Enhanced dye-sensitized up-conversion luminescence of neodymium-sensitized multi-shell nanostructures
WANG Dan,XUE Bin,TU Lang-ping,ZHANG You-lin,SONG Jun,QU Jun-le,KONG Xiang-gui
(1.Key Laboratory of Optoelectronic Devices and Systems of the Ministry of Education/Guangdong Province,College of Physics and Optoelectronic Engineering, Shenzhen University, Shenzhen 518060, China;2.College of Information Engineering, Shenzhen University, Shenzhen 518060, China;3.State Key Laboratory of Luminescence and Applications, Changchun Institute of Optics, Fine Mechanics and Physics, Chinese Academy of Sciences, Changchun 130033, China)
Abstract:Lanthanide-ion-doped upconversion luminescence is limited by the small absorption cross-section and narrow absorption band of lanthanide ions,which results in weak luminescence.Recently,a dye-sensitized method has proven to be an effective strategy of increasing upconversion luminescence.However,simply attaching dye molecules to nanoparticles with classic Yb-doped nanostructures cannot effectively activate the sensitizing ability of the dye molecules.In response to this problem,we designed Nd-sensitized core/shell/shell(NaYF4:Yb/Er(20/2%)@NaYF4:Yb(10%)@NaYF4:Nd(80%))nanostructures,compared with the classic IR-806 sensitized NaYF4:Yb/Er nanostructure,their upconversion luminescence(500 to 700 nm)was approximately enhanced by a factor of 38.Through analysis of the nanostructure’s emission and luminescence lifetime data,the enhancement was confirmed by the effective overlap of Nd absorption with the emission of near-infrared dye molecules and the protective effects of the shell structure on the luminescent center(the lifetime of Er(4S3/2→4I15/2)was increased by 1.7 times).In addition,we found that the doping Yb3+in the outermost layer will decrease the dye-sensitized luminescence intensity.Furthermore,this Ndsensitized core/shell/shell structure also achieved enhancement in the sensitized upconversion luminescence of the luminescence centers of Ho and Tm,which establishes a foundation for enhanced dye-sensitized upconversion luminescence.
Key words:upconversion luminescence;dye-sensitized;lanthanide ion;nanoparticles
1 Introduction
Due to the unique electron transitions in 4f electron configuration and between 4f and 5d,rare earth ions can generate the photon radiation of various wavelengths from ultraviolet,visible light to infrared light[1-2].The Up-Conversion NanoParticles(UCNPs)doped with rare earth ions have the characteristic of converting two or more low-energy near-infrared photons into a high-energy photon.In particular,the photon emission generated by UCNPs has the advantages such as narrow spectral band,resistance to bleaching,and low background noise[3].This up-conversion luminescence induced by nearinfrared light has been applied in many fields,such as super-resolution imaging,fluorescent labeling,photodynamic therapy,and optical anti-counterfeiting[4-8].
In spite of the above wide applications,the further practical application of the up-conversion luminescence generated by the doped rare earth ions has been limited by its low luminous intensity.How to enhance the up-conversion luminescence has always been an urgent problem to be solved in upconversion luminescence research.In recent years,the techniques such as core-shell nanostructure[9],plasma field-enhanced luminescence[10]and dyesensitized luminescence[11]have realized the enhancement of up-conversion luminescence intensity.In particular,the study of dye-sensitized luminescence has not only enhanced the intensity of up-conversion luminescence,but also broadened the excitation range of up-conversion luminescence.Instead of the rare earth ions with weak absorption(absorption coefficient:0.1~10 M?1cm?1),near-infrared dyes(absorption coefficient:1 000~10 000 M?1cm?1)have been used to absorb near-infrared light so as to realize the up-conversion luminescence enhanced by sensitization.However,the iterated integral of most of the near-infrared dye emission(800~900 nm)and the Yb absorption in the classical Yb-sensitized doping system(950~1,000 nm)is small,thus limiting the enhancement of dye-sensitized up-conversion luminescence.Furthermore,Nd/Er-ion sensitization system has been designed as the recipient of dye sensitization[12-13]to achieve more effective enhancement of dye-sensitized up-conversion luminescence.It is in recent years that Nd3+-sensitized upconversion luminescence system has been developed.Especially,its partitioned doping strategy can realize efficient up-conversion luminescence[14-16].However,the dye-sensitized Nd-ion doping system usually adopts the optimum structural design of Ndsensitized up-conversion luminescence.Considering that the dye will interact with rare earth luminescence system in the dye sensitization process to weaken the luminescence[17],the design and optimization of dye-sensitized Nd-doped up-conversion luminescence system will be more effectively applied in biochemical analysis,tumor diagnosis and treatment,luminescence display and other fields[18-22].
In this paper,Nd-sensitized core/shell/shell structure was designed as the recipient of enhanced dye-sensitized up-conversion luminescence.The Nd-sensitized core/shell/shell structure was successfully prepared by high-temperature thermal decomposition,and then was coupled with the dye IR-806 molecules to enhance the intensity of dye-sensitized up-conversion luminescence.The related structural characterization confirmed the successful preparation of this nanostructure.The enhancement mechanism behind it was further studied through the analysis of emission spectrum and fluorescence lifetime spectrum.Meanwhile,by optimizing the doping concentration of Yb ions in the outermost shell,the intensity of the dye-sensitized up-conversion luminescence without Yb doping proved to be the strongest.
2 Experiment
2.1 Experimental materials
The experimental materials include ytterbium chloride(YbCl3·6H2O,99.99%),yttrium chloride(YCl3·6H2O),erbium chloride(ErCl3·6H2O),sodium hydroxide(NaOH,98%),ammonium fluoride(NHF4,99.99%),IR-780 iodide(99%),4-mercaptobenzoic acid(99%),1-octadecene(ODE),oleylamine(90%)(OM)and oleic acid(90%)(OA),all of which were purchased from Sigma-Aldrich.According to the reference[14],Nd(CF3COO)3was obtained through the reaction between Nd2O3powder and excessive trifluoroacetic acid and then removing the remaining trifluoroacetic acid by evaporation.Ytterbium trifluoroacetate(Yb(CF3COO)3),yttrium trifluoroacetate(Y(CF3COO)3)and sodium trifluoroacetate(CF3COONa)were purchased from GFS Chemicals.Dichloromethane,trichloromethane and dimethyl formamide(DMF)were purchased from Beijing Chemical Works.All the chemical reagents were of analysis purity.
