我國地表水中典型DBPs的暴露水平及生態風險
羅 瑩,劉 娜,孫善偉,侯 嵩,郭昌勝*,徐 建
我國地表水中典型DBPs的暴露水平及生態風險
羅 瑩1,2,3,劉 娜3,孫善偉1,侯 嵩1,郭昌勝1,3*,徐 建1,3
(1.中國環境科學研究院,國家環境保護化學品生態效應與風險評估重點實驗室,北京 100012;2.北京師范大學水科學研究院,北京 100875;3.中國環境科學研究院,環境基準與風險評估國家重點實驗室,北京 100012)
氯消毒過程中可能生成有毒有害的副產物,會對水生態系統和環境健康產生直接和間接的次生危害.三鹵甲烷(THMs)和鹵乙酸(HAAs)是地表水中檢出率最高的消毒副產物(DBPs),其毒性效應受到廣泛關注.本文檢索整理了三氯甲烷(TCM)、三溴甲烷(TBM)、二氯乙酸(DCAA)和三氯乙酸(TCAA)在我國地表水暴露濃度和對水生生物的毒性效應濃度,了解我國重點流域地表水環境中TCM、TBM、DCAA和TCAA的濃度水平,分別利用急、慢性毒性數據推導預測無效應濃度(PNEC),并使用風險商(RQ)和概率生態風險評價法(PERA)對我國重點流域水環境中TCM、TBM、DCAA和TCAA進行多層次生態風險評估.結果表明,我國地表水環境中TCM、TBM、DCAA和TCAA暴露濃度范圍為n.d.~51μg/L.以致死和生長、繁殖等為測試終點的急、慢性毒性數據,構建物種敏感度分布(SSD)曲線,推導出TCM、TBM、DCAA和TCAA的PNEC值分別為0.586,0.857,0和44.880mg/L; 0.006,0.064,0.956和0.012mg/L.基于急、慢性毒性數據計算出的RQ小于1.我國重點流域中TCM和TCAA對1%的水生生物造成生長、繁殖等慢性毒性影響的概率分別為78.86%和20.61%,存在潛在的生態風險.
消毒副產物;地表水;生態風險評估
目前我國新冠肺炎疫情形勢依然嚴峻,為防止傳染病毒快速傳播,含氯消毒劑大量使用,消毒劑在阻隔緩解病毒擴散的同時,也產生大量含氯消毒副產物,通過醫療廢水和生活污水等途徑由城鎮排水系統進入河流水體.同時,為保證公眾飲用水安全,氯化消毒通常也是國內外集中式供水最主要的消毒方式.但氯消毒劑會與水中的天然有機物反應,生成對人體和水生生物有害的消毒副產物(DBPs)[1],對人體健康和水生態系統產生直接和間接的次生危害.DBPs種類繁多[2],主要包括三鹵甲烷(THMs)、鹵乙酸(HAAs)、鹵乙腈、鹵代酚、鹵代酮等物質[3],其中THMs和HAAs含量較高,并且已納入飲用水監管體系[2].近年來,THMs和HAAs在地表水中均有檢出,其濃度達到μg/L的水平[3-13].
20世紀70年代,國內外陸續對THMs和HAAs展開毒性研究,結果表明,兩者具有“三致效應”[14-17].同時,相關研究證實,THMs和HAAs對水生生物具有死亡[18-40]、發育[18,32,41-43]和繁殖效應[27,44],評估THMs和HAAs存在的潛在生態風險對保護水生生物至關重要.
生態風險評估是預測環境中污染物對生態系統或其中一部分產生有害影響可能性的過程[45],主要將污染物暴露濃度與效應濃度進行比較.暴露濃度通常指實測或預測的環境中化合物的濃度,效應濃度指化合物對生物造成不良效應的濃度,通常由預測無效應濃度(PNEC)來表示.風險商(RQ)和概率生態風險評價法(PERAs)是風險評估中常用的方法. RQ是污染物暴露濃度與預測無效應濃度PNEC的比值[46].但RQ的不確定性較大,因而適用于低水平或多層次中較低層次的風險評估.為了得到更確切的風險概率,一般采用PERAs作為多層次風險評估中較高層次的評估方法[47].為了獲得更準確的風險結果,一些學者和研究機構提出連續應用低層次到高層次的風險評價方法,即將RQ和PERAs進行綜合,充分利用各種方法進行從簡單到復雜的風險評價[47].本文通過文獻檢索和資料收集,獲取THMs和HAAs在我國地表水環境中的暴露濃度和毒性效應濃度,對我國地表水中THMs和HAAs進行全面的多層次生態風險評估,為THMs和HAAs的環境管理提供理論依據和科學支撐.
1 材料與方法
1.1 THMs和HAAs環境暴露濃度
THMs和HAAs是水環境中最主要的DBPs[8]. THMs包括三氯甲烷(TCM)、一溴二氯甲烷(BDCM)、一氯二溴甲烷(DBCM)和三溴甲烷(TBM)[48];HAAs包括一氯乙酸(MCAA)、二氯乙酸(DCAA)、三氯乙酸(TCAA)、一溴乙酸(MBAA)和二溴乙酸(DBAA)[48].通過檢索國內外期刊文獻、碩博學位論文等,獲取我國重點流域及其水源地THMs和HAAs的暴露濃度數據,以全面評估THMs和HAAs在我國地表水環境中的暴露水平.結果表明,THMs和HAAs在水源水[3,6-8,10-12]和地表水[4-5,9,13]中均有檢出.低于方法檢出限的同一流域中不同檢測點位的濃度數據按最低檢出限的50%計算.使用IBM SPSS Statistics 24的Kolmogorov-Smirnov檢驗對THMs和HAAs在我國地表水中的濃度分布數據進行正態分布檢驗.
1.2 THMs和HAAs毒性效應評估
THMs和HAAs的水生生物毒性數據來自毒理數據庫(如美國的ECOTOX數據庫,http://cfpub.epa. gov/ecotox/)、已發表論文及期刊文獻[47,49].數據篩選遵循相關性、可靠性、精確性的原則[50].根據暴露時間和測試終點將THMs和HAAs水生生物毒性數據分為:急性毒性(測試終點為致死)和慢性毒性,由于現有研究中THMs和HAAs的慢性毒性數據較少,因而將生長、繁殖、生物化學與分子生物學及其他行為學等慢性毒性測試終點合并分析.根據水生生物的測試終點,以半致死濃度(LC50)或半效應濃度(EC50)為測試終點的毒性數值作為急性毒性數據,以無觀察效應濃度(NOEC)為測試終點的毒性數值作為慢性毒性數據.當NOEC數據不足時,采用最大可接受濃度、最低可觀察效應濃度或EC50替代[47].當一個物種同一測試終點具有多個數值時,使用幾何平均值;同一個生物有不同測試終點時,選擇最敏感測試終點的毒性數據[51].
