Impacts of Metal Ions, Cysteine, Phosphate and Ethanol on Browning and Antioxidant Activity of Glycosylated Whey Protein Isolate-Inulin Conjugate
MA Ling, SUN Dongxue, LI Tianqi, WANG Yu, Abdul QAYUM, HOU Juncai, JIANG Zhanmei*
(Key Laboratory of Dairy Science, Ministry of Education, Northeast Agricultural University, Harbin 150030, China)
Abstract: The impacts of metal ions, cysteine, phosphate and ethanol on the browning and antioxidant activity of glycosylated whey protein isolate (WPI)-inulin conjugate were investigated in this study. With the increase in the concentrations of iron and zinc ions, the browning and antioxidant activity of glycosylated WPI-inulin conjugate increased initially and afterward decreased, whereas the content of free amino groups content firstly reduced and then increased. With increasing copper ion concentration from 0 to 150 mg/L, the browning and antioxidant activity increased whereas the content of free amino groups decreased. Cysteine could not only inhibit the browning, but also enhance the antioxidant activity of WPI-inulin conjugate. Phosphate buffer and ethanol could facilitate the glycosylation reaction, with the former being more effective. Therefore, the metal ions, phosphate and ethanol could promote the glycosylation reaction and enhance the antioxidant activity of WPI-inulin conjugate.
Keywords: whey protein isolate; inulin; glycosylation; antioxidant activity
Glycosylation is an effective way to improve or enhance the functional properties of food proteins[1]. Although a variety of techniques are available for the preparation of glycoproteins, the glycoconjugates obtained between the proteins and polysaccharides by the Maillard reaction have received much attention in recent years[2]. Glycation is based on the Amadori rearrangement steps in the Maillard reaction[3]. Glycosylation does not require any chemical reagent as a catalyst, and heating alone allows the reaction to proceed spontaneously[4]. Moreover, glycosylated protein products have the advantages of low toxicity, good antioxidant activity, solubility and thermal stability, making the glycosylation an ideal method for protein modification.
Whey protein is widely used as functional ingredients in the food industry[5]. Recent years, glycosylation has been used to improve functional properties[6]and antioxidant activity[7]of whey protein. However, taking into account the extremely complex composition of real food systems, the glycosylated reaction conditions and the interaction between glycosylated reaction products and other food components are mostly varied[8]. It was not only influenced by reactive condition factors, such as type and ratio of reactants, reaction temperature and pH, but also by exogenous substances,including metal ions, cysteine and ethanol. For instance,whey protein treated with 10 kDa dextran had higher levels of glycosylation than that treated with 20 kDa dextran[9]. At the reaction temperatures of 60 and 70 ℃, the browning of whey protein isolate (WPI)-maltose conjugate was deepened with the mass ratio of WPI to maltose increased[10]. Heat stability of WPI-chitosan oligosaccharide was increased with increasing temperature from 35 to 55 ℃[11]. WPI-glucose mixtures showed more browning intensity at pH 8 than at pH 6 and 7[12]. Cu2+, Fe2+, Na+have been reported to activate the advanced stage of Maillard reaction through increasing the conversion rate of the Amadori product to dicarbonyl compounds[13]. Furthermore, cysteine can contribute not only to inhibition of color formation but also to the continuity,mouthfulness and overall acceptance of soybean peptide glycosylated reaction products[14]. The addition of inorganic phosphate could increase the degree of glycosylated reaction,because it acts as a nucleophile catalyzing the conversion of the glycosylamine into the Amadori rearrangement product[15].On the other hand, it is noteworthy that a higher level of ethanol enhances browning of glucose-glycine system[16].Although there are a few reports on effects of individual exogenous substances on glycosylation, there have has been no comparison study of impacts of exogenous factors on glycosylated reaction. Furthermore, metal ions, cysteine,phosphate, and ethanol are substances that are often present in food systems, so it is necessary to study the effects of these four exogenous substances on glycosylation.