2.2 Synthesis of up-conversion nanoparticles
The core-shell-shell up-conversion nanostructure is fabricated based on the published chemical process[14-15].Firstly,synthesize the core structure.Dissolve YbCl3·6H2O(0.1 mmol),YCl3·6H2O(0.39 mmol)and ErCl3·6H2O(0.01 mmol)in a three-mouth flask containing 3 mL OA and 7.5 mL ODE.Heat the mixture to 150℃for 30 minutes,and then cool it to room temperature under the protection of argon.Then,dissolve NH4F(2 mmol)and NaOH(1.25 mmol)into 5 mL methanol,add the mixture to the above three-mouth flask with rare earth salts,and heat it to 70℃to remove methanol and then heat to 300℃for 1h.Then,add 0.25 mmol NaYF4:Yb(10%)active shell to ODE(synthesized by trifluoroacetate process)and then add to the above mixture for 10 min curing.Then,add 0.5 mmol NaYF4:Nd(20%)active shell(synthesized by trifluoroacetate process)and cure it for 10 min.Finally,cool the solution to room temperature,centrifuge it with ethanol,and dissolve it into 6 mL trichloromethane.The core-shell-shell up-conversion nanostructures doped with different rare earth elements were all synthesized in the similar way.
2.3 Synthesis of IR-780 molecules
Similarly,dissolve organic IR-780 molecules(250 mg),4-mercaptobenzoic acid(115.5 mg)and DMF(10 mL)in a 50-mL three-mouth flask under nitrogen protection according to the Ref.[11].Then keep the mixture under nitrogen protection for 17 h.Filter the product solution with 0.45μm PTFE and remove DMF through reduced-pressure distillation.Then dissolve the residue into 5 mL dichloromethane,filter the mixture again with 0.45μm PTFE and precipitate it with ice ethyl ether.Finally,filter and dry the reactant under vacuum,and keep it in dark place.
2.4 Synthesis of IR-806 molecules
In the similar way as Refs.[8],[17],dissolve 1 mL IR-806(xmg/mL,x=0~20 mg/mL)into trichloromethane,and mix it with 1 mLβ-NaYF4:Yb/Er(20/2%)@NaYF4:Yb(10%)@NaYF4:Nd(20%)nanoparticles(Er3+:~1.67 mmol).Stir the entire reaction mixture for 24 h at room temperature,centrifuge it,and redisperse it into 1 mL trichloromethane.Test the up-conversion spectrum of IR 806-sensitized UCNPs under the Er3+concentration of about 16μmol.
2.5 Experimental characterization testing
Transmission Electron Microscope(TEM)was tested at 200 kV by use of Tecnai G2 F20 S-Twin electron microscopy.X-ray diffraction(XRD)test was performed on Rigaku D/Max-2000 by using Cu Kαradiation(λ=0.154 1 nm)as diffraction radius.Absorption spectrum was tested on a Maya 2000 spectrometer(Ocean Optics).The up-conversion spectrum was recorded by an externally coupled 808 nm laser on an ocean optical spectrometer(Maya2000).Energy Dispersive Spectrum(EDS)analysis was characterized by Hitachi S-4800.In the fluorescence lifetime test of up-conversion luminescence,500 MHz TDS 3052 was used as the excitation light source,and the fluorescence lifetime data was obtained through an OPO(Sunlite 8000)and an oscilloscope.
3 Experimental results and discussion
3.1 Morphology and characterization of nanoparticles