通過查閱文獻和毒理數據庫收集到THMs和HAAs的水生生物毒性數據,滿足使用物種敏感度分布曲線(SSD)推導5%物種受到危害時的濃度(HC5)的要求,采用SSD推導HC5,以不同生物毒性數據的對數濃度值為橫坐標,以累計概率為縱坐標作圖.其中累計概率是將所有已篩選物種的最終毒性值按從小到大的順序進行排列,并且給其分配等級,最小的最終毒性值等級為1,最大的最終毒性值等級為,依次排列,計算公式為[52]:
/1(1)
式中:為累計概率,%;為物種排序的等級;為物種的個數.
如果有2個或者2個以上物種的毒性值是相等的,則將其任意排成連續的等級,計算每個物種的最終毒性值的累計概率.該研究采用荷蘭國家公共衛生與環境研究院(RIVM)開發的ETX 2.0推導基于50%置信度的HC5[47].
由于非本地物種數據、物種種類以及野外實際暴露等會對PNEC產生影響,最終PNEC值為:
PNEC=HC5/AF(2)
式中:HC5為5%物種受到危害時的化合物濃度, mg/L;AF取5[53].
1.3 消毒副產物的生態風險評價
本文采用多層次生態風險評估法對我國包括水源在內的地表水中的THMs和HAAs進行生態風險評估.首先,采用風險商進行評估,見下式:
RQ=MEC/PENC(3)
式中:MEC為實測環境濃度, μg/L;PNEC為預測無效應濃度,μg/L.
當RQ<0.1,該化合物對水生生物的風險可忽略;當0.1£RQ<1,該化合物對水生生物的風險較低;當1£RQ<10,該化合物對水生生物為中等風險;當RQ310,該化合物對水生生物的風險高[47].
其次,采用聯合概率分布曲線法(JPCs)作為概率生態風險評價對THMs和HAAs進行高層次風險評價.聯合概率分布曲線法(JPCs)可以計算出某一特定百分比物種引起不利影響的濃度在地表水中出現的概率,反映各毒性損傷水平下暴露濃度超出相應臨界濃度值的概率,體現暴露情況和暴露風險之間的關系[46-47].
2 結果與討論
2.1 暴露水平
我國地表水中THMs的濃度范圍為0~51μg/L; HAAs的濃度范圍為n.d~12.61μg/L(表1),其中, TCM、TBM、DCAA和TCAA分別是THMs和HAAs中檢出率較高化合物,濃度范圍分別為0~51,0.22~ 1.46,0.01~2.56和n.d.~12.61μg/L.從THMs和HAAs來看,除大連市地表水中DCAA和TCAA的平均濃度(6μg/L)外,水體中THMs的平均濃度(0.14~2.8μg/ L)高于HAAs的平均濃度(0.01~3.37μg/L),其中,THMs中TCM的最高濃度(51μg/L)高于HAAs中DCAA的最高濃度(12.61μg/L).同區域不同種類的消毒副產物,TCM的平均濃度高于DCAA和TCAA的平均濃度水平.由表1可知,THMs中主要的消毒副產物為TCM,其平均濃度的范圍為0.33~ 2.80μg/L,遠高于其余3種化合物;HAAs中, DCAA (0.01~6μg/L)和TCAA(0.12~1.30μg/L)是主要的化合物.
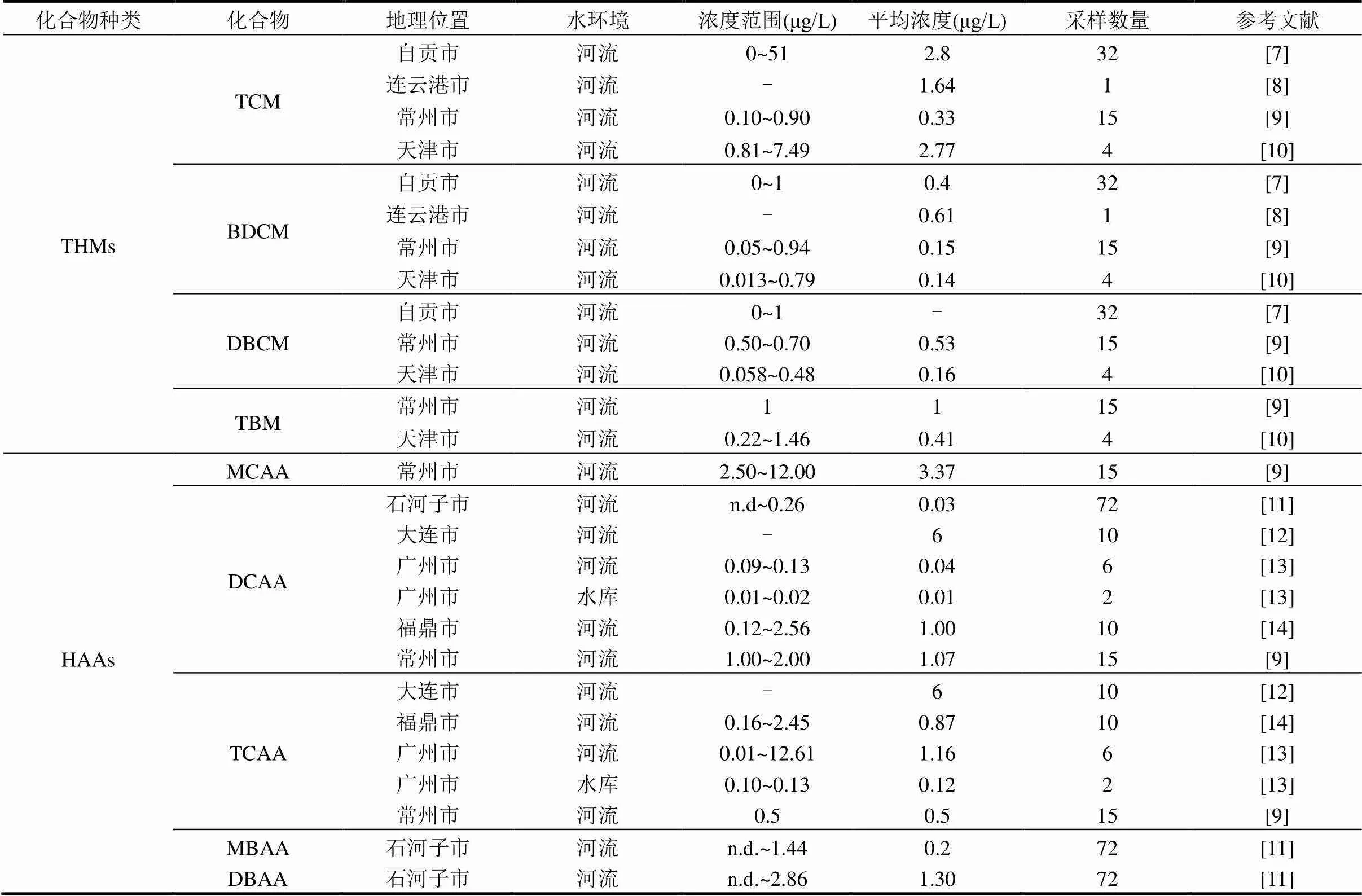
表1 我國地表水中THMs和HAAs污染水平
注:“-”表示數據不可獲得.