Inulin, a polydisperse fructan[17]has the biological functions, including the control of blood fat and blood sugar, promotion of mineral absorption and prevention of constipation[18]. Meanwhile, it also serves as a carbonyl donor for glycosylation reactions with proteins[19]. In order to elucidate the effects of exogenous substances on glycosylation of proteins, we established a WPI-inulin model system in this study. In addition, the influences of metal ions, cysteine, phosphate and ethanol on the browning and antioxidant activity of WPI-inulin conjugates were also investigated.
1 Materials and Methods
1.1 Materials and reagents
WPI (protein, 93.77%) was purchased from Milky Way commercial company (Beijing, China); Inulin was purchased from Dingxi Longhai dairy limited liability company (Gansu,China); 1,1-diphenyl-2-picrylhydrazyl (DPPH) was purchased from Sigma-Aldrich Co. Ltd. (St. Louis, MO, USA).
Sodium dihydrogen phosphate was purchased from Yaohua Chemical Reagent Co. Ltd. (Tianjin, China);disodium phosphate and potassium ferricyanide were purchased from Bodie Chemical Co. Ltd. (Tianjin, China);ferric chloride was purchased from Double Boat Chemical Reagent Factory (Tianjin, China); absolute ethanol was purchased from Benchmark Chemical Reagent Co. Ltd.(Tianjin, China); zinc sulfate was purchased from Yongda Chemical Reagent Co. Ltd. (Tianjin, China); copper sulfate was purchased from East Tianzheng Fine Chemical Reagent Factory (Tianjin, China); ferrous sulfate was purchased from Tianli Chemical Reagent Co. Ltd. (Tianjin, China); all the other chemicals were of analytical grade.
1.2 Instruments and equipment
UV-2401PC Spectrophotometer was purchased from Shimadzu Co. Ltd. (Kyoto, Japan); TGL20M desktop highspeed refrigerated centrifuge was purchased from Baimisi Instrument Co. Ltd. (Changzhou, China); thermostatic water bath was purchased from Aisite Instrument Co. Ltd. (Tianjin, China);AL-104 precision electronic balance was purchased from Mettler Toledo Instruments Co. Ltd. (Shanghai, China).
1.3 Methods
1.3.1 Preparation of glycosylated WPI-inulin conjugate
WPI (30 g/L) and inulin (30 g/L) were dissolved in deionized water. Ferrous sulfate (0, 15, 30, 75, 100, 125 and 150 mg/L), zinc sulfate (0, 7.5, 15, 37.5, 75 and 100 mg/L),copper sulfate (0, 1.5, 3, 7.5 and 15 mg/L) or cysteine (0,30, 60, 100, 130 and 160 mg/L) were selected as exogenous substances and added into the mixture of WPI and inulin.Meanwhile, phosphate buffer (0.05, 0.1, 0.25, 0.5 mol/L)or ethanol (0, 30, 60, 90 and 120 g/L) was a substitute for deionized water as a reaction solution during the preparation of glycosylated conjugate. Subsequently, initial pH value of all the sample mixture was adjusted to 9.0 by the addition of 6 mol/L NaOH, and heated in sealed screw-top glass tubes at 95 ℃. After treatment for 12 h, all the mixed solutions were immediately submerged in ice water. Control experiments with WPI (30 g/L) being heated without inulin were also carried out.
1.3.2 Determination of browning intensity
All the samples were diluted to the concentration of WPI of 7.5 g/L using deionized water, and the browning intensity of the samples was determined by a UV-VIS spectrophotometer at 420 nm.
1.3.3 Determination of free amino groups
The free amino groups of the samples were determined according to the o-phthalaldehyde (OPA) method with some modifications[20]. The OPA reagent was freshly prepared before used. Samples were diluted to a final concentration of 5 mg/mL. 3 mL of OPA reagent was mixed with 100 μL of each diluted sample, then put in the dark and incubated for 5 min at room temperature. Absorbance was recorded at 340 nm.Reagent blank was made using distilled water instead of samples. The content of free amino groups of sample was calculated from its absorbance using a calibration curve obtained with L-leucine (0.1-0.5 mg/mL) as a standard.