The highly uniform Nd3+-sensitized core-shellshell(NaYF4:Yb/Er(20/2%)@NaYF4:Yb(10%)@NaYF4:Nd(80%))UCNP structure was successfully prepared through high-temperature thermal decomposition.The TEM photo showed that the UCNPs were in uniform size.As shown in Fig.1,the average sizes of core(NaYF4:Yb/Er(20/2%),denoted as“C”),core/shell(NaYF4:Yb/Er(20/2%)@NaYF4:Yb(10%),denoted as“CS”)and core/shell/shell(NaYF4:Yb/Er(20/2%)@NaYF4:Yb(10%)@NaYF4:Nd(80%)),denoted as“ CSS”)were 23.5 nm,26.3 nm and 33.6 nm,respectively.This increasing size confirmed that an Yb transition layer of about 1.4 nm and a 3.7 nm Nd-sensitized nanoshell layer were gradually growing on the NaYF4:Yb/Er nano-core.The Fig.2(a)shows that the UCNPs have a classical hexagonal phase structure(JCPDS-16-0334).The EDS confirmed that Nd,Y,Yb and other rare earth elements were effectively doped into the nanoparticles(Fig.2(b)).Furthermore,we synthesized the IR-806 molecule according to the reference method(Fig.2(c)).As seen from the absorption diagram(Fig.2(d)),its absorption peak shifted from 780 nm to 806 nm,which confirmed the successful synthesis of IR-806 molecule.Furthermore,the IR-806 molecules was modified to the surface of UCNPs according to the above method.As shown in Fig.2(d),after an IR-806 molecule was modified to UCNPs,the absorption peak of the UCNPs was masked by the absorption spectrum of IR-806,thus confirming the successful modification of dye molecule to UCNPs.
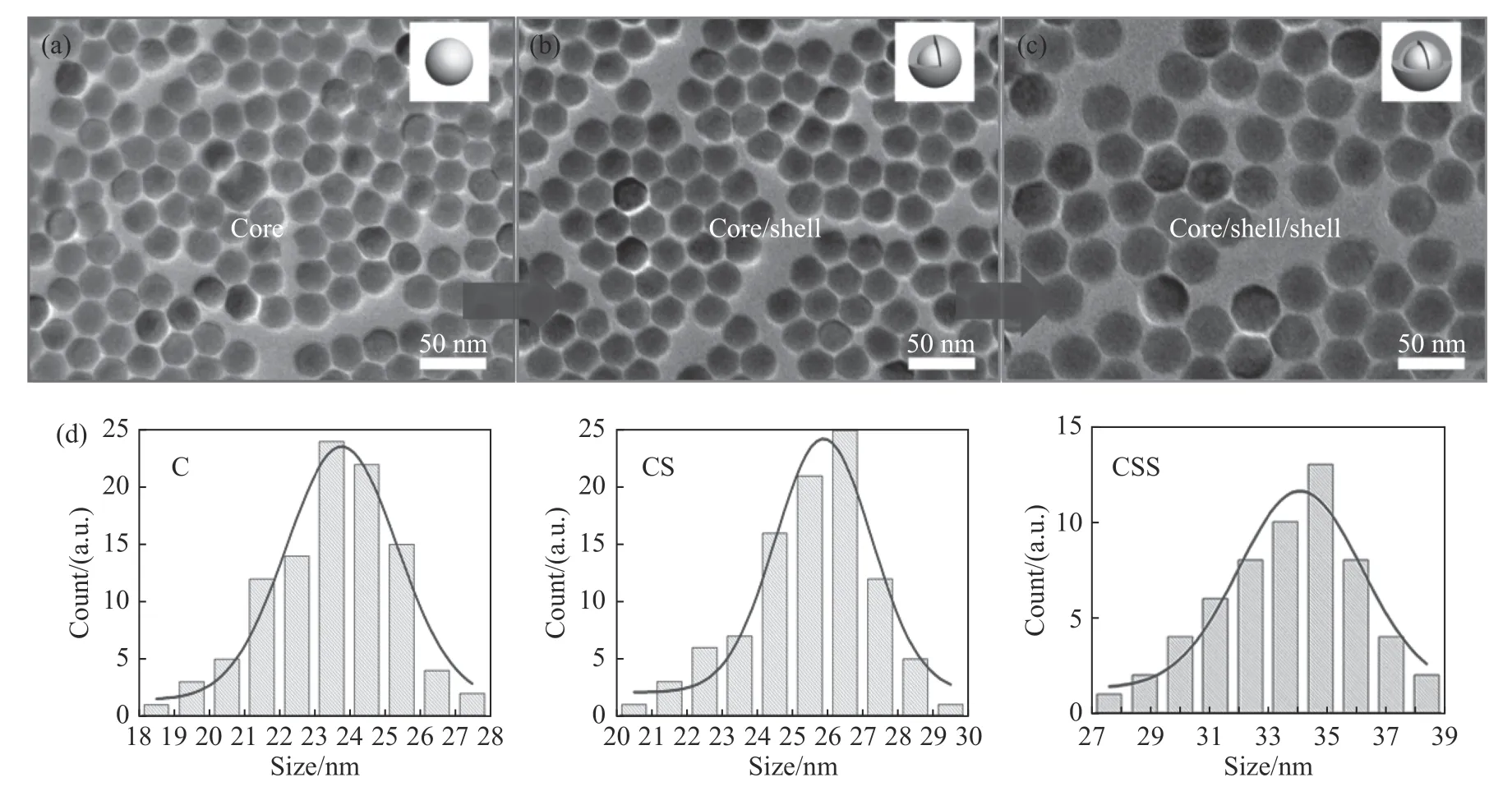
Fig.1 TEM images of core(a),core/shell(b)and core/shell/shell(c)of up-conversion nanoparticles and their size distributions(d)圖1 上轉換納米粒子的核(a),核/殼(b),核/殼/殼(c)電鏡表征圖及尺寸分布(d)
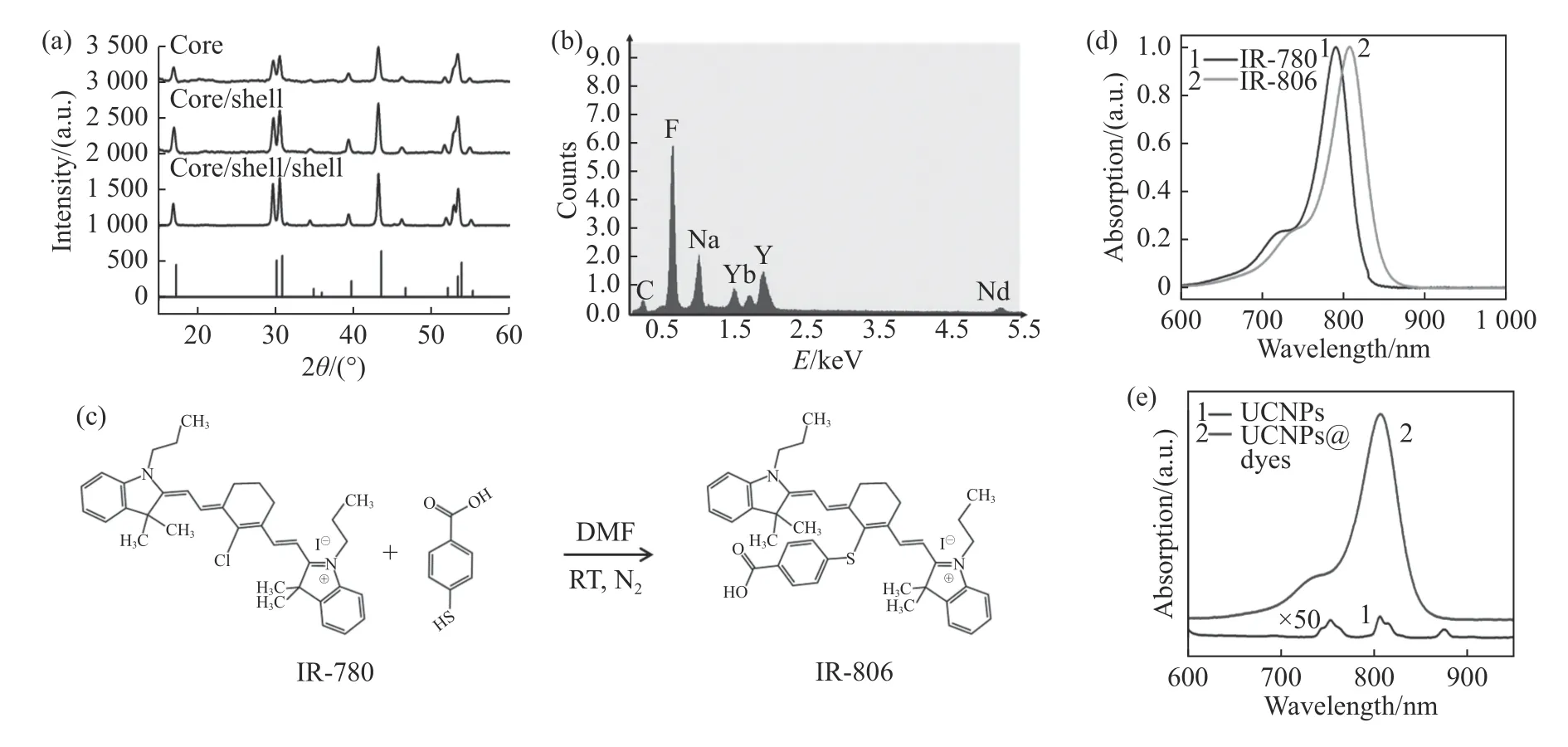
Fig.2 (a)XRD data of C,CS and CSS of UCNPs and theβ-NaYF4(JCPDS-16-0334,bottom);(b)EDS data of CSS;(c)IR-806 synthesis process;(d)absorptions of IR-780 and IR-806 before and after synthesis;(e)absorption of UCNPs and dye conjugated UCNPs after IR-806 connection圖2 (a)上轉換納米粒子的核、核/殼、核/殼/殼XRD 及標準卡片β-NaYF4(JCPDS-16-0334,底部)結果,(b)上轉換CSS 的EDS 數據,(c)IR-806合成過程圖,(d)合成前后IR-780和IR-806 的吸收,(e)連接IR-806 之后UCNPs 吸收和UCNPs 本身的吸收
3.2 Confirmation and discussion of dye-sensitized enhancement mechanism
As shown in Fig.3(a),the up-conversion luminescence intensity of the dye-sensitized CSS structure proposed in this paper is about 38 times stronger than that of the classical IR-806-sensitized NaYF4:Yb/Er(20/2%)nanoparticle reported at the earliest[11].This proves that the dye-sensitized structure has achieved the enhancement of up-conversion luminescence intensity.In addition,under the excitation of 808 nm near-infrared light,the intensities of both the up-conversion red and green light emissions of dye-sensitized CSS structure were nonlinearly dependent on excitation light power(Fig.3(b)).The corresponding multiphoton indexes were 1.67(540 nm green light emission:4S13/2→4I15/2)and 2.0(655 nm red light emission:4F9/2→4I15/2).Therefore,the luminescence of this structure proves to be nonlinear up-conversion luminescence.
