH4.Early transition metal amidinate and guanidinate complexeshave attracted much research interest recently[6-18].In certain reactions,amidinate and guanidinate ligands act as spectators.We reported earlier the reaction of guanidinate amide Zr(NMe2)2[iPrNC(NMe2)NiPr]2(1)with O2(Scheme 1)[19],as part of the studies of O2reactions with d0transition metal complexes[20-37].The d0metal centers in the complexes formally have no d electrons.Their reactions with the oxidant O2thus typically involve the ligand oxidation.This is in contrast to the reactions of dncomplexes,in which the metal centers are often oxidized.From the chemistry shown in Scheme 1[19],it appears to be a good candidate to test the radical trapping[38-40].Because of CH2(NMe2)2formed as a side product in this reaction in Scheme 1[19],the reaction is believed to follow a radical mechanism.In addition,the rate of the reaction was enhanced several folds with the addition of the radical initiator 2,2′-azobis(2-methylpropionitrile)(AIBN)[19].
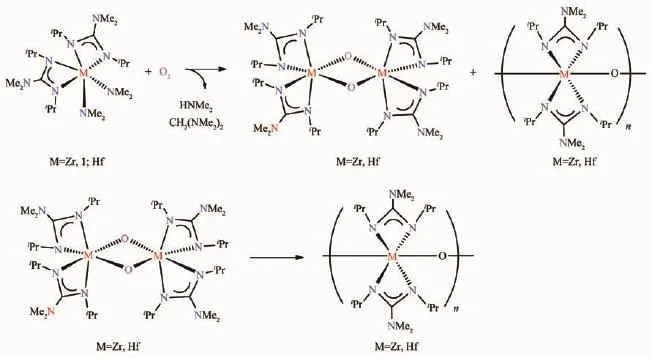
Scheme 1 Reaction of 1 and its Hf analog with O2[19]
CBrCl3and CCl4have been used extensively for radical trapping[38-40].For example,cyclopropyl radical is known to behave as a rapidly inverting σ radical of high reactivity.Experiments have been performed using radical trapping to determine the extent to which a variety of substituted cyclopropyl radicals are capable of maintaining their original configuration[38].BrCCl3was chosen as a trap for the cyclopropyl radical(Eq.(1)).
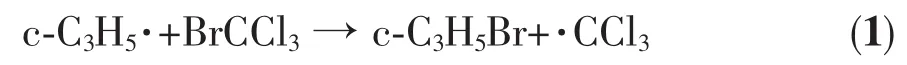
Radical trapping has also been used to probe organometallic reactions[39].For example,irradiation of metal-metal bonded complexes leads to the formation of radicals that may be captured by chlorine atom abstraction from CCl4(Eq.(2),Cp=C5H5)[39].
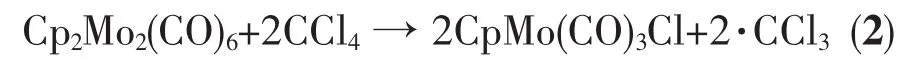
Prior to the use of CCl4as a radical trap to probe the reaction of Zr(NMe2)2[iPrNC(NMe2)NiPr]2(1)with O2,it is,however,necessary to investigate whether 1 would react with CCl4itself in the absence of O2.Indeed we have found that Zr(NMe2)2[iPrNC(NMe2)NiPr]2(1)does react with CCl4,yielding sequentially ZrCl(NMe2)[iPrNC(NMe2)NiPr]2(2)and ZrCl2[iPrNC(NMe2)NiPr]2(3).Complex 2 is a new compound.In addition to observing 2 from the reaction between CCl4and 1,2 has been prepared by a different route-direct insertion ofiPr-N=C=N-iPr into Zr-NMe2bonds in ZrCl(NMe2)3.Our studies of the reaction between CCl4and 1,preparation of 2,and its characterization are reported.
1 Experimental
All manipulations were performed under a dry nitrogen atmosphere with the use of either a drybox or standard Schlenk techniques.All solvents such as pentane,tetrahydrofuran (THF),hexanes were dried over potassium/benzophenone,distilled,and stored under nitrogen.Benzene-d6was dried over activated molecular sieves and stored under nitrogen.CCl4was also dried over activated molecular sieves and stored under nitrogen.NMR spectra were recorded on a Varian 500 MHz Fourier transform spectrometer unless otherwise noted,and were referenced to solvents.Elemental analyses were conducted via Complete Analysis Laboratories,Inc.,Parsippany,NJ.
