兩種形貌納米Fe2O3對TKX-50熱分解的催化性能研究
張明,趙鳳起,楊燕京,李輝,張建侃,馬文喆,高紅旭,李娜
西安近代化學(xué)研究所,燃燒與爆炸技術(shù)重點實驗室,西安 710065
1 Introduction
Energy components used in solid rocket propellants help to improve the energy performance, and their thermal decomposition characteristics will significantly affect the combustion properties of propellants1-3. Owing to the favorable physical and chemical stability, ammonium perchlorate (AP) is a commonly used oxidizer of solid propellant. However,hydrogen chloride formed from AP decomposition could corrode the nozzle of engine, and hydrochloric acid formed from the reaction between hydrogen chloride and air has a great harm to the environment4-6. Replacing AP with high energy, low sensitivity and environment friendly energetic materials is an inevitable development trend in the field of solid rocket propellants7-9.
As a kind of energetic material with both high energy and low sensitivity, 5,5’-bistetrazole-1,1’-diolate (TKX-50) can not only improve the energy and safety characteristics of solid propellant,but also avoid the drawbacks of equipment corrosion and environmental pollution caused by AP10,11. However, most of the current studies focus on the thermal decomposition and combustion characteristics of AP based propellant, while the thermal decomposition and combustion performances of TKX-50 are seldom reported.
Burning catalyst is another important component of solid propellant, which could significantly improve the burning rate of propellant and reduce the pressure exponent. Nanometer transition metal oxides used as burning catalysts can effectively promote the thermal decomposition of energetic components, and thus enhance the combustion properties of solid propellants12-15.Among various metal oxides, nano-Fe2O3presents excellent catalytic activity on the thermal decomposition of energetic ionic salts including 5,5’-bistetrazole-1,1’-diolate (TKX-50)16-18.Previous studies confirmed the reduction of the thermal decomposition peak temperature and apparent activation energy of TKX-50 after mixed with spherical Fe2O319. However, the catalytic effect comparison of the Fe2O3with different morphologies for thermal decomposition of TKX-50 has not been studied.
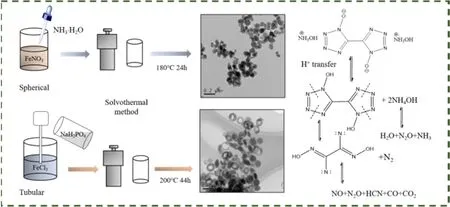
Fig. 1 Illustration of Fe2O3 fabrication and used for thermal decomposition of TKX-50.
Based on this, shape-dependent catalytic activity of nano-Fe2O3 for thermal decomposition of TKX-50 were studied. As can be seen from Fig. 1, the Fe2O3samples with spherical and tubular microstructures were preparedviathe simple one-pot solvothermal method and characterized using SEM, TEM, XRD,FT-IR and XPS, respectively. Then, the catalytic action of Fe2O3(spherical and tubular microstructures) on the thermal decomposition of TKX-50 were studied by TG and DSC method.The apparent activation energies of TKX-50 were also calculated according to the DSC data. Besides, the possible catalytic mechanism of Fe2O3for thermal decomposition of TKX-50 was also analyzed.
2 Experimental and computational section
2.1 Materials
All the chemicals used were analytical grade and used without further purification. Ferric nitrate hydrate (Fe(NO3)3·9H2O,99.99%), ferric trichloride hexahydrate (FeCl3·6H2O, 99.9%)and sodium dihydrogen phosphate (NaH2PO4, 99%) were purchased from Aladdin Inc. Ammonia (FuYu Chemical Co.,Ltd. of TianJin, 25%) was used to adjust pH value. Distilled water and ethanol (EA) (Xi’an Chemical Reagent Factory, 95%)were used for Fe2O3samples fabrication. TKX-50 (99.5%) was obtained from Xi’an Modern Chemistry Research Institute.