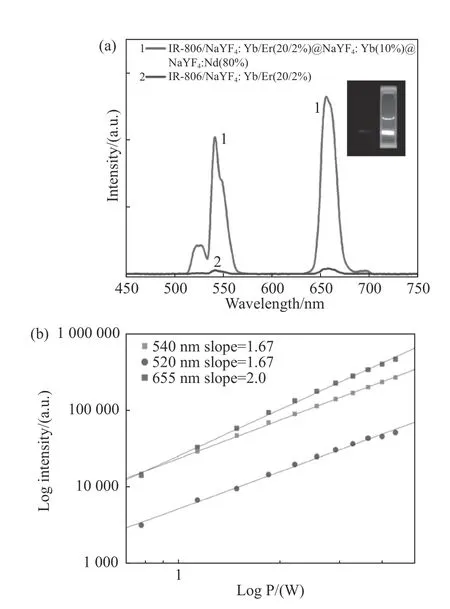
Fig.3 (a)Up-conversion luminescence(UCL)spectra of IR-806-sensitized CSS structure and IR-806-sensitized core structure under 808 nm excitation wavelength;(b)log-log plots of the UCL intensity versus laser power for the IR-806 dye-sensitized CSS under 808 nm excitation圖3 (a)IR-806 敏化CSS 結構的上轉換光譜及IR-806 敏化核結構的上轉換光譜,激發波長為808 nm,(b)808 nm 激發下的IR-806 敏化的CSS 結構的上轉換發光強度隨功率變化的log-log 關系
According to our analysis,the outermost layer of CSS nanostructure is Nd3+-doped shell,where Nd can be sensitized by IR-806 molecules efficiently due to the serious overlap between the Nd absorption and the emission of IR-806 dye molecules,as shown in Fig.4(a)(Color online).On the other hand,the nanoshell in CSS structure can effectively protect the luminescence center.As seen from Fig.4(b)(Color online),the luminescence lifetime of Er(253μs)in the dye-sensitized CSS structure was significantly longer than that of Er in the dyesensitized core nanoparticle(146μs)or core nanoparticle(169μs).In other words,the luminescence lifetime of Er has increased by 1.73 times and 1.50 times,respectively.Thus,it is proved that the nanoshell can insulate the luminescence center from the external interference environment so as to guarantee a long life of the luminescence center.It is worth noting that although the designed dye-sensitized Nd3+-doped nanostructure has a better excitation wavelength near 800 nm,but NaYF4:Yb/Er(20/2%)nanoparticles can only be excited by 980 nm wavelength.As shown in Fig.4(b),the exicitation wavelength was 980 nm,not the conventional 808 nm wavelengh.Furthermore,the Fig.4(c)shows that the luminescence life of CSS nanostructure remains unchanged whether dye molecules are connected or not.This further proves that the nanoshell can effectively prevent the interaction between the luminescence center and the external environment,thus enhancing the up-conversion luminescence
The difference of Nd3+sensitization system lies in the fact that its outermost nanoshell is doped with only Nd3+ions,rather than the previously reported Nd-Yb ions[16].This structural design is based on the results of our experiments.As shown in Fig.5,with the increase of Yb3+-doping concentration in the outermost layer,the up-conversion luminescence intensity of dye-sensitized CSS structure will gradually decrease.According to our previous research of dye-sensitized rare earth up-conversion nanosystem,the excitation energy absorbed by dye needs to be gradually transferred to the internal luminescence center[17].In this process,the energy loss in the migration of excitation energy to the surface is very heavy.The doped Yb3+ions are likely to transfer the excitation energy to the surface[9,23-24],thus reducing the excitation energy transferred to the interior and weakening the up-conversion luminescence.Therefore,the strongest dye-sensitized up-conversion luminescence is produced in the outermost nanoshell without doped Yb3+ions,as shown in Fig.5.