2.2 不同測試終點PNEC值的推導
本文通過文獻和毒理數據庫收集TCM、TBM、DCAA和TCAA的急性和慢性毒性數據(表2). 基于致死為測試終點,本文收集了大量脊椎和無脊椎水生動物以及藻類的急性毒性數據, TCM,TBM, DCAA和TCAA的急性毒性數值范圍分別為2~7771.18, 2.90~75, 23~4060和1.2~9300mg/L,均值分別為341.77,29.10,1468.20和3576.45mg/L.從THMs和HAAs兩類主要DBPs來看,THMs對水生生物的急性毒性遠高于HAAs,因而THMs水生生物的生命具有較高的威脅.從不同化合物來看,TBM對水生生物的致死效應濃度最高,TCAA水生生物的致死效應濃度最低,TBM對水生生物的生存影響可能高于其他3種DBPs,地表水環境中TBM對水生生物的生存存在一定影響.基于生長、繁殖和分子生物學等為測試終點,本文收集了脊椎和無脊椎水生動物以及藻類的慢性毒性數據,TCM,TBM,DCAA和TCAA的毒性濃度范圍分別為0.1~185, 0.24~38.6, 0.0002~1485和0.01~1740mg/L,均值分別為36.07, 13.45, 235.24和512.39mg/L.從THMs和HAAs 2類主要DBPs來看,THMs對水生生物的慢性毒性高于HAAs,地表水環境中THMs對水生生物的慢性毒性影響大于HAAs.從不同化合物來看,TBM對水生生物的生長、繁殖和分子生物學等慢性毒性效應濃度最高,TBM對水生生物的生長和繁殖等毒性影響可能高于其他3種DBPs.
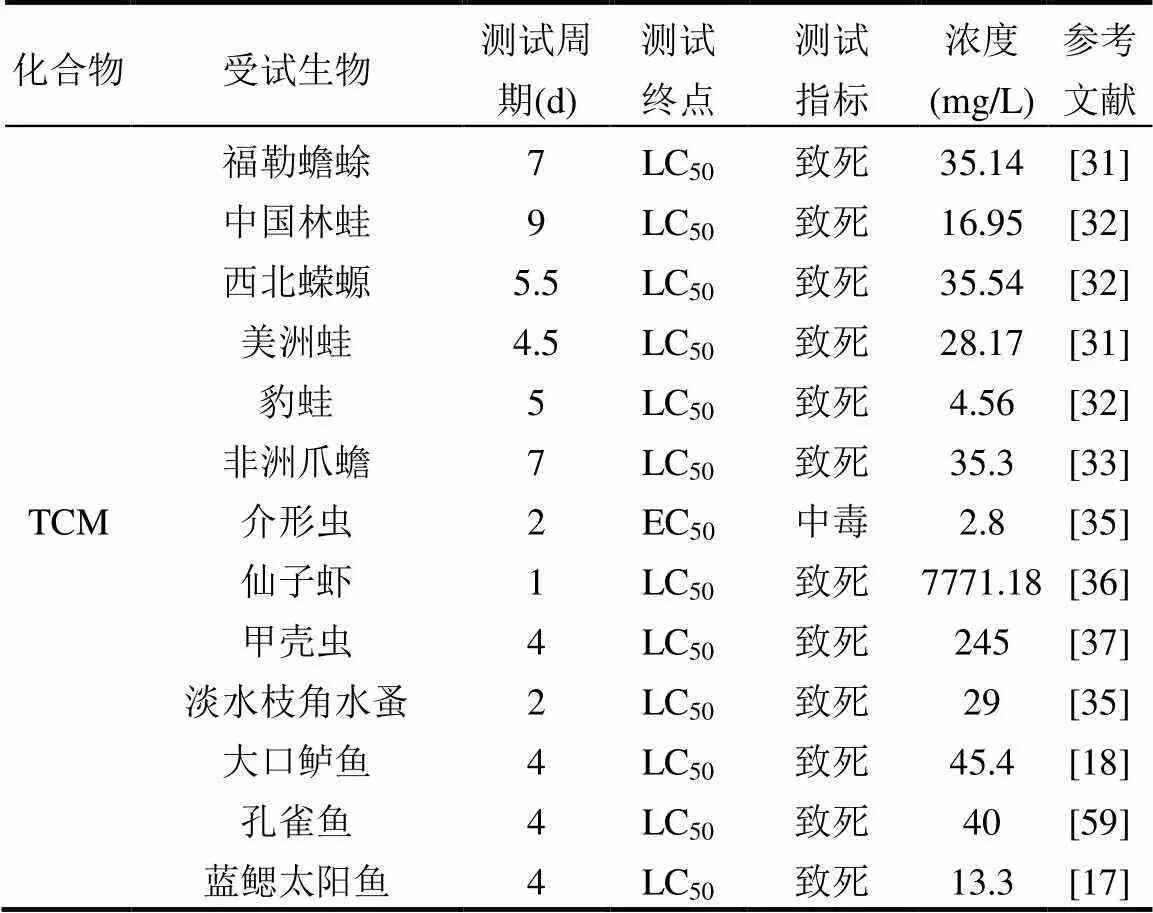
表2 THMs和HAAs對水生生物的毒性數值
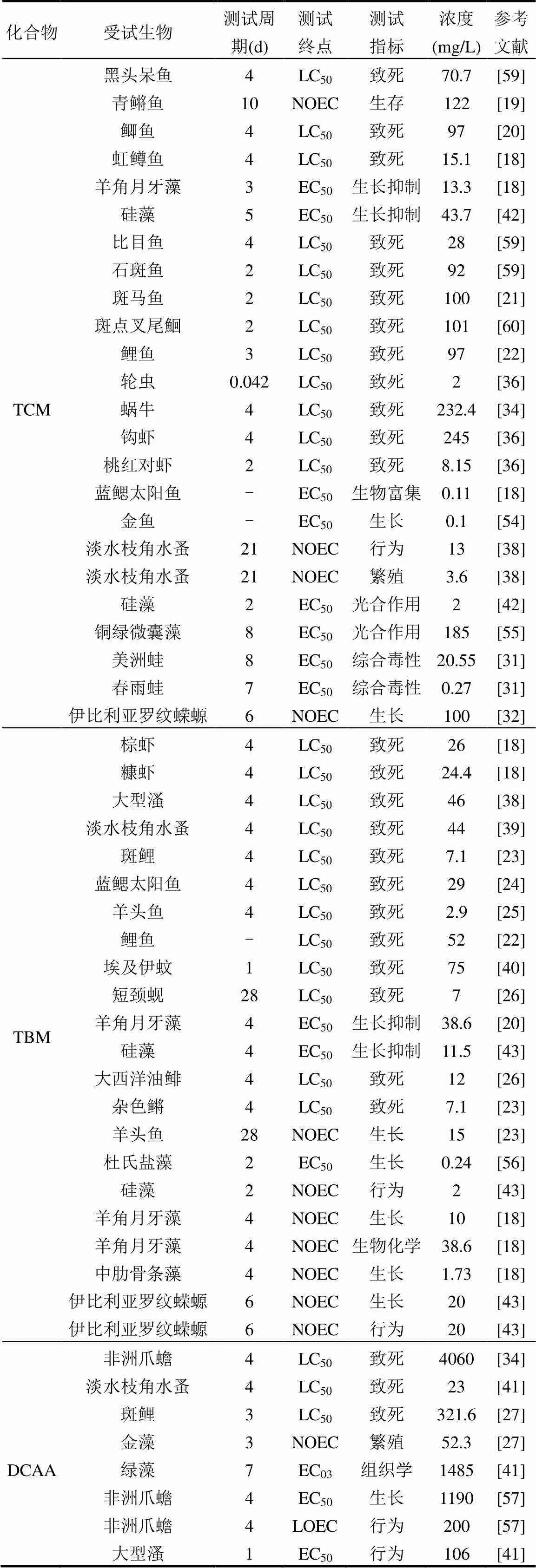
續表2
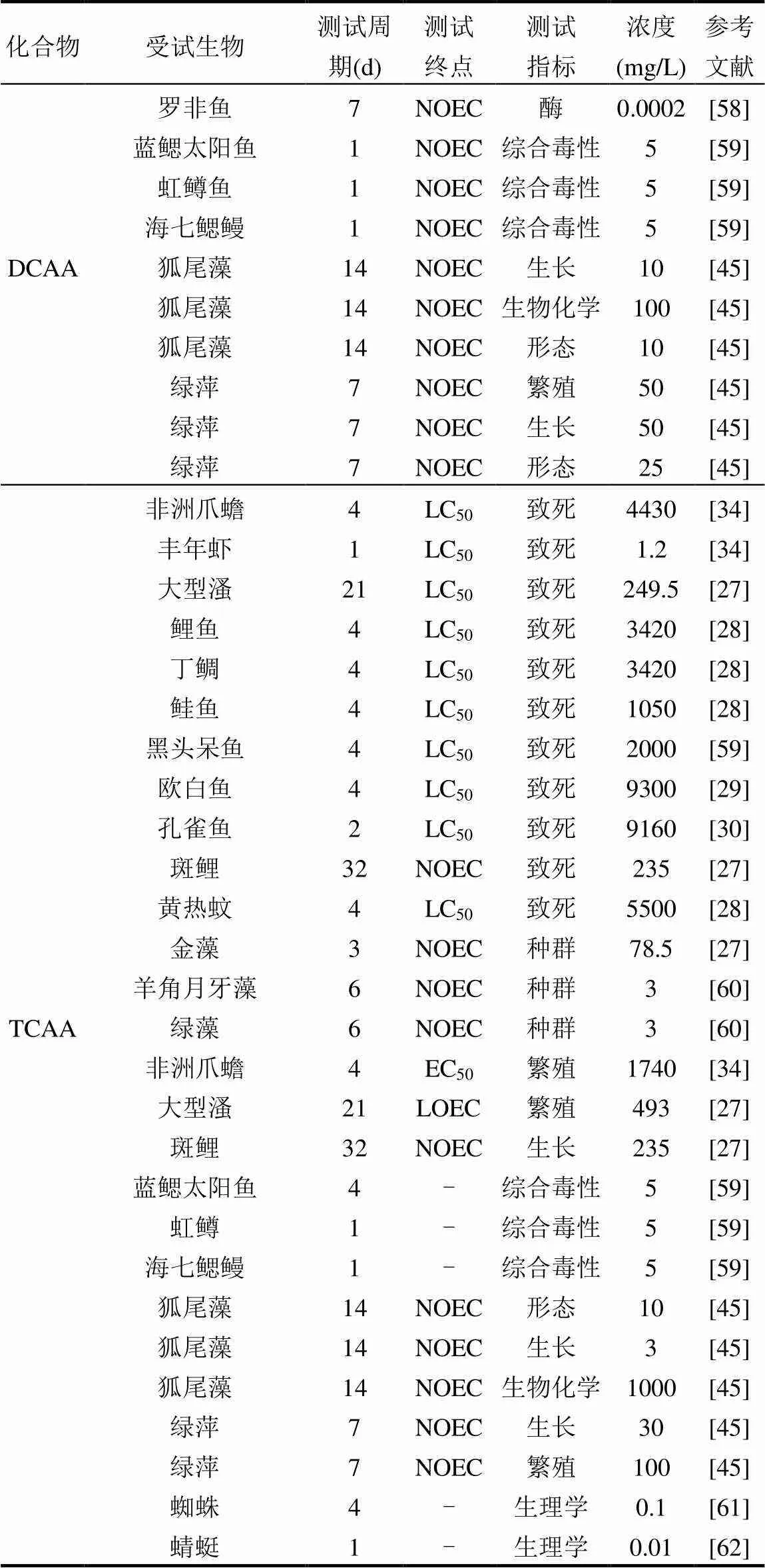
續表2
注:“-”表示信息不可獲得.