2.2 Preparation of spherical Fe2O3
Certain amounts of Fe(NO3)3·9H2O (0.2 g) was dissolved in 60 mL distilled water under magnetic agitation. Then, the pH value of the solution was adjusted to 9-10 by dropping ammonia.The reactants were converted into 100 mL Teflon-sealed autoclave and heated for 24 h at 180 °C. After cooling, the products were washed several times by distilled water and absolute alcohol and cured at 60 °C in a vacuum oven over night.The obtained powder was ground for further characterization18.
2.3 Preparation of tubular Fe2O3
Certain amounts of FeCl3·6H2O (0.3745 g) was dissolved in 30 mL distilled water. Then, the 30 mL solution of sodium dihydrogen phosphate (NaH2PO4, 2.5 mmol·L?1) was mixed with the above solution under magnetic agitation. The reactants were transformed into 100 mL Teflon-sealed autoclave and heated at 200 °C for 44 h. After cooling, the products were washed several times by distilled water and absolute alcohol and cured at 60 °C in a vacuum oven over night. The obtained powder was ground for further characterization.
2.4 Characterization
The morphology and size of Fe2O3 were characterized by scanning electron microscope (SEM, Quanta600,Quantachrome, America) and transmission electron microscopy(TEM, Tecnai G2 F20, FEI, America). The structure and composition were analyzed using X-ray diffraction (XRD,EMPYREAN, PANayltical, Netherlands), Fourier transform infrared spectroscopy (FT-IR, Tensor 27, Bruker, Germany) and X-ray photoelectron spectroscopy (XPS, NEXSA, Thermo scientific, UK) methods. XRD were collected with CuKαsource in the measurement angle range 2θ= 5°-90° with a scan rate of 8 (°)·min?1. The catalytic activities of Fe2O3samples on the thermal decomposition of TKX-50 (the mass ratio of TKX-50 to Fe2O3is 10) were studied by differential scanning calorimeter(DSC, 200 F3, NETZSCH, Germany) and TG-DSC (NETZSCH STA 449C with TASC 414/4 controller, Germany) with a heating rate of 5, 10, 15 and 20 K·min?1, respectively (N2flow rate of 50 mL·min?1, sample mass of (0.5 ± 0.2) mg).Iso-conversional methods (Kissinger and Flynn-Wall-Ozawa) were employed to obtain the activation energies of TKX-50 before and after mixed with different Fe2O3samples (Eqs. (1) and (2))20-22.
HereEis activation energy;Tis temperature;βis heating rate;Ris universal gas constant;Ais pre-exponential factor;
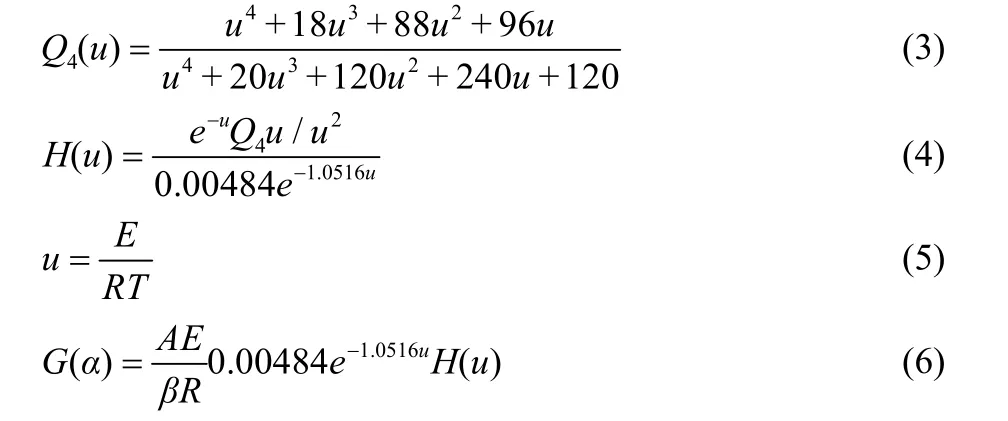
3 Results and discussion
3.1 Morphology and size characterization
The morphologies and sizes of Fe2O3samples were characterized using SEM and TEM instruments, and the results were shown in Fig. 2. The SEM results indicated that the Fe2O3samples with spherical and tubular microstructures were successfully fabricated. The spherical Fe2O3sample is composed of agglomerated Fe2O3nano-particles with average particle size of 110 nm. SEM and low-magnification TEM results showed the hollow structure of the tubular Fe2O3sample with an average diameter of 120 nm and a length about 200 nm. The HRTEM images of the spherical and tubular Fe2O3samples were shown in Fig. 2c,f, respectively. Both of them showed clear lattice fringes with the inter planar distance of 0.368 nm, which can be indexed to the (012) plane of the hexahedron Fe2O3structure.