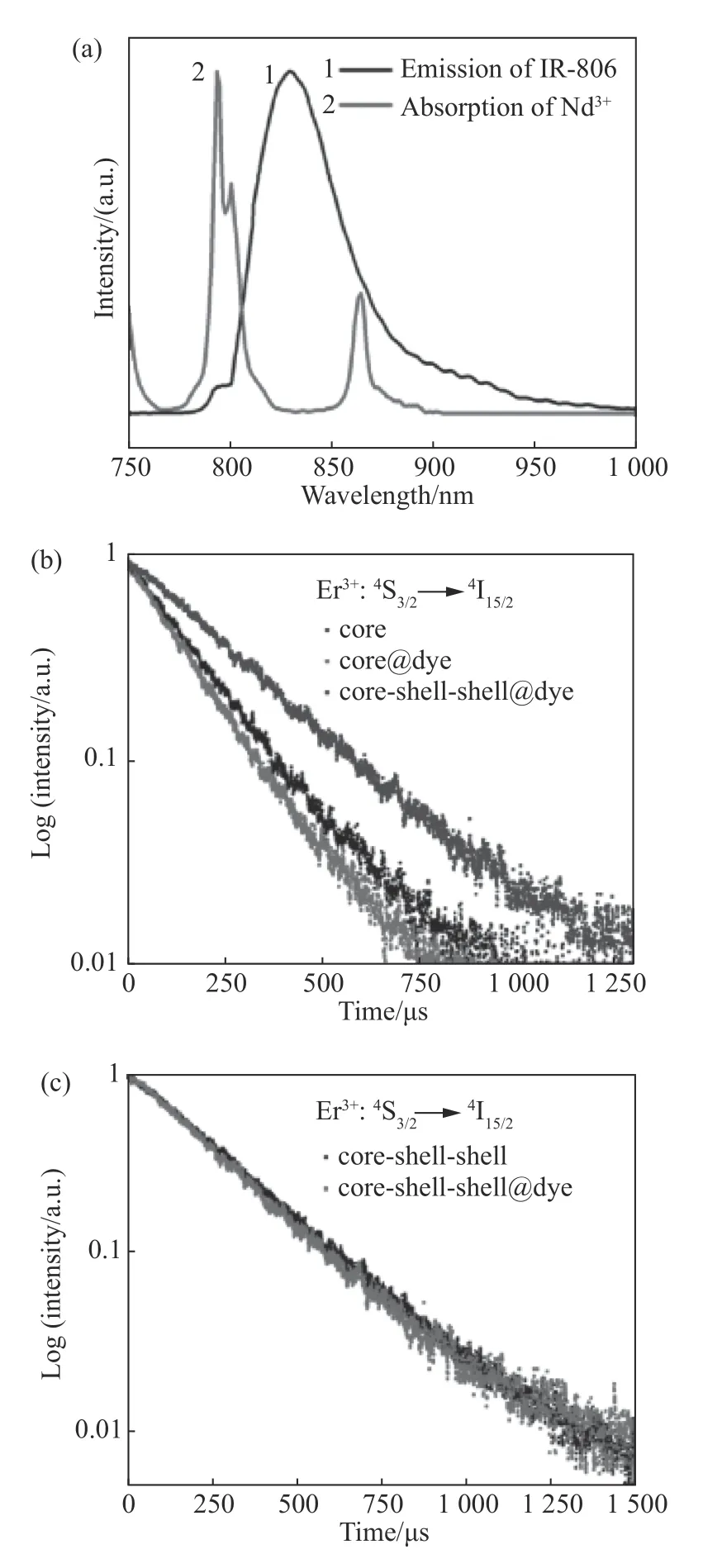
Fig.4 (a)Overlap between the emission spectrum of IR-806 molecules and the absorption spectrum of Nd3+;(b)the lifetimes of Er3+(4S3/2→4I15/2)in core nanoparticles(black,NaYF4:Yb/Er(20/2%),dye-sensitized core nanoparticles(red)and dye-sensitized CSS nanostructure(blue)under 980 nm excitation;(c)the lifetimes of Er3+(4S3/2→4I15/2)in CSS nanostructure and dye-sensitized CSS nanostructure under 808 nm excitation圖4 (a)IR-806 分子的發射光譜與Nd3+的吸收交疊圖;(b)980 nm 激發下,測試得到的核納米粒子(黑色,NaYF4:Yb/Er(20/2%)),染料敏化核納米粒子(紅色)及染料敏化的CSS 納米結構(藍色)的Er3+(4S3/2→4I15/2)的壽命測試結果;(c)808 nm 激發下CSS 納米結構及染料敏化的CSS 納米結構的Er3+(4S3/2→4I15/2)的壽命測試結果
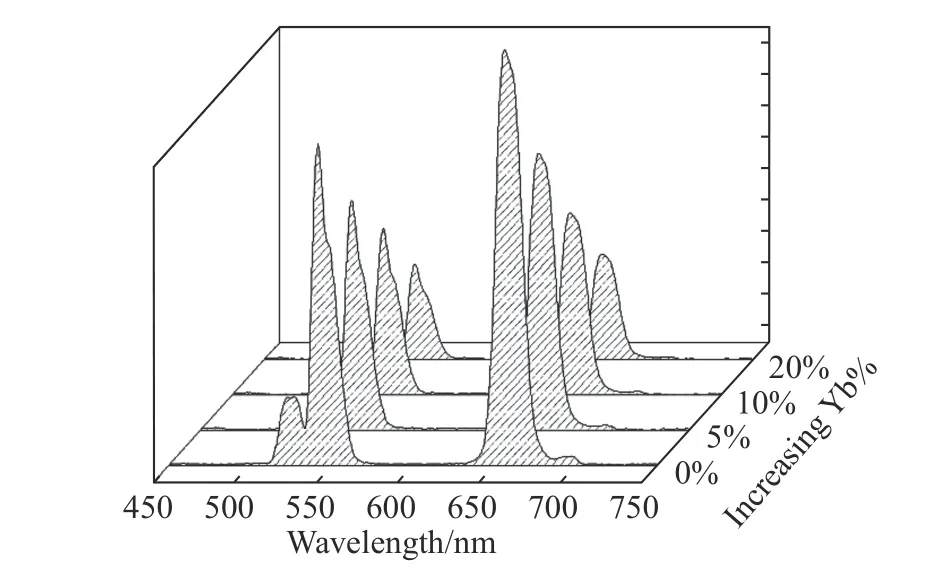
Fig.5 Upconversion spectra of dye-sensitized NaYF4:Yb/Er(20/2%)@NaYF4:Yb(10%)@NaYF4:Nd/Yb(80/x%)(x=0,5,10,20)nanoparticles under 808 nm excitation圖5 染料敏化的NaYF4:Yb/Er(20/2%)@NaYF4:Yb(10%)@NaYF4:Nd/Yb(80/x%)(x=0,5,10,20)的上轉換光譜(808 nm 激發)
3.3 Enhancement of dye-sensitized luminescence by using Ho or Tm as luminescence center
Furthermore,the replacement of luminescence center in the core of CSS structure by Ho(NaYF4:Yb/Ho(20/1%))@NaYF4:Yb(10%)@NaYF4:Nd(80%))or Tm(NaYF4:Yb/Tm(20/1%)@NaYF4:Yb(10%)@NaYF4:Nd(80%))also realized the enhancement of dye-sensitized up-conversion luminescence(Fig.6(a)and 6(b),Color online).When the luminescence center was Ho or Tm,the typical nonlinear dependence of luminescence intensity on excitation light power was also shown(Fig.6(c)and 6(d),Color online).When the luminescence center was Ho,the multi-photon indexes were 1.57(540 nm emission,4S13/2→4I15/2)and 1.88(645 nm emission,4F9/2→4I15/2)respectively.When the luminescence center was Tm,the multi-photon indexes were 2.82(450 nm emission,1D2→3F4),1.74(470 nm emission,1G4→3H6),1.80(645 nm emission,1G4→3F4)and 1.34(695 nm emission,3F2→3H6)respectively.It should be noted that,for Tm ions,the dye-sensitized up-conversion luminescence had hardly been seen in the NaYF4:Yb/Tm(20/1%)nano-core structure.This is because the 800 nm emission level(3H4→3H6)of Tm heavily overlapped with the absorption of IR-806 molecules,thus quenching the Tm luminescence.However,the outer shell of CSS structure successfully blocked the transfer of Tm to IR-806,thus realizing the dye-sensitized up-conversion luminescence.