基于對TCM、TBM、DCAA和TCAA各類水生生物急慢性毒性數據進行統計分析,獲得相關參數(表3).通過Kolmogorov-Smirnov檢驗,數據均符合對數正態分布(>0.05).采用SSD曲線推導TCM、TBM、DCAA和TCAA的HC5.基于TCM的急性和慢性毒性數據推導的HC5分別是2.932和0.029mg/L,推導出的PNEC值分別為0.586和0.006mg/L;基于TBM的急性和慢性毒性數據推導的HC5分別是4.286和0.320mg/L,推導出的PNEC值分別為0.857mg/L和0.064mg/L;基于DCAA以生長、繁殖等為測試終點的慢性毒性數據推導的HC5是1.912mg/L,推導出的PNEC值為0.956mg/L,由于DCAA的急性毒性數據不滿足構建SSD曲線最低條件,因此選擇最敏感物種的急性毒性數據與AF(AF=1000)[59]相比,計算得到DCAA基于急性毒性數據的PNEC值為23mg/L;基于TCAA的急性和慢性毒性數據推導的HC5分別是224.40和0.061mg/L,推導出的PNEC值分別為44.880和0.012mg/L.綜上所述,TCM、TBM、DCAA和TCAA對水生生物的生長、繁殖等慢性毒性更敏感,其中TCM對水生生物的慢性毒性高于其他化合物,DCAA對水生物的生長、繁殖、生物化學和分子生物學等慢性毒性相對較不敏感.從2類不同DBPs來看,THMs基于急性毒性數據推導出的PNEC值遠低于HAAs,水生生物的生存對水環境中THMs更加敏感,尤其是TCM,這一結果與毒性數據進行均值比較的結果存在一些差異,為獲得更準確的結果,不能僅僅進行簡單的分析,應該使用更科學的統計分析方法,例如SSD[47].從不同化合物來看, TCM對水生生物的生長、繁殖和分子生物學等毒性更加敏感,TCAA次之,同樣的,與利用毒性均值比較時存在一些差異,根據國內外研究人員的結果表明[47,51-52,63-64,66],采用SSD曲線進行毒性效應分析更加科學,準確.
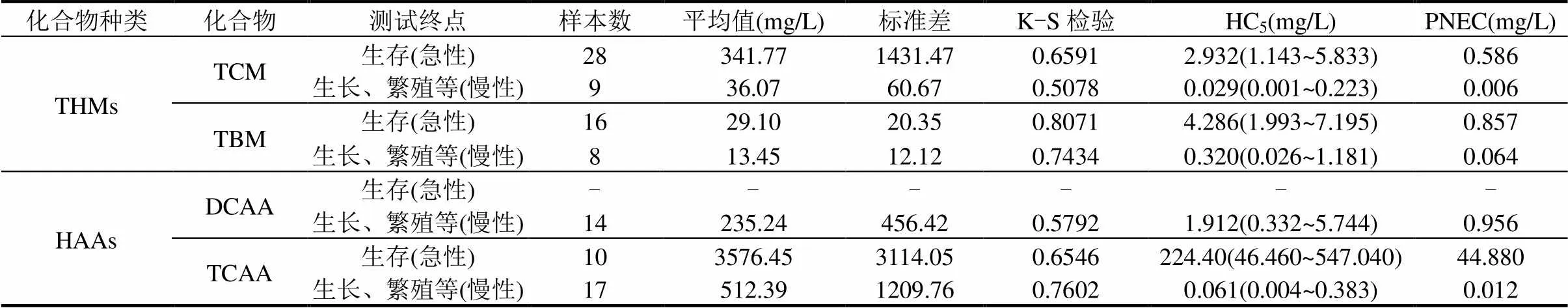
表3 基于不同測試終點構建THMs和HAAs的SSD曲線相關參數
注:“-”表示數據不可獲得.
2.3 我國地表水中THMs和HAAs生態風險評估
該研究采用RQ對我國地表水中的THMs(TCM和TBM)和HAAs(DCAA和TCAA)平均濃度進行風險評估,我國地表水中TCM、TBM、DCAA和TCAA的平均濃度分別與急性毒性數據推導出的PNEC相比,求出RQ值(圖1).
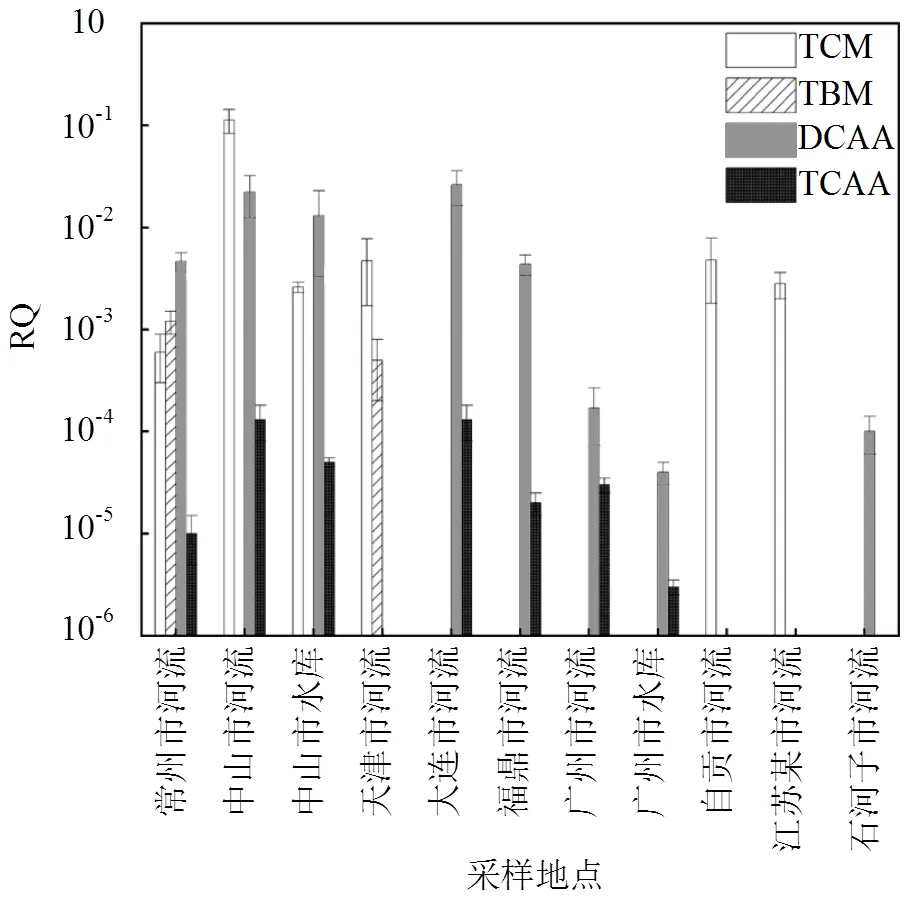
圖1 基于急性毒性計算TCM、TBM、DCAA和TCAA的RQ
由圖1可知,基于TCM、TBM、DCAA和TCAA急性毒性數據計算出的RQ均小于1.從不同流域來看,除中山市河流中TCM的RQ超過0.1,其他流域中基于TCM、TBM、DCAA和TCAA的平均濃度與急性毒性數值計算得出的RQ均小于0.1,表明TCM、TBM、DCAA和TCAA在我國地表水中的生態風險較小,可以忽略.從不同消毒劑副產物的種類來看,我國地表水中THMs基于急性毒性數據計算出的RQ高于HAAs基于急性毒性數據計算出的風險商,中山市河流中TCM的RQ最高(RQ=0.11).此外,DCAA和TCAA在大連市地表水中的RQ最高,分別為0.026和0.00013,表明THMs對我國地表水中水生生物的致死風險高于HAAs.此外,我國地表水中TCM 基于急性毒性數據計算出的RQ高于TBM基于急性毒性數據計算出的RQ;DCAA基于急性毒性數據計算出的RQ高于TCAA的RQ.