3.2 Composition and structure characterization

Fig. 2 SEM (a, d), TEM (b, e) and HRTEM (c, f) images of spherical (a-c) and tubular (d-f) Fe2O3 samples.
XRD results confirmed the successful fabrication of spherical and tubular Fe2O3using solvothermal methods (Fig. 3). The diffraction peaks of Fe2O3appear at 24.2°, 33.2°, 35.6°, 40.9°,49.5°, 54.1°, 62.4°, and 64.0°, which correspond well with the crystal planes of (012), (104), (110), (113), (024), (116), (214)and (300) of hematite (JCPDS No. 33-0664)23. Besides, there are no unknown crystalline phases and impurities in the spherical and tubular Fe2O3samples. Average crystallite size of the spherical and tubular Fe2O3samples were calculated using(d110) according Scherrer’s equation. The calculation indicated that the average crystallite sizes of spherical and tubular Fe2O3samples are 28.65 and 30.33 nm, respectively.
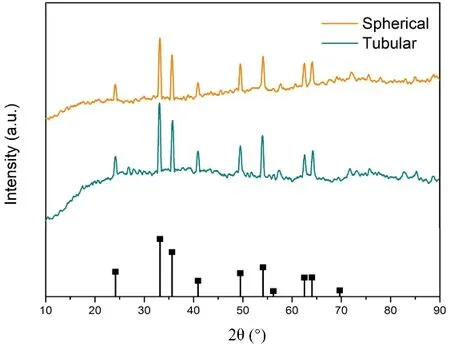
Fig. 3 XRD results of spherical and tubular Fe2O3 samples.
FT-IR measurement was also conducted to investigate the composition of Fe2O3, and the corresponding spectra are shown in Fig. 4. For both spherical and tubular Fe2O3samples,absorption peaks appeared around 3500 cm?1and 1650 cm?1deriving from the O―H stretching vibration and bending vibration of adsorbed water molecules, respectively.Additionally, strong peaks at 564 cm?1and 477 cm?1(derived from the stretching vibration of Fe―O) consist well with the previous study that the peaks of Fe―O located around 580 cm?1and 470 cm?124. The peaks appeared around 1040 cm?1deriving from the Fe―O bending vibration, and the difference between spherical and tubular Fe2O3may result from the different fabrication method25.
XPS was carried out to study the surface chemical composition of spherical and tubular Fe2O3samples, and the results were shown in Fig. 5. The wide scan XPS confirmed the existence of Fe and O elements in both Fe2O3samples. The peaks at 711 and 725 eV appeared in spherical Fe2O3sample are related to Fe 2p3/2and Fe 2p1/2, providing clear evidence for the existence of Fe3+. Besides, the accompanying satellite peaks of Fe 2p3/2at 719.0 eV also confirm the existence of Fe3+26.
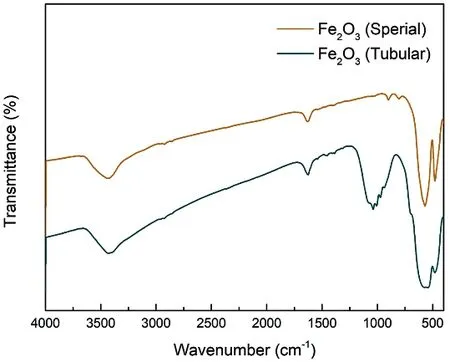
Fig. 4 FTIR results of spherical and tubular Fe2O3 samples.