1.3.4 DPPH radical scavenging activity
The DPPH radical scavenging activity of the samples was determined according to the modified method of Yen et al.[21]. One millilitre of sample solutions (WPI 7.5 g/L)was mixed with 4.0 mL of 0.1 mmol/L DPPH-ethanol solution, prepared freshly. The reaction solution was allowed to stand in the darkness at 25 ℃ for 30 min. The mixture was centrifuged at 4 000 r/min for 10 min. The absorbance of the supernatant was recorded at 517 nm.The percentage of DPPH radical scavenging activity was calculated as follows:

Where Ablankis the absorbance of ethanol and 0.1 mmol/L DPPH without the samples; Asampleis the absorbance of samples and 0.1 mmol/L DPPH; Acontrolis the absorbance of ethanol instead of 0.1 mmol/L DPPH.
1.3.5 Determination of ferrous reducing power
The ferrous reducing power of all the samples was determined according to the method of Oyaizu[22]with minor modification. Samples was diluted to a concentration of 15 g/L, and mixed with 5 mL of 0.2 mol/L sodium phosphate buffer (pH 6.6) and 2.5 mL of 1% potassium ferricyanide. The mixture was incubated at 50 ℃ for 20 min and cooled down rapidly. After adding 10% trichloroacetic acid (2.5 mL, 10%),the mixture was centrifuged at 3 000 r/min for 10 min. An aliquot of supernatant (0.5 mL) was blended with 2.5 mL of deionized water and 0.5 mL of 0.1% FeCl3. The mixture was left for 10 min. The absorbance was monitored at 700 nm.The results were expressed as absorbance units.
1.4 Statistical analysis
Independent trials were carried out three times.Experimental results were expressed as ± s. The data were subjected to one-way analysis of variance (ANOVA) and independent samples t-test to determine the signi ficance of the main effects by using the SPSS system (SPSS 22.0). Multiple comparisons were determined by the Duncan multiple range test with the level of signi ficance set at P < 0.05.
2 Results and Analysis
2.1 Effects of ferric sulfate on browning and antioxidant activity of WPI-inulin conjugate
The effects of ferrous sulfate on browning of WPI-inulin and WPI were presented in the Fig. 1A. The absorbance of WPI-inulin at 420 nm was significantly higher than that of WPI (P < 0.05). With the increase in ferrous sulfate concentration ranged from 0 to 150 mg/L, the browning of WPI was remarkably increased, while browning intensity of WPI-inulin conjugate was firstly increased and afterward decreased (P < 0.05). For instance, browning intensity of WPI-inulin conjugate at the ferrous sulfate concentration of 100 mg/L was 1.79 times higher than that of WPI-inulin conjugate without ferrous sulfate. This indicated that ferrous sulfate could promote the browning of glycosylated WPI-inulin conjugate as compared with ferrous sulfate-untreated one. However, the browning degree of WPI-inulin conjugate was decreased with increase in the ferrous sulfate concentration from 100 to 150 mg/L. Furthermore, more metal ions might be chelated on the amino and carboxyl groups of amino acids or the hydroxyl groups of sugars, leading to a decrease in the formation of glycosylated conjugate[8].