基于我國水源中TCM、TBM、DCAA和TCAA的平均濃度與慢性毒性數據推導出的PNEC相比,求出RQ,如圖2所示.

圖2 基于慢性毒性計算TCM、TBM、DCAA和TCAA的RQ
圖2表明,基于TCM、TBM、DCAA和TCAA慢性毒性數據計算出RQ,除中山市河流中TCM的RQ,其余均小于1.從不同化合物來看,我國地表水中THMs(TCM和TBM)和HAAs(DCAA和TCAA)基于慢性毒性數據計算出的RQ排序依次為TCM、TCAA、DCAA和TBM.從THMs和HAAs的空間分布來看,除常州市河流中TCM的RQ外(RQ= 0.056),其余流域中TCM基于慢性毒性數據計算出的RQ均高于0.1,中山市河流中TCM的RQ最高(RQ=1.12).其次,我國地表水中TCAA的RQ較高,范圍為0.002~0.099. TCM基于慢性毒性數據計算出的RQ值高于TBM基于慢性毒性數據計算出的RQ值,TCAA和DCAA基于慢性毒性數據計算出的RQ值呈現相同的規律.綜上,TCM對地表水中的水生生物具有一定的生態風險,且對我國地表水中水生生物的繁殖、生長及生物化學及分子生物學的毒性影響高于TBM、TCAA和DCAA.
TCM和TCAA對我國地表水中的水生生物具有一定的慢性毒性生態風險,因而選擇JPC進行較高層次的生態風險評估.JPC是以所有生物毒性數據的累積函數和污染物暴露濃度的反累積函數作圖,將風險評價的結論通過連續分布曲線的形式表現.聯合概率曲線的軸表示不良效應產生的強度,即水生生物受到影響的百分比;軸表示事件發生的概率;聯合概率曲線上的每一個點表示在目標水體(評價對象)中,一定百分比的生物受到影響(事件)的概率.聯合概率曲線越靠近軸,生物受到影響的可能性越小,則目標水體越安全[63-65].
根據地表水中TCM和TCAA濃度分別與慢性毒性數據建立聯合概率曲線(圖3).結果表明,我國地表水中TCM對1%、5%和10%的水生生物造成繁殖、生長及其他行為學等慢性毒性影響的概率分別為78.86%、3.6%和0.07%;我國地表水中TCAA對1%和5%的水生生物的繁殖、生長及其他行為學等造成慢性毒性影響的概率分別為20.61%和0.01%.綜上所述,我國地表水中TCM濃度對水生生物的風險高于地表水中TCAA濃度對水生生物的風險,TCM對我國地表水中水生生物存在一定的生態風險, TCAA對我國地表水具有潛在生態風險.
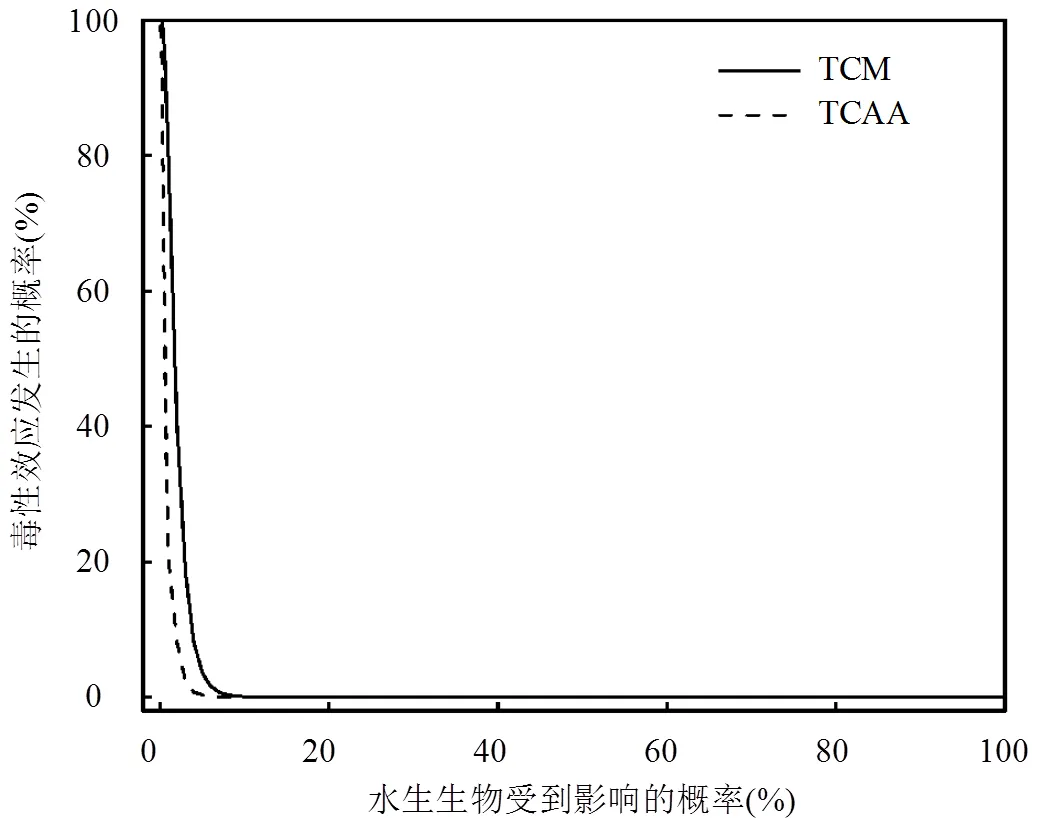
圖3 TCM和TCAA水生態風險聯合概率曲線
2.4 不確定性分析
不確定性在生態風險評估過程中是不可避免的,無論是低層次的生態風險評估還是較高層次的生態風險評估.水體中THMs和HAAs濃度的實際變化,毒性數據與生態的關聯性以及風險表征模型的選擇是生態風險評估過程中產生不確定性的主要因素.