3.3 Catalytic performance
3.3.1 DSC analysis

Fig. 5 XPS results of spherical and tubular Fe2O3 samples.
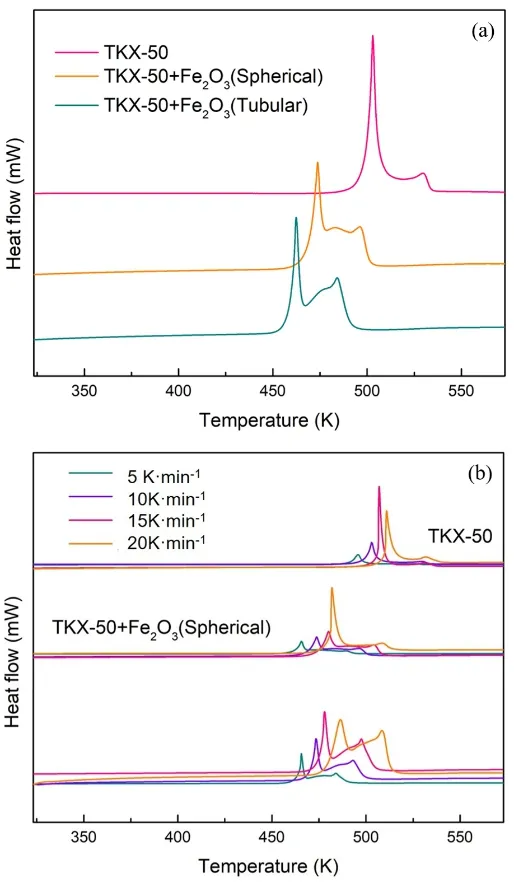
Fig. 6 DSC curves of pristine TKX-50, TKX-50 mixed withs pherical and tubular Fe2O3 at different heating rates.
The catalytic performance of spherical and tubular Fe2O3samples for thermal decomposition of TKX-50 was investigated by DSC, and the results were shown in Fig. 6. The shape dependence of Fe2O3on the thermal decomposition peak temperature of TKX-50 was explored according to the DSC curves. DSC curves of pristine TKX-50 contains two exothermic decomposition peaks appeared at 509.95 K and 533.95 K, which correspond to the decomposition processes of TKX-5022.
As can be seen from Fig. 6, theTFDPand the second thermal decomposition peak temperature (TSDP) of TKX-50 decreased obviously after mixed with spherical and tubular Fe2O3(listed in Table 1). TheTFDP(TSDP) of TKX-50 + Fe2O3(spherical) and TKX-50 + Fe2O3 (tubular) samples are decreased by 26.3 (30.1)and 36.5 K (40.9) in comparison with the pristine TKX-50 at 10 K·min?1. The better catalytic activity of tubular Fe2O3is resulting from its hollow structure, which can provide more active sites are beneficial for the thermal decomposition of TKX-50.
3.3.2 TG and DTG analyses
The TG and the corresponding DTG curves of TKX-50 before and after mixed with different Fe2O3samples at 10 K·min?1were shown in Fig. 7. The decomposition process can be divided into two stages as shown in the TG and DTG curves. The TG results indicated that the mass loss of pristine TKX-50 after the first decomposition is 81.80%. The total mass loss until 450 °C is 95.20%, which suggests that the decomposition of TKX-50 is nearly complete. After the addition of Fe2O3, the reduction of mass loss can be attributed to the addition of Fe2O3. Additionally,the advanced mass loss of TKX-50 also showed the catalytic effect of Fe2O3for thermal decomposition of TKX-50. As shown in Fig. 7, tubular Fe2O3 possessed better catalytic activity than spherical Fe2O3, which coincide with the DSC results.