1.1 Reaction of 1 with CCl4
In a Young′s NMR tube,1(15 mg,0.029 mmol)was dissolved in benzene-d6.Excess CCl4was then added to this Young′s tube.Immediately after the addition of CCl4,the intensities of the peaks corresponding to 1 started decreasing.The progress of the reaction was followed with1H NMR spectroscopy.The1H NMR peaks of 2 first started to appear after~2 h.This process eventually led to the formation of 3.
1.2 Synthesis of ZrCl(NMe2)[iPrNC(NMe2)NiPr]2(2)from the reaction of ZrCl(NMe2)3 with iPr-N=C=N-iPr
ZrCl4(1.620 g,6.952 mmol)in THF was added LiNMe2(1.062 g,20.84 mmol)in THF.After stirring overnight,the solution was filtrated to remove LiCl,and volatiles were removed in vacuo to give crude ZrCl(NMe2)3(0.963 g,3.72 mmol,Yield:59.4%).This crude product was then re-dissolved in hexanes and cooled to give pure ZrCl(NMe2)3as crystals.These crystalswerethen separated from motherliquor solution and washed with cooled hexanes.
ZrCl(NMe2)3(282.6 mg,1.092 mmol)was then reacted withiPr-N=C=N-iPr(275.7 mg,2.185 mmol)in pentane overnight.The volatiles were then removed in vacuo to give the crude product of 2 as an off-white solid (isolated solid:198 mg,0.388 mmol,Yield:70.1%).Repeated attempts to grow the crystals of 2 in different solvents did not yield crystals suitable for single-crystal X-ray diffraction.1H NMR(benzene-d6,499.7 MHz,25 ℃):δ 3.59 (m,4H,CHMe2),3.39(s,6H,Zr-NMe2),2.43 (s,12H,C-NMe2),1.37 (d,12H,3JH-H=6.43 Hz,CHMe2),1.32 (d,12H,3JH-H=6.42 Hz,CHMe2).13C{1H}NMR(benzene-d6,125 MHz,25℃):δ 172.01 (C-NMe2),47.58 (CHMe2),47.12(Zr-NMe2),39.75(C-NMe2),25.18(CHMe2),24.99(CHMe2).Anal.Calcd.for C20H46ClN7Zr(%):C,46.98;H,9.07;N,19.18.Found(%):C,46.91;H,9.13;N,19.11.
2 Results and discussion
2.1 Reaction of Zr(NMe2)2[iPrNC(NMe2)NiPr]2(1)with CCl4
The reaction between 1 and CCl4is given in Scheme 2.1H NMR spectrum of 1 itself is given in Fig.S1 (Supporting information)for comparison.In a Young′s tube,1 in benzene-d6was added CCl4,and the progress of the reaction was followed by1H NMR spectroscopy.Right after the addition of CCl4,new NMR peaks were observed,which were assigned to ZrCl(NMe2)[iPrNC(NMe2)NiPr]2(2),a mono-chloride derivative of 1(Scheme 2,Fig.S2).With the passage of time,1H NMR peaks of 1 decreased in intensity.After 2~3 d at room temperature,1H NMR peaks corresponding to ZrCl2[iPrNC(NMe2)NiPr]2(3),a dichloride derivative of 1,started to appear as well(Scheme 2).After ca.one week,1H NMR spectrum of the solution showed only 3 (Fig.S3).Complex 3 has been reported by Arnold,Bergman and coworkers[41],and it was prepared by direct insertion ofiPr-N=C=N-iPr into the Zr-NMe2bonds in (Me2N)2ZrCl2(THF)2.Comparison of its1H(Fig.S3)and13C{1H}NMR spectra with those reported confirmed the formation of 3 in the reaction in Scheme 2.