首先,THMs和HAAs在地表水中的暴露濃度數據有限,為了更全面精確地了解THMs和HAAs的暴露情況,需要進一步搜集整理并開展全國范圍地表水中THMs和HAAs暴露濃度的時空變化數據監測.其次,研究表明THMs和HAAs對水生生物的繁殖、生長等慢性毒性更為敏感,該研究中THMs和HAAs的毒性數據均以致死為測試終點的急性毒性數據為主,不能充分反映THMs和HAAs對水生生物的慢性毒性的影響.若考慮對水環境中THMs和HAAs開展更加全面的毒性評估,則需要更多魚類、無脊椎動物等水生生物慢性毒性相關試驗.此外,由于生物具有地域性的特點,收集的所有毒性數據是否具有代表性仍存在爭論[45].Jin等[64]的研究表明當本地物種毒性數據不足時,使用一定的安全系數(AF=2~10)即可將非本土物種的毒性數據納入風險評估的研究.生態風險評估過程中,數據分析模型的選擇影響SSD曲線的構建[66],例如對數正態分布、對數邏輯斯蒂、波爾III模型等.因而,通過數據的隨機性、評估過程和數據分析模型的選擇所產生的誤差,導致風險評估結果具有一定的不確定性.
3 結論
3.1 通過文獻檢索和資料收集,我國地表水中TCM、TBM、DCAA和TCAA的濃度范圍分別為0~51,0.22~1.46,0.01~2.56和n.d.~12.61μg/L.
3.2 根據RQ的評價結果,TCM基于急性和慢性毒性數據計算得出的RQ值大于1,其余THMs和HAAs的RQ值均小于1.利用JPCs得到TCM和TCAA對1%的水生生物產生慢性毒性影響的概率分別為78.86%和20.61%,TCM對我國地表水中水生生物具有一定的生態風險.
3.3 地表水中TCM和TCAA對水生生物的繁殖、發育等具有較低影響,毒性影響不可忽略,其余化合物對地表水中的水生生物具有潛在的影響.
[1] Regli S, Chen J, Messner M, et al. Estimating potential increased bladder cancer risk due to increased bromide concentrations in sources of disinfected drinking waters [J]. Environmental Science& Technology, 2015,49(22):13094-13102.
[2] Richardson S D, Plewa M J, Wagner E D, et al. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: a review and roadmap for research [J]. Mutation Research/Reviews in Mutation Research, 2007,636(1/3):178-242.
[3] 錢洪智.不同飲用水源水氯化消毒副產物的特征與風險評價[D]. 杭州:浙江大學, 2013. Qian H Z. Characteristics and risk assessment of DBPs in different chlorination drinking water source [D]. Hangzhou: Zhejiang University, 2013.
[4] Hao R J, Zhang Y, Li Y, et al. Effect of water chemistry on disinfection by-product formation in the complex surface water system [J]. Chemosphere, 2017,172:384-391.
[5] Du Y, Wu Q Y, Hu H Y, et al. Increase of cytotoxicity during wastewater chlorination: Impact factors and surrogates [J]. Journal of Hazardous Materials, 2017,324:681-690.
[6] 梅玉琴,李 謙,劉天潔,等.自貢市鄉鎮自來水廠氯化消毒副產物調查[J]. 預防醫學情報雜志, 2012,28(11):851-854. Mei Y Q, Li Q, Liu T J, et al. Investigation of chlorinated disinfection by products in township waterworks in Zigong [J]. Journal of Preventive Medical Information, 2012,28(11):851-854.
[7] 劉曉琳.飲用水中THMs、HAAs、含氮類和鹵代酮類消毒副產物檢測識別與風險評估[D]. 上海:復旦大學, 2013. Liu X L.Determination and risk assessment of THMs, HAAs,nitrogen containing DBPs and haloketones in drinking water [D]. Shanghai: FudanUniversity, 2013.
[8] 劉麗穎.南方某地農村集中供水消毒副產物水平研究[D]. 北京:中國疾病預防控制中心, 2012. Liu L Y. Study on disinfection by-product levels in centralized water supplyfrom a southern rural area [D]. Beijing:Chinese Center for Disease Control and Prevention, 2012.
[9] 李肖楠.海河口三鹵甲烷分布和生態風險評價[D]. 天津:天津大學, 2013. Li X N. Distribution of trihalomethanes in Haihe river estuary and ecologicalriskassessment [D]. Tianjin: Tianjin University, 2016.
[10] 蔡 靖.石河子市飲用水中消毒副產物的污染水平研究及風險評估 [D]. 石河子:石河子大學, 2018. Cai J. Study on the pollution level and risk assessment of disinfectionbyproducts in drinking water in Shihezi City [D]. Shi Hezi: Shi Hezi University, 2018.
[11] 宋 月,魏麟歡,郭維靜,等.大連市飲用水中消毒劑副產物調查[J]. 中國消毒學雜志, 2015,32(5):517-518. Song Y, Wei L H, Guo W J, et al. Investigation of disinfectant by-products in drinking water, Dalian City [J]. Chinese Journal of Disinfection, 2015,32(5):517-518.
[12] 劉祖發,周月英,張駿鵬,等.廣州市飲用水中消毒副產物鹵乙酸的毒性及其降解研究[J]. 亞熱帶資源與環境學報, 2015,10(3):1-10. Liu Z F, Zhou Y Y, Zhang J P, et al. Disinfection by-products halo acetic acids of Guangzhou drinking water: toxicity and degradation [J]. Journal of Subtropical Resources and Environment, 2015,10(3):1-10.
[13] 王曉云,李啟明,付愛明.福鼎市桐江溪鹵乙酸分布特征及潮汐影響分析[J]. 河北工業科技, 2019,36(4):263-270. Wang X Y, Li Q M, Fu A M. Distribution characteristics and tidal effects of halo acetic acids in Tongjiang river, Fuding City [J]. Hebei Journal of Industrial Science and Technological, 2019,36(4):263-270.