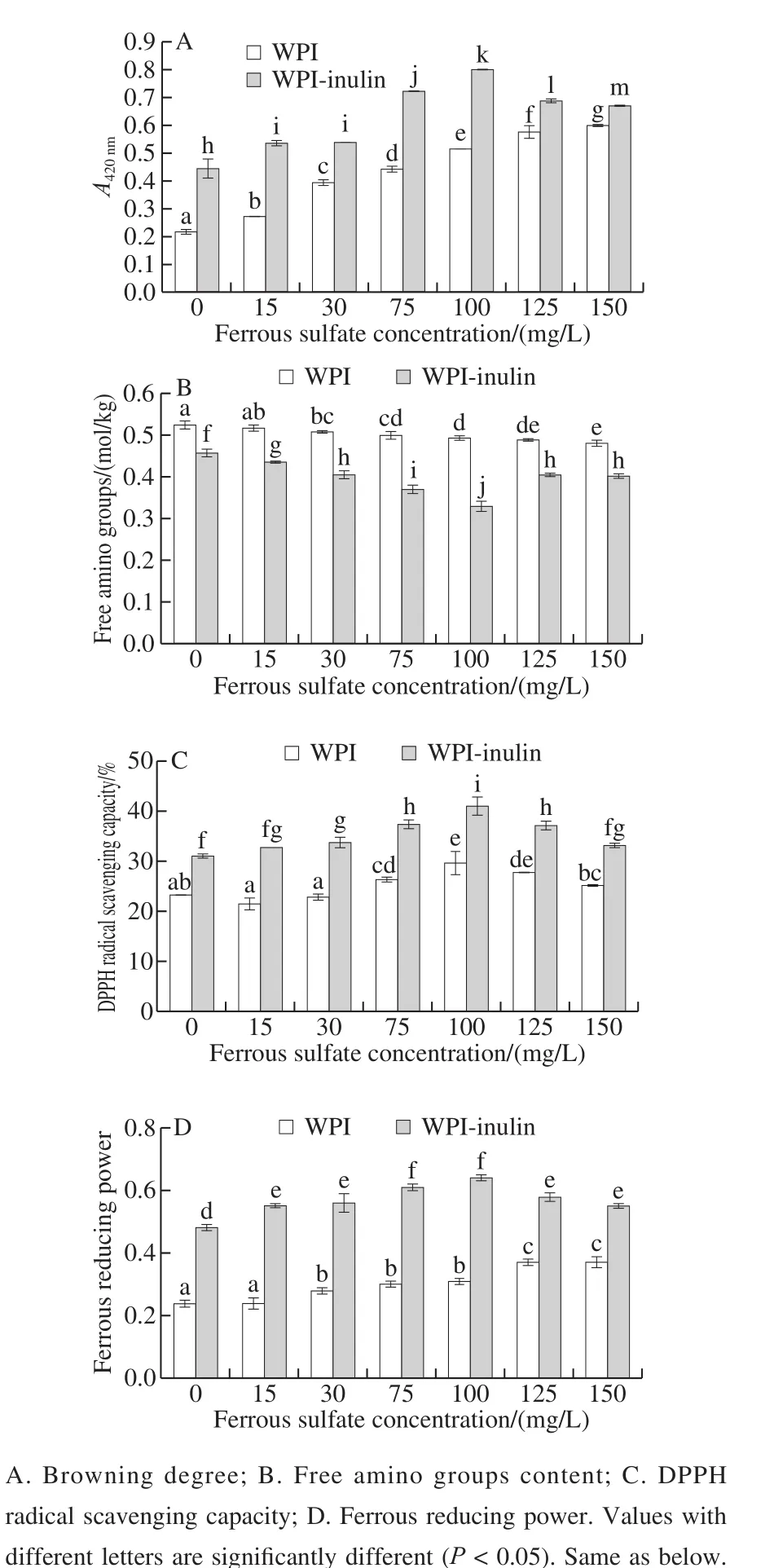
Fig. 1 Effects of ferrous sulfate on browning degree and antioxidant activity of WPI and WPI-inulin MRPs
In the glycosylation reaction, the terminal α-amino groups of peptides or proteins and ε-amino groups of lysine residues react with the carbonyl groups of reducing sugars[23].The loss of available amino groups is another indicator of the reactivity of glycosylation reaction. The effects of ferrous sulfate on free amino groups of WPI-inulin and WPI were shown in Fig. 1B. The content of free amino groups of WPI-inulin was significantly lower than that of WPI (P < 0.05). With the increase in ferrous sulfate concentration ranged from 0 to 150 mg/L, the content of free amino groups of WPI-inulin conjugate was decreased and afterward increased (P < 0.05).When the concentration of ferrous sulfate was 100 mg/L, the free amino group of WPI-inulin polymer was the minimum,and its browning degree was the maximum. This result further proved that the browning degree of glycosylation was increased and afterward decreased with increasing ferrous sulfate concentration. The result was consistent with that reported by Shen et al.[16], who also showed that the glycosylation was positively catalyzed by low concentration of iron ions while higher concentration of iron ions could suppressed glycosylation.