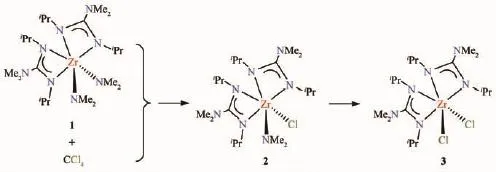
Scheme 2 Reaction of 1 with CCl4,yielding amide chlorides 2 and 3
2.2 Synthesis of 2 via the reaction of ZrCl(NMe2)3 withiPr-N=C=N-iPr and characterization of 2
The mono-chloride ZrCl(NMe2)[iPrNC(NMe2)NiPr]2(2)is a new compound.In the reaction between Zr(NMe2)2[iPrNC(NMe2)NiPr]2(1)with CCl4,it is an intermediate in the formation of the di-chloride 3(Scheme 2)and it was difficult to control the reaction to just form 2.Thus,2 from this reaction was not isolated.Instead,2 was directly prepared through the insertion ofiPr-N=C=N-iPr into two Zr-NMe2bonds in ZrCl(NMe2)3.ZrCl(NMe2)3,as solvent-free[Cl(Me2N)2Zr(μ-NMe2)]2,has been prepared by the reaction of ZrCl4with LiNMe2in ether[42].Its X-ray structure showed a dimer with two NMe2bridges.Our group has earlier prepared ZrCl(NMe2)3as a THF adduct,(Me2N)3Zr(μ-Cl)2(μ-NMe2)Zr(NMe2)2(THF),from either the reaction between ZrCl4and LiNMe2(nZrCl4∶nLiNMe2=1∶3)in THF or Zr(NMe2)4and(Me2N)2ZrCl2(THF)2(nZr(NMe2)4∶n(Me2N)2ZrCl2(THF)2=1∶1)in THF[43].The THF adduct is a dimer bridged by one chloride and one amide ligand.
In the current work,ZrCl(NMe2)3was synthesized by the direct reaction of 3 equiv of LiNMe2with ZrCl4in THF.After filtration to remove LiCl and volatiles were removed in vacuo,the crude product was recrystallized in hexanes to give THF-free ZrCl(NMe2)3,as its1H NMR spectrum shown in Fig.S4.ZrCl(NMe2)3was then reacted withiPr-N=C-N-iPr(nZrCl(NMe2)3∶niPr-N=C-N-iPr=1∶2),resulting in the formation of ZrCl(NMe2)[iPrNC(NMe2)NiPr]2(2,Schme 3).This compound was then characterized by1H,13C{1H}and HSQC NMR spectroscopies(Fig.S5~S7).In1H NMR spectrum(Fig.S5),two doublets at δ 1.32 and 1.37 are assigned to the two different CHMe2groups.One peak at δ 2.43 was observed for the C-NMe2groups on the guanidinate ligands.These may be understood by the Bailar twist mechanism in Scheme 4 (There is a mirror plane in B)[44].The exchange leads to an intermediate B in which there is a mirror plane through the molecule.As a result,the twoiPr groups in the bottom face of B from two different guanidinate ligands are chemically equivalent.Similarly the twoiPr groups on the top face,also from two different guanidinate ligands,are chemically equivalent.The two C-NMe2groups on the two guanidinate ligands were equivalent.The multiplet at δ 3.59 was assigned to CHMe2,and the resonances from two differentiPr groups may overlap here.The peak at δ 3.39 was assigned to the Zr-NMe2group.
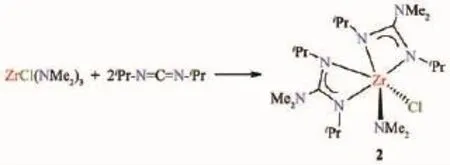
Scheme 3 Synthesis of 2
In the13C{1H}NMR spectrum (Fig.S6)of ZrCl(NMe2)[iPrNC(NMe2)NiPr]2(2),the two peaks at δ 24.99 and 25.18 were assigned to the CHMe2groups.The CNMe2group was observed at δ 39.75.The Zr-NMe2group appeared at δ 47.12.The peak at δ 47.58 was assigned to the CHMe2group.Finally the peak at δ 172.01 was assigned to the quaternary carbon atom of the C-NMe2group.These assignments were confirmed with an HSQC experiment(Fig.S7),and it is consistent with the Bailar twist mechanism in Scheme 4.
The solid product of 2 from the reaction in Scheme 3, without further purification, passed elemental analysis.
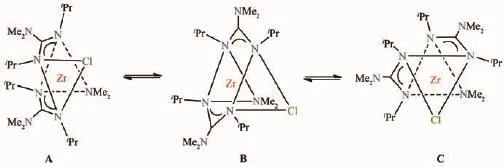
Scheme 4 Bailar twist mechanism for the exchange in 2
2.3 Mechanistic considerations for the reaction between 1 and CCl4
The mechanistic pathway in the reaction between 1 and CCl4was not investigated in the current work.The following are considerations that based in part on observations in the current studies and in part on the reported properties of CCl4and Zr(NMe2)2[iPrNC(NMe2)NiPr]2(1).It should be pointed out that these considerations are essentially speculations.