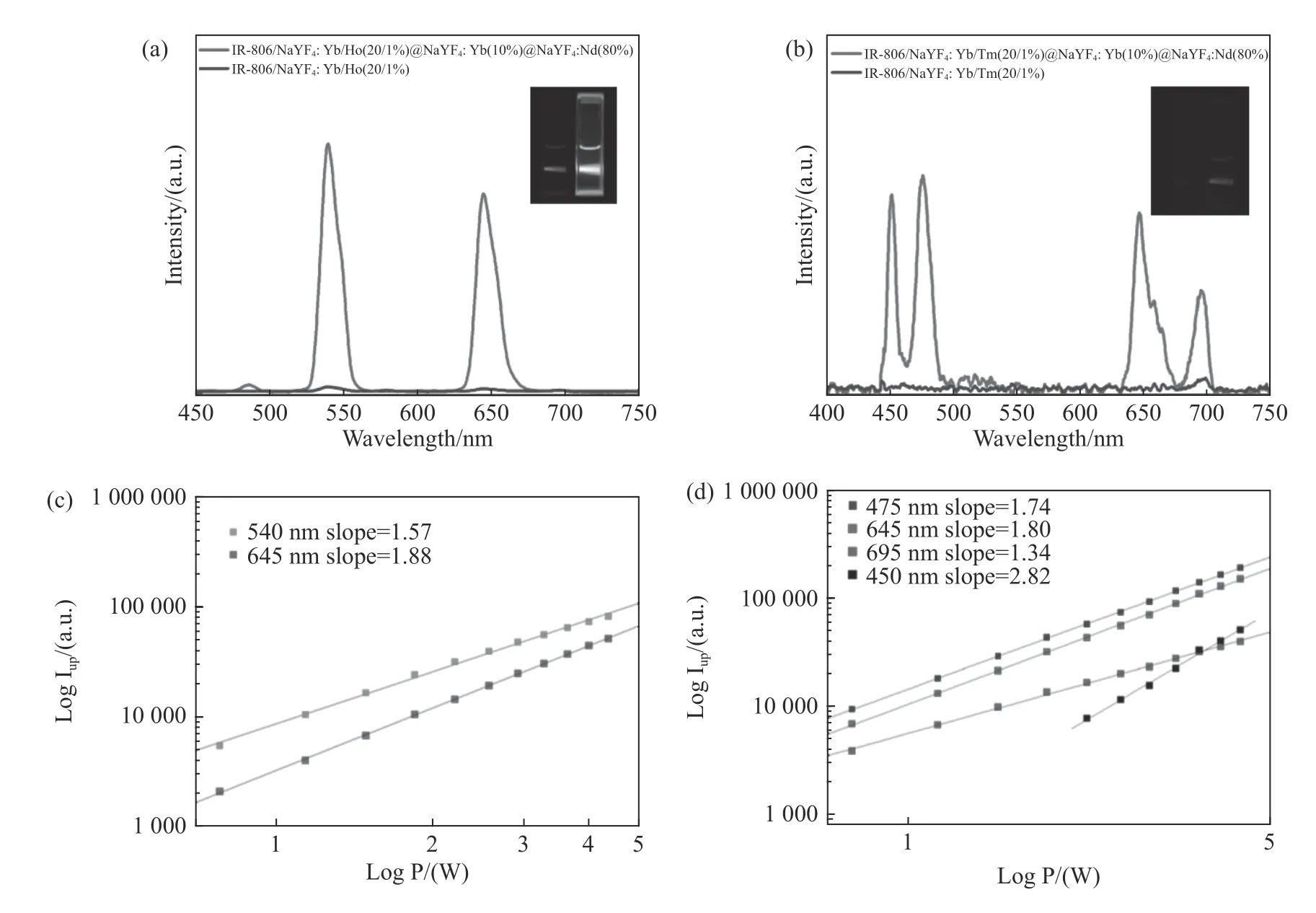
Fig.6 (a)The UCL of the IR-806 sensitized Ho core nanostructure and IR-806 sensitized Ho-CSS nanostructure,(b)the UCL of the IR-806 sensitized Tm core nanostructure and IR-806 sensitized Tm-CSS nanostructure,(c)log-log plots of the UCL intensity over laser power for the green and red emissions of the dye-sensitized Ho-CSS under 808 nm excitation,(d)log-log plots of the UCL intensity versus laser power for the green and red emissions of the dye-sensitized Tm-CSS under 808 nm excitation圖6 (a)IR-806 敏化Ho 核結構及IR-806 敏化Ho-CSS 結構的上轉換光譜,(b)IR-806 敏化Tm 核結構及IR-806 敏化Tm-CSS 結構的上轉換光譜,(c)808 nm 激發下的IR-806 敏化的Ho-CSS 結構的上轉換發光強度隨功率變化的loglog 關系,(d)808 nm 激發下的IR-806 敏化的Tm-CSS 結構的上轉換發光強度隨功率變化的log-log 關系
4 Conclusion
Highly uniform NaYF4:Yb/Er(20/2%)@NaYF4:Yb(10%)@NaYF4:Nd(80%)up-conversion nanoparticles were successfully prepared.Their up-conversion luminescence intensity was about 38 times stronger than that of dye-sensitized NaYF4:Yb/Er(20/2%)core nanostructure.Further studies showed that there were two reasons for this enhancement.On the one hand,the heavy overlap between the Nd absorption in the outmost layer and the emission of dye IR-806 molecules led to the effective absorption of the excitation energy of dye.On the other hand,due to the protective effect of nanoshell layer on the luminescence center,the luminescence life of this structure was 1.73 times longer than that of dye-sensitized core nanostructure.By changing the doping concentration of Yb3+ions in the outermost layer,we demonstrated that the dye-sensitized up-conversion luminescence would be weakened by the doping of Yb3+ions,and could become the strongest without the doping of Yb3+ions.Furthermore,this dye-sensitized CSS structure has realized the enhancement of dye-sensitized up-conversion luminescence intensity when the luminescence center is Ho or Tm.