As shown in the Fig. 1C, the DPPH radical scavenging activity of WPI-inulin was significantly higher than that of WPI (P < 0.05) in the ferrous sulfate concentration range of 0 to 150 mg/L (P < 0.05). Furthermore, with the increase of ferrous sulfate concentration, DPPH radical scavenging activity of glycosylated WPI-inulin conjugate and WPI increased and afterward decreased (P < 0.05). Antioxidant activity of L-ascorbic-glycine model system also first increased and afterward decreased with the increase of Fe2+and Cu2+concentration[24]. It is known that metal ions can not only form complexes with Maillard reaction products, but also can oxidize Amadori compounds and their derivatives,and catalyze further interactions of these compounds[25].
As shown in the Fig. 1D, the ferrous reducing power of WPI-inulin was significantly higher than that of WPI in the ferrous sulfate concentration range of 0 to 150 mg/L (P < 0.05).The ferrous reducing power of WPI was increased with the increase in ferrous sulfate concentration, while ferrous reducing power of WPI-inulin conjugate was increased and afterward decreased (P < 0.05). Furthermore, ferrous sulfate could probably accelerate WPI oxidation and form more WPI oxidized products, thereby improving the browning, DPPH radical scavenging activity and ferrous reducing power of heated WPI. It is consistent with the findings that protein oxidation of soy protein isolate can be induced by heat and soybean oil[26].
In this test, it was also found that DPPH radical scavenging activity and ferrous reducing power of WPI-inulin conjugate had a positive relation with its browning at the ferrous sulfate concentration range of 0 to 150 mg/L.Moreover, WPI-inulin conjugate showed the maximum browning, DPPH radical scavenging activity and ferrous reducing power at ferrous sulfate concentration of 100 mg/L.
2.2 Effects of the zinc sulfate on browning and antioxidant activity of WPI-inulin conjugate
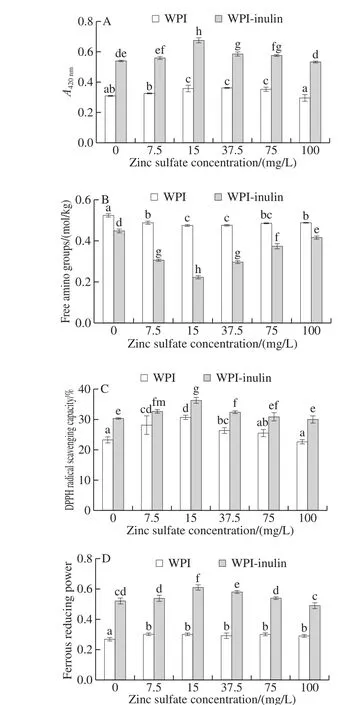
Fig. 2 Effects of zinc sulfate on browning degree and antioxidant activity of WPI and WPI-inulin MRPs
The results in Fig. 2A show the effects of zinc sulfate on browning of WPI-inulin and WPI. The browning degree of WPI-inulin was significantly higher than that of WPI(P < 0.05). Accordingly, as the zinc sulfate concentration was changed from 0 to 100 mg/L, browning of WPI-inulin and WPI conjugate was increased and afterward decreased(P < 0.05). Moreover, zinc sulfate concentration of 15 mg/L significantly enhanced the browning degree of WPI-inulin conjugate. It indicated that zinc sulfate could promote the browning of glycosylated WPI-inulin conjugate.However, higher concentration zinc sulfate could also inhibit the browning degree of glycosylation. It was also found that coagulation of melanoidin could be suppressed in the presence of a large amount of metal ions[27].
Analysis of the contents of free amino groups in WPI-inulin and WPI which were added with zinc sulfate were shown in Fig. 2B. WPI-inulin contained lower free amino groups than WPI did (P < 0.05). The content of free amino groups in WPI-inulin was decreased and afterward increased with increase in zinc sulfate concentration ranged from 0 to 100 mg/L (P < 0.05). The free amino content of WPI-inulin was negatively correlated with its browning degree(correlation coefficient about 0.71).