CCl4is a radical trap,forming·CCl3through its reaction with another radical,as discussed earlier(Eq.(2))[39].The C-Cl bond in CCl4may also undergo homolytic splitting to give two radicals,·CCl3and·Cl,especially under photo-irradiation (Eq.(3))[45-46].Our earlier studies of the reactions of 1 with O2(Scheme 1)showed 1 may undergo reactions with radicals such as O2[19].
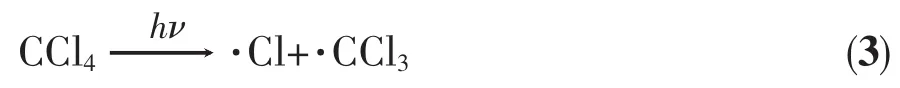
One possible pathway in the reaction of 1 with CCl4in Scheme 2 is that CCl4undergoes the homolytic splitting in Eq.(3) in its solution with Zr(NMe2)2[iPrNC(NMe2)NiPr]2(1)in benzene-d6,perhaps during the initial brief exposure of CCl4to room light during the sample preparation.The newly formed·Cl radical attacks a Zr-NMe2bond in 1,forming ZrCl(NMe2)[iPrNC(NMe2)NiPr]2(2)and radical·NMe2.2 may react with another·Cl radical,giving the dichloride complex ZrCl2[iPrNC(NMe2)NiPr]2(3).The radicals·CCl3,·NMe2and·Cl may react with each other or attack other bonds of the molecules,including those of CCl4,in the solution,giving new radicals such as·Cl.The newly formed·Cl may repeat the process described above,giving more products 2 and 3.It is not clear why 2 and 3 are the major products,but not other possible complexes such as(Cl3C)(Me2N)Zr[iPrNC(NMe2)NiPr]2from the hypothetical attack of·CCl3on 1.Giving the nature of radical reactions,it is perhaps not surprising that no major organic products appeared in1H NMR spectrum of the reaction mixture at the end of the reaction(Fig.S3).
Metathesis or substitution reactions,also known as double displacement reactions,are those involving the exchanges between two reacting chemical species[47-50].If metathesis occurs in the reaction of Zr(NMe2)2[iPrNC(NMe2)NiPr]2(1)with CCl4,formation of Me2N-CCl3and/or(Me2N)2CCl2as major products is expected.Since no major organic product appeared to be obvious in the reaction mixture(Fig.S3),metathesis is probably unlikely the pathway.
3 Conclusions
CCl4has been used in various reactions as a radical trap.It is essential to first make sure that CCl4does not directly react with reactants.It is not surprising to discover that CCl4in fact reacts with 1,first giving monochloride 2 and then dichloride 3.Complex 2 is a new compound,and it has been prepared from direct insertion reaction between ZrCl(NMe2)3and diisopropyl carbodiimide(iPr-N=C=N-iPr,(nZrCl(NMe2)3∶niPr-N=C-N-iPr=1∶2)).
Acknowledgments:The authors thank financial support by the U.S.National Science Foundation (Grants No.CHE-1362548,CHE-1900296).
Supporting information is available at http://www.wjhxxb.cn
- 無機化學學報的其它文章
- 《無機化學學報》投稿須知(NOTICE TO AUTHORS)
- An N-Alkylated 2-(5-Bromo-4-methylthiophen-2-yl)-imidazo[4,5-f]-[1,10]-phenanthroline Rhenium(Ⅰ)Tricarbonyl Compound Showing Aggregation-Induced Emission Enhancement
- Syntheses,Structures and Magnetic Properties of Manganese Phosphonates
- Two Zn(Ⅱ) and Cd(Ⅱ) Metal-Organic Frameworks with Mixed Ligands:Synthesis,Structure,Sorption and Luminescent Properties
- Syntheses and Crystal Structures of Two Copper Complexes Based on Quinolyl and Pyridyl Substituted Triaryltriazoles
- Plasma-Assisted Fabrication of Ferroelectric Flakes with Single-Unit-Cell Thickness and Characterization of Ferroelectricity