——中文對照版——
1 引言
稀土離子由于其具有4f 電子組態內及4f 到5d 之間的電子躍遷的特異性,導致其可產生從紫外、可見光區到紅外光區的多種波長的光子輻射[1-2]。尤其是,稀土離子摻雜的上轉換納米粒子(UCNPs)具備將兩個及兩個以上的低能量近紅外光子轉換為一個高能量光子的特性,其產生的光子發射具有譜帶窄、抗漂白、背景噪聲低等優點[3]。這種近紅外光激發的上轉換發光特性將產生諸多應用,如超分辨成像、熒光標記、光動力治療、光學防偽等[4-8]。
盡管稀土離子摻雜產生的上轉換發光有諸多應用,但其相對較低的發光強度限制了其進一步實際應用。如何增強上轉換發光一直是上轉換發光研究中亟待解決的問題。近年來,如核殼納米結構[9]、等離子體場增強發光[10]、染料敏化發光[11],實現了上轉換發光強度的增強。特別是,染料敏化發光的研究不僅增強了上轉換發光的強度,也拓寬了上轉換發光的激發范圍。相對于吸收弱的稀土離子(吸收系數為0.1~10 M?1cm?1),改用近紅外染料(吸收系數為1 000~10 000 M?1cm?1)來吸收近紅外光可實現敏化增強上轉換發光。然而,大部分近紅外染料發射波長(800~900 nm)和經典的Yb 敏化摻雜體系的Yb 的吸收波長(950~1 000 nm)的交疊積分較小,從而限制了染料敏化上轉換發光的增強。進一步,設計采用Nd 和Er離子敏化體系作為染料敏化的受主[12-13],實現更有效的染料敏化上轉換發光的增強。Nd3+敏化上轉換發光是近些年發展的上轉換發光體系,尤其是其分區摻雜策略可以實現高效的上轉換發光[14-16]。目前為止,染料敏化的Nd 離子摻雜體系通常采用的是Nd 敏化上轉換發光的最佳結構設計。考慮到染料敏化過程中染料會與稀土發光體系相互作用從而減弱發光[17],因此,設計優化染料敏化增強的Nd 摻雜的上轉換發光體系,將能更有效地應用于生化分析、腫瘤診療、發光顯示等領域[18-22]。
本文設計采用Nd 敏化的核/殼/殼結構作為增強染料敏化上轉換發光的受主,通過采用高溫熱分解方法成功制備Nd 敏化的核/殼/殼結構,并與染料IR-806 分子耦連實現染料敏化上轉換發光強度的增強。相關的結構表征證實納米結構的有效性。進一步通過發射光譜,熒光壽命光譜分析等研究了其背后的增強機制。通過優化最外層殼中Yb 離子的摻雜濃度,證實無Yb 摻雜情況下染料敏化上轉換發光最強。
2 實 驗
2.1 實驗原料
氯化鐿(YbCl3·6H2O(99.99%)、氯化釔(YCl3·6H2O)、氯化鉺(ErCl3·6H2O)、氫氧化鈉(NaOH,98%)、氟化銨(NHF4,99.99%)、IR-780 iodide(99%)、4-巰基苯甲酸(99%)、1-十八烯(ODE)、油胺(90%)(OM)和油酸(90%)(OA),購于Sigma-Aldrich 公司。根據參考文獻[14],Nd(CF3COO)3通過將Nd2O3粉末與過量的三氟醋酸反應,然后蒸發除去過量的三氟醋酸獲得。三氟醋酸鐿(Yb(CF3COO)3)、三氟醋酸釔(Y(CF3COO)3)、三氟醋酸鈉(CF3COONa)購于GFS Chemicals。二氯甲烷及三氯甲烷、二甲基甲酰胺(DMF)購買于北京化工廠。所有的化學試劑都是分析純度。
2.2 合成上轉換納米粒子
該核殼殼層上轉換納米結構基于已發表的化學方法制備[14-15]。首先,合成核結構,將YbCl3·6H2O(0.1 mmol),YCl3·6H2O(0.39 mmol)和ErCl3·6H2O(0.01 mmol)溶解在3 mL OA,7.5 mL ODE 的三口瓶中,并加熱到150℃,保持30 min,之后在氬氣保護下冷卻至室溫。然后,配置NH4F(2 mmol),NaOH(1.25 mmol),使其溶解在5 mL 甲醇中,并加入到以上稀土鹽的三口瓶中,加到70℃去除甲醇,再加熱到300℃并保持1 h。然后,加入0.25 mmol NaYF4:Yb(10%)活性殼在ODE 中(通過三氟醋酸鹽法合成),加熱并熟化10 min。然后加入0.5 mmol NaYF4:Nd(20%)活性殼(通過三氟醋酸鹽法合成),并熟化10 min。最后,使溶液冷卻到室溫,并使用乙醇離心,將上述混合物溶解在6 mL 三氯甲烷中。不同稀土元素摻雜的核殼殼層上轉換納米結構均采用類似方法合成。
2.3 合成IR-780 分子
根據文獻[11]方法,類似地,在氮氣保護下,將有機IR-780 分 子(250 mg),4-巰基苯甲酸(115.5 mg),和DMF(10 mL)溶解在50 mL 三口瓶中,使混合溶液在氮氣環境下維持17 h。產物溶液用0.45μm PTFE過濾后,減壓蒸餾去除DMF。然后,將殘余物溶解在5 mL 二氯甲烷中,再次通過0.45μm PTFE過濾,并用冰乙醚實施沉淀。最后,將反應物在真空下過濾、干燥并避光保存。
2.4 合成IR-806 分子
采用類似于文獻[8,17]的方法,將1 mL IR-806(xmg/mL,x為0~20 mg/mL)溶解在三氯甲烷中,并與1 mLβ-NaYF4:Yb/Er(20/2%)@NaYF4:Yb(10%)@NaYF4:Nd(20%)納米粒子混合,其中(Er3+~1.67 mM)。將得到的反應混合物攪拌24 h在室溫下離心,重新分散在1 mL 三氯甲烷中。對IR-806敏化的UCNPs 進行上轉換光譜測試時,在稀土納米粒子中的Er3+離子濃度約為16μM的條件下進行。
2.5 實驗表征測試
透射電鏡(TEM)測試:采用Tecnai G2 F20 S-Twin電子顯微鏡在200 kV 電壓下測試。X-ray衍射(XRD)測試通過Rigaku D/max-2000 完成,衍射半徑采用Cu Kαradiation(λ=0.154 1 nm)。吸收光譜利用Maya 2000光譜儀完成測試(Ocean optics)。利用外在耦合的808 nm激光在海洋光學光譜儀(Maya2000)記錄上轉換光譜。通過Hitachi,S-4800 表征能譜(EDS)。上轉換發光的熒光壽命測試采用500 MHz TDS 3052作為激發光源,通過OPO(Sunlite 8000)及示波器獲得熒光壽命數據。
3 實驗結果與討論
3.1 納米粒子形貌及表征