As shown in Fig. 2C and D, the effects of the zinc sulfate concentration range of 0 to 100 mg/L on the DPPH radical scavenging activity of WPI-inulin was significantly higher than that of WPI (P < 0.05). The ferrous reducing power of WPI was increased slightly with increase in zinc sulfate concentration, while the content of ferrous reducing power of WPI-inulin conjugate was increased and afterward decreased (P < 0.05).
It was also found that DPPH radical scavenging activity and ferrous reducing power of WPI-inulin conjugate were also positively related with its browning at the zinc sulfate concentration range of 0 to 100 mg/L. It was also verified that a low concentration of zinc ion increased the browning rate in the lactose-glycine model system, whereas a high concentration of zinc ion suppressed the formation of brown compounds[8].
2.3 Effects of the copper sulfate on browning and antioxidant activity of WPI-inulin conjugate
The effects of copper sulfate on browning of WPI-inulin and WPI were presented in Fig. 3A. Compared with the WPI,the absorbance of WPI-inulin was significantly increased at 420 nm (P < 0.05). With the increase in copper sulfate concentration ranged from 0 to 15 mg/L, browning of WPI was decreased and afterward increased, while browning of WPI-inulin conjugate was increased (P < 0.05). It indicated that copper ion could promote the browning of glycosylated WPI-inulin conjugate. It was reported that ferric and copper ions could accelerate the browning rate of the glycosylation reaction model system[28].
Effects of copper sulfate concentration on the content of free amino groups of WPI-inulin and WPI were shown in Fig. 3B. The content of free amino groups of WPI-inulin conjugates was significantly decreased (P < 0.05) with copper sulfate concentration being increased from 0 to 15 mg/L.Accordingly, the content of free amino groups was the lowest at the copper sulfate concentration of 15 mg/L. The result further proved that adding copper sulfate could remarkably enhance the degree of glycosylation. It may be due to the fact that copper ion catalyzes the Maillard reaction by the oxidative pathway[29].

Fig. 3 Effect of copper sulfate on browning degree and antioxidant activity of WPI and WPI-inulin MRPs
It can be seen from Fig. 3C and D that the DPPH radical scavenging activity and ferrous reducing power of WPI-inulin were significantly higher than those of WPI in the copper sulfate concentration range of 0 to 15 mg/L (P < 0.05).Moreover, with the increase of copper sulfate concentration,DPPH radical scavenging activity of WPI and glycosylated WPI-inulin conjugate were significantly increased (P < 0.05).Meanwhile, WPI-inulin conjugate showed the maximum browning, DPPH radical scavenging activity and ferrous reducing power at copper sulfate concentration of 15 mg/L.It was also revealed that certain metal ions can promote the formation of Schiff base by forming complexes[30]. It had also been shown that Cu2+could form stable complexes with proteins and sugars, thereby reducing the free energy of glycosylated reaction[31]. In addition, it was obtained that correlation coefficient between ferrous reducing power and browning was about 0.95, and correlation between DPPH radical scavenging activity and browning of WPI-inulin was approximately 0.71. Thus, DPPH radical scavenging activity and ferrous reducing power of WPI-inulin exhibited a positive trend with its browning intensity.
2.4 Effects of cysteine on browning and antioxidant activity of WPI-inulin conjugate
The effects of cysteine on browning of WPI-inulin and WPI were presented in the Fig. 4A. With increase in concentration of cysteine concentration ranged from 0 to 160 mg/L, browning degree of WPI-inulin conjugate and WPI were remarkably increased and afterward decreased(P < 0.05). It indicated that cysteine could promote the browning of glycosylated WPI-inulin conjugate at a lower concentration, however, higher concentration cysteine could inhibit the glycosylation browning of WPI-inulin. It was probably due to the fact that cysteine could inhibit the formation of brown pigment[32].
As was shown in Fig. 4B, the free amino groups content of WPI-inulin and WPI was significantly increased with cysteine concentration extended up from 0 to 160 mg/L(P < 0.05). Free amino content of WPI-inulin was lower than that of WPI. This result was consistent with that reported by Church et al.[20], in which the free amino groups content was increased significantly (P < 0.05) after the addition of cysteine. It may be due to the free amino group brought by cysteine[33]. On the other hand, the addition of cysteine may lead to competition to react with reducing sugar[34].