3.3.3 Activation energy
The activation energies (Ea) of TKX-50 thermal decomposition mixed with the Fe2O3samples were evaluated by Kissinger and Ozawa’s methods using DSC data, and the results were shown in Fig. 8. The results showed that the activation energies calculated by Kissinger method are in good agreement with the Ozawa results. Besides, both spherical and tubular Fe2O3can effectively reduce theEaof TKX-50 thermal decomposition, and theEawas significantly reduced after mixed with the tubular Fe2O3. Combined with the DSC, TG-DTG and activation energy results, both Fe2O3 could effectively promote the thermal decomposition of TKX-50, and the tubular Fe2O3presents better catalytic activity than spherical Fe2O3.
3.4 Catalytic mechanism analysis
The thermal decomposition of TKX-50 involved several processes including protonation, hydroxylamine decomposition,breaking of the tetrazole ring and the formation of stable products (see Fig. 1). First of all, the protons are transferred from hydroxylamine to tetrazole ring. The decomposition of hydroxylamine corresponds to the initial stage of exothermic decomposition. After that, the N―N bonds in the tetrazole ring are broken to form nitrogen and intermediate products. The process corresponding to the first decomposition peak of TKX-50 is accompanied by rapid heat release. Finally, the intermediates decomposed into stable products, corresponding to the second decomposition peak of TKX-5022,26,27.

Table 1 Thermal decomposition peak temperatures of TKX-50 at different heating rates.
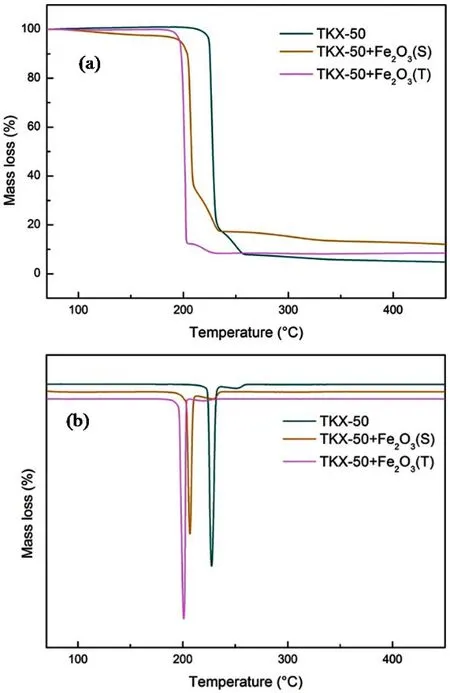
Fig. 7 TG-DTG curves of TKX-50 before and after mixed with different Fe2O3 samples at 10 K·min?1.

Fig. 8 Activation energy of TKX-50 decomposition obtained from DSC data by iso-conversional methods.
DSC curves indicated that the spherical and tubular Fe2O3samples not only affect the initial stage of thermolysis, but also play a significant role for tetrazole ring breaking. Besides, the catalytic effect of Fe2O3 samples on tetrazole ring breaking resulted in an obvious reduction ofTFDPandEa. Combined with DSC and SEM results, the better catalytic activity of tubular Fe2O3 sample may results from the hollow structure, which is beneficial for the thermal decomposition process of TKX-50.
4 Conclusions
The spherical and tubular Fe2O3particles were successfully fabricatedviathe solvothermal method, and the catalytic action of the nano-Fe2O3for thermal decomposition of TKX-50 were studied according DSC and TG-DTG curves. SEM and TEM results showed the hollow structure of tubular Fe2O3 with an average diameter of 120 nm and a length about 200 nm. DSC and TG-DTG results indicated that the tubular Fe2O3presents better catalytic activity for TKX-50 thermal decomposition. The first thermal decomposition peak temperature of TKX-50 were reduced by 36.5 K and 26.3 K in the presence of tubular Fe2O3 and spherical Fe2O3, respectively. Besides, the activation energies of TKX-50 reduced obviously after mixed with Fe2O3samples, and tubular Fe2O3possesses better effect than spherical Fe2O3sample. The better catalytic activity of the tubular Fe2O3is attributed to the hollow structure, which owns more active site are beneficial for TKX-50 thermal decomposition.