采用高溫熱分解法制備高度均勻的Nd3+敏化核/殼/殼上轉換NaYF4:Yb/Er(20/2%)@NaYF4:Yb(10%)@NaYF4:Nd(80%)納米核結構。透射電鏡照片顯示上轉換納米粒子的尺寸均勻。圖1 為上轉換納米粒子的核、核/殼、核/殼/殼電鏡表征圖及尺寸分布圖。由平均粒徑大小統計結果可知,核(NaYF4:Yb/Er(20/2%),記 為C),核/殼(NaYF4:Yb/Er(20/2%)@NaYF4:Yb(10%),記 為CS),核/殼/殼(NaYF4:Yb/Er(20/2%)@NaYF4:Yb(10%)@NaYF4:Nd(80%)),記為CSS)的平均尺寸分別為23.5、26.3、33.6 nm。這證實結構存在約1.4 nm 的Yb 過渡層,3.7 nm 的Nd 敏化納米殼層逐步生長在NaYF4:Yb/Er 納米核上面。圖2(a)為上轉換納米粒子的核、核/殼、核/殼/殼XRD圖,顯示合成的UCNPs 為經典的六角相結構(JCPDS-16-0334),EDS 證實Nd、Y、Yb 等稀土元素有效地摻雜進納米粒子內(圖2(b))。進一步,根據文獻的方法(圖2(c)),合成了IR-806 分子,由吸收圖(圖2(d))可見,其吸收峰位從780 nm移動到806 nm,結果證實成功合成了IR-806 分子。進一步,根據前面的方法將IR-806 分子修飾到上轉換納米粒子表面。由圖2(d)可知,當IR-806 分子修飾到UCNPs 上之后,其吸收峰位被IR-806 的吸收所掩蓋,從而證實染料分子成功修飾到UCNPs 上。
3.2 染料敏化增強機制證實與討論
相對于最早報道的經典的IR-806 敏化NaYF4:Yb/Er(20/2%)納米粒子[11],本文設計的染料敏化CSS 結構的上轉換發光強度(圖3(a))增強了約38 倍。證實本文設計的染料敏化結構實現了上轉換發光強度的增強。另外,在近紅外(808 nm)光激發下,染料敏化CSS 結構的上轉換紅光發射與綠光發射均表現出發光強度隨激發光功率的非線性依賴特性(圖3(b)),其多光子指數分別為1.67(綠 光540 nm發 射4S13/2→4I15/2),2.0(紅光655 nm 發射4F9/2→4I15/2),證實其發光特性為非線性的上轉換發光。
本文設計的染料敏化CSS 結構的上轉換發光強度相對于染料敏化的核納米粒子增強約38 倍。對于這種增強,分析認為:一方面,在CSS 納米結構中最外層為Nd3+離子摻雜殼層,而由圖4(a)(彩圖見期刊電子版)可知,Nd 的吸收與IR-806 染料分子的發射有大量交疊,因而,能夠被IR-806 分子高效敏化;另一方面,CSS 結構中的納米殼層可以有效保護發光中心。由圖4(b)(彩圖見期刊電子版)可見,經染料敏化的具有CSS 結構的Er 的發光壽命(253μs)明顯長于染料敏化核納米粒子(146μs)及核納米粒子(169μs)Er 的壽命,發光壽命值分別延長了1.73 倍及1.50倍。證實了納米殼層隔絕了發光中心與外部環境干擾,使得發光中心產生長的發光壽命。值得注意的是,盡管所設計的染料敏化Nd3+離子摻雜納米結構較佳的激發波長在800 nm 附近,但是在研究發光壽命時,NaYF4:Yb/Er(20/2%)納米粒子只能在980 nm 波長處激發。
傳統(20/2%)核納米粒子只能被980 nm 光激發產生上轉換發光,因此,圖4(b)的測試激發波長選擇為980 nm 而非通常采用的808 nm。由圖4(c)可知,對于CSS 納米結構,無論是否連接染料分子,其發光壽命均保持不變。進一步證實納米殼層有效地阻隔了發光中心與外界環境的相互作用,從而增強了上轉換發光。
本文Nd3+敏化體系的不同之處在于,其最外層的納米殼層中僅摻雜了Nd3+,而沒有像以往文獻報道的將Nd-Yb 共摻雜到納米殼層中[16]。這種結構設計是根據實驗結果所得。本文所設計的釹敏化多層殼納米結構的上轉換光譜如圖5 所示。可見,隨著最外層Yb3+摻雜濃度的增加,染料敏化CSS 結構的上轉換發光強度反而逐漸減弱。根據前面的研究可知,染料敏化稀土上轉換納米體系中,染料吸收的激發能需要逐步傳遞到內部的發光中心[17],而激發能傳遞過程中,能量損耗非常大,摻雜Yb3+離子極易將激發能傳遞到表面[9,23-24],從而使傳遞到內部的激發能降低,最終導致上轉換發光降低。由此可知,最外層的納米殼層在不摻雜Yb3+離子的情況下,產生的染料敏化上轉換發光最強。
3.3 發光中心為Ho 及Tm 時染料敏化發光增強
進一步,對于CSS 結構,將核內的發光中心換 為 Ho(NaYF4:Yb/Ho(20/1%))@NaYF4:Yb(10%)@NaYF4:Nd(80%))或Tm(NaYF4:Yb/Tm(20/1%)@NaYF4:Yb(10%)@NaYF4:Nd(80%)),同樣實現了染料敏化上轉換發光的增強(圖6(a)和6(b),彩圖見期刊電子版)。對于發光中心為Ho 及Tm,發光強度同樣隨激發光功率呈非線性的依賴關系(圖6(c)和6(d),彩圖見期刊電子版)。對于發光中心為Ho 的情況,其多光子指數分別為1.57(540 nm發 射4S13/2→4I15/2),1.88(645 nm 發射4F9/2→4I15/2)。對于發光中心為Tm的情況,其多光子指數分別為2.82(450 nm 發射1D2→3F4)、1.74(470 nm 發射1G4→3H6)、1.80(645 nm發射1G4→3F4)、1.34(695 nm發射3F2→3H6)。值得注意的是,對于Tm 離子,NaYF4:Yb/Tm(20/1%)納米核結構,幾乎沒有染料敏化的上轉換發光出現。這是由于Tm 的800 nm 發射能級(3H4→3H6)與IR-806 分子的吸收交疊嚴重,從而猝滅了Tm的發光。而CSS 結構使得外面的殼層成功地阻隔了Tm 向IR-806 傳遞,從而實現了染料敏化上轉換發光。
4 結論
本文成功制備了高度均勻的NaYF4:Yb/Er(20/2%)@NaYF4:Yb(10%)@NaYF4:Nd(80%)上轉換納米粒子,其染料敏化上轉換發光強度相對于染料敏化的NaYF4:Yb/Er(20/2%)核納米結構增強了約38 倍。進一步研究表明,這種增強一方面源自最外層Nd 吸收與染料IR-806 分子的發射交疊大,導致其能有效吸收染料的激發能。另一方面源自納米殼層對發光中心的保護作用,相對于染料敏化的核納米結構,其發光壽命延長了1.73 倍。通過改變最外層Yb3+的摻雜濃度,證實摻雜Yb3+將導致染料敏化上轉換發光減弱,而無摻雜Yb3+的條件下上轉換發光最強。最終,采用這種染料敏化的CSS 結構實現了發光中心為Ho 及Tm 的染料敏化上轉換發光強度的增強。
- 中國光學的其它文章
- Line-scanning confocal microscopic imaging based on virtual structured modulation
- Polarization changes of partially-coherent Airy-Gaussian beams in a slanted turbulent atmosphere
- Hybrid plasmonic leaky-mode lasing on subwavelength scale
- 10?9 量級高靈敏度點源透射比測試設備研究
- 大孔徑靜態干涉成像光譜儀徑向畸變導致的譜線偏移誤差的校正
- 表面損傷衍射雙向反射分布函數模型建立及分析