As shown in the Fig. 4C and D, the DPPH radical scavenging activity and ferrous reducing power of WPI-inulin were significantly higher than those of WPI in the cysteine concentration range of 0 to 160 mg/L (P < 0.05). It has been demonstrated that cysteine was a thiol-containing compound with high antioxidant capacities, and nonenzymatically interacted with reactive electrophile and free radicals[35],potentially leading to an increase in antioxidant activities of WPI-inulin conjugate.
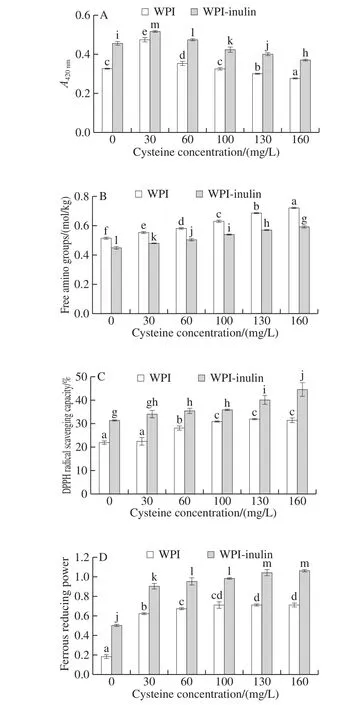
Fig. 4 Effect of cysteine on browning degree and antioxidant activity of WPI and WPI-inulin MRPs
2.5 Effects of the phosphate buffer on browning and antioxidant activity of WPI-inulin conjugate
The effects of phosphate buffer on browning of WPI-inulin and WPI were shown in the Fig. 5A. With the increase in phosphate buffer concentration ranged from 0 to 0.5 mol/L,the browning degree of WPI-inulin conjugate and WPI were remarkably increased and afterward decreased (P < 0.05).Especially, browning degree of WPI-inulin conjugate at the phosphate buffer concentration of 0.25 mol/L, was 1.85 times higher than that of WPI-inulin conjugate without phosphate buffer. It meant that phosphate buffer could promote the browning of glycosylated WPI-inulin conjugate. However,higher phosphate buffer could inhibit the glycosylation browning. Previous works also validated that the phosphate buffer solution could promote the glycosylation reaction,probably due to the reason that it can accelerate the nucleophilic reaction of amine and carbonyl[36]. Furthermore,phosphate buffer solution had an obvious catalytic effect on glycosylation of soybean protein-konjac[37]. Additionally,phosphate is an acid-base catalyst that accelerates the nucleophilic reaction of amines and carbonyls[15].
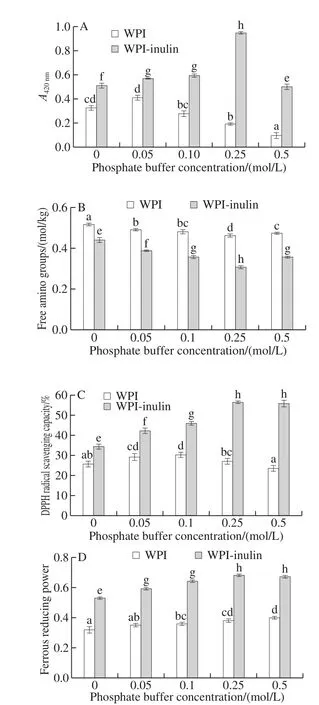
Fig. 5 Effect of phosphate buffer on browning degree and antioxidant activity of WPI and WPI-inulin MRPs
As was shown in Fig. 5B, the contents of free amino groups of WPI-inulin and WPI were decreased and afterward increased with phosphate concentration extended up from 0 to 0.5 mol/L (P < 0.05). WPI-inulin had the lowest free amino group and the largest browning degree at the concentration of phosphate of 0.25 mol/L. This could further testify that phosphate and other polyatomic anions might accelerate the rate of Maillard reaction through providing reactive intermediates directly from sugars[38].
As presented in the Fig. 5C and D, the DPPH radical scavenging activity and ferrous reducing power of WPI-inulin were significantly higher than those of WPI in the phosphate buffer concentration range of 0 to 0.5 mol/L(P < 0.05). With the phosphate buffer concentration increased,DPPH radical scavenging activity and ferrous reducing power of glycosylated WPI-inulin conjugate and WPI were increased(P < 0.05). It was also found that DPPH radical scavenging activity and ferrous reducing power of WPI-inulin conjugate were positively related with its browning at the phosphate buffer concentration range of 0 to 0.25 mol/L.
2.6 Effects of the ethanol on browning and antioxidant activity of WPI-inulin conjugate
As it is shown in the Fig. 6A, the effects of ethanol on browning of WPI and WPI-inulin were presented. With the increase in ethanol concentration ranged from 0 to 120 g/L,browning degree of WPI-inulin conjugate and WPI remarkably were increased (P < 0.05). It indicated that ethanol could promote the browning degree of glycosylated WPI-inulin conjugate. It was also found that the glycineglucose reaction system reacted more severely brown in ethanol than in aqueous solution[39]. It has been noted that the presence of ethanol could accelerate the glucose consumption in a glycine-glucose system[40]. Although the ethanol solution could promote the glycosylation reaction, the glycosylated mechanisms between the ethanol and aqueous solution were different[41]. Baltes et al.[42]studied the glycosylation of glucose with chloroaniline in an ethanol solution and found that ethanol might form O-2 ethyl glucoside with the product of Amadori rearrangement.
The effects of ethanol concentration on free amino groups content of WPI-inulin and WPI were shown in Fig. 6B. The content of free amino groups of WPI-inulin was reduced significantly with increasing ethanol concentration from 30 to 120 g/L (P < 0.05). At the amount of ethanol of 120 g/L, WPI-inulin contained the the lowest amount of free amino and the largest browning degree. Lu et al.[43]also reported that the content of Maillard reaction products derived from an ethanolic solution were higher than that from an aqueous solution, indicating that ethanol promotes the Maillard reaction.
As shown in the Fig. 6C and D, the DPPH radical scavenging activity and ferrous reducing power of WPI-inulin were remarkably higher than those of WPI in the ethanol concentration range of 0 to 120 g/L (P < 0.05). Furthermore,with the increase of ethanol concentration, the DPPH radical scavenging activity of glycosylated WPI-inulin conjugate was increased and afterward decreased (P < 0.05). Meanwhile, the ferrous reducing power of WPI-inulin conjugate was increased with the increase in ethanol concentration (P < 0.05).

Fig. 6 Effects of ethanol on browning degree and antioxidant activity of WPI and WPI-inulin MRPs
DPPH radical scavenging activity and ferrous reducing power of WPI-inulin conjugate have a positive relation with its browning at the ethanol concentration range of 0 to 90 g/L.Moreover, WPI-inulin conjugate showed the maximum browning, DPPH radical scavenging activity and ferrous reducing power at the ethanol concentration of 90 g/L.The glucose consumption in the glycine-glucose ethanol system was higher than that in the glycine-glucose aqueous system[44]. When the ethanol was used as the reaction solution,it could promote the glycosylation reaction to produce more antioxidant active substances, but its reaction mechanism needed to be further studied.
3 Conclusions
The present study for the first time has presented the effects of the metal ions, cysteine, phosphate and ethanol on browning and antioxidant activity of glycosylated WPI-inulin conjugate. Browning intensity, antioxidant activity and consumption of free amino groups of WPI-inulin conjugate were increased and afterward decreased with the increase in iron and zinc ion concentration. Furthermore, antioxidant activity and consumption of free amino groups of WPI-inulin conjugate were found to be proportional to the number of copper ions added. In summary, the browning intensity and antioxidant activity of WPI-inulin conjugate were increased and afterward decreased with increasing iron and zinc ion concentrations, and were proportional to the amount of copper ions added. After the addition of cysteine, the antioxidant activity of WPI-inulin conjugate was increased,while its browning was inhibited. Phosphate buffer can promote greater glycosylation progress of WPI and inulin than ethanol. Thus, metal ion, phosphate and ethanol could remarkably enhance the browning and antioxidant activity of glycosylated WPI-inulin conjugate.