[14] Zhao W J, Boyd J M, Qin F, et al. Formation of-Nitrosamines and two new N-containing disinfection by-products from chlorination of water containing diphenylamine [J]. Environmental Science& Technology, 2009,43:8443-8448.
[15] Choi J, Valentine R L. Formation of-Nitroso dimethylamine (NDMA) from reaction of monochloramine: a new disinfection by-product [J]. Water Research, 2002,36:817-824.
[16] Gerecke A C, Sedlak D L. Precursors of N-Nitroso dimethylamine in natural waters [J]. Environmental Science& Technology, 2003,37: 1331-1336.
[17] Chen W Y, Yong T M. Influence of nitrogen source on NDMA formation during chlorination of diuron [J]. Water Research, 2009, 43:3047-3056.
[18] Kaydos E H, Soarez J D, Roberrts N L, et al. Haloacid induced alterations in fertility and the sperm biomarker SP22in the rat are additive: validation of an ellsa [J]. Toxicological Sciences, 2004,81:430-442.
[19] Schell J D J. Interactions of halogenated hydrocarbon mixtures in the embryo of the japanese medaka (Oryzias latipes) [D]. New Brunswick: The State University of New Jersey, 1987.
[20] Cherkin A, Catchpool J F. Temperature dependence of anesthesia in golfish [J]. Science, 1964,144:1460-1462.
[21] Slooff W. Detection limits of a biological monitoring system Based on fish respiration [J]. Bulletin of Environmental Contamination and Toxicology, 1979,23(4/5):517-523.
[22] Mattice J S, Tsai S C, Burch M B, et al. Toxicity of trihalomethanes to common carp embryos [J]. Transactions of the American Fisheries Society, 1981,110(2):261-269.
[23] Ward G S, Parrish P R, Rigby R A. Early life stage toxicity tests with a saltwater fish: effects of eight chemicals on survival, growth, and development of sheepshead minnows [J]. Journal of Toxicology and Environmental Health, 1981,8(1/2):225-240.
[24] Buccafusco R J, Ells S J, Leblance G, et al. Acute toxicity of priority pollutants to bluegill (Lepomis macrochirus) [J]. Bulletin of Environmental Contamination and Toxicology, 1981,26(4):446-452.
[25] Heitmuller P T, Hollister T A, Parrish P R. Acute toxicity of 54industrial chemicals to sheepshead minnows (cyprinodon variegatus) [J]. Bulletin of Environmental Contamination and Toxicology, 1981, 27(5):596-604.
[26] Gibson C I, Tone F C, Wilkinson P, et al. Bioaccumulation and depuration of bromoform in five marine species [R]. Richland: Batelle Northwest National Laboratories, 1979.
[27] Fisher D L, Yonkos G, Ziegler E, et al. Acute and chronic toxicity of selected disinfection byproducts to daphnia magna, cyprinodont variegatus, and isochores galbanum [J]. Water Research, 2014,55:233-244.
[28] Knapek R, Lakota S. Biological testing to determine toxic effects of pesticides in water [J]. Tauscher Ditch Demark Redub Ditch Akkad Land Berl, 1974,126:105-109.
[29] Linden E, Bengtsson B E, Sundstrom G, et al. The acute toxicity of 78chemicals and pesticide formulations against two brackish water organisms, the bleak (alburnus) and the harpacticoid Nicotra snipiness [J]. Chemosphere, 1979,8(11/12):843-851.
[30] Jirasek J, Adamek Z, Kepr T. Toxicity of some herbicides for fish [J]. Zivoc Vyroba, 1980,53(11):857-862.
[31] Birge W J, Black J A, Kuehne R A. Effects of organic compounds on amphibian reproduction [R]. Lexington: Research Report No. 121, Water Resources Research Institute, 1980.
[32] Black J A, Birge W J, Ramey B A, et al. The aquatic toxicity of organic compounds to embryo-larval stages of fish and amphibians [R]. Lexington: Research Report No.133, Water Resources Research Institute, 1982.
[33] Byrne T D. The Effects of Four Trihalogenated methanes on the embryonic development of rana pipiens and xenopus laevis [D]. Minnesota: St Cloud State University, 1978.
[34] Fort D J, Stover E L, Rayburn J R, et al. Evaluation of the developmental toxicity of trichloroethylene and detoxification metabolites using xenopus [J]. Teratog Carcinog Mutagen, 1993,13(1):35-45.
[35] Khangarot B S, Das S. Acute toxicity of metals and reference Toxicants to a freshwater ostracod, cypris subglobosa sowerby, 1840 and correlation to EC50values of other test models [J]. Journal of Hazardous Materials, 2009,172(2/3):641-649.
[36] Calleja M C, Persoone G, Geladi P. Comparative acute toxicity of the first 50multicentre evaluation of in vitro cytotoxicity chemicals to aquatic non-vertebrates [J]. Archives of Environmental Contamination and Toxicology, 1994,26(1):69-78.
[37] Horne J D, Oblad B R. Aquatic toxicity studies of six priority pollutants [J].Bulletin of environmental contamination and toxicology, 1983,32(5):586-594.
[38] Leblanc G A. Acute toxicity of priority pollutants to water flea (Daphnia magna) [J]. Bulletin of Environmental Contamination and Toxicology, 1980,24(5):684-691.
[39] Jolley R L, Brungs W A, Jacobs V A, et al. Investigation of the effects of halogenated organic compounds produced in cooling systems and process effluents on aquatic organisms [M]. Ann Arbor: Environmental Impact and Health Effects, 1978:163-173.
[40] Richie J P, Mills B J, Lang C A. The verification of a mammalian toxicant classification using a mosquito screening method [J]. Fundamental and Applied Toxicology, 1984,4(6):1029-1035.
[41] Cowgill U M, Milazzo D P, Landenberger B D. Toxicity of nine benchmark chemicals to speleothems causatum, a marine diatom [J]. Environmental Toxicology and Chemistry, 1989,8(5):451-455.
[42] Erickson S J, Hawkins C E. Effects of halogenated organic Compounds on photosynthesis in estuarine phytoplankton [J]. Bulletin of Environmental Contamination and Toxicology, 1980,24(6):910-915.
[43] Curieux F L, GauthierA L, Erb F, et al. Use of the SOS chromates, the ames-fluctuation test and the new micronucleus test to study the genotoxicity of four trihalomethanes [J]. Mutagenesis, 1995,10(4): 333-341.
[44] Hanson M L, Solomon K R. Halo acetic acids in the aquatic environment. part I: Macrophyte Toxicity [J]. Environmental Pollution, 2004,130(3):371-383.
[45] 雷炳莉,黃圣彪,王子健.生態風險評價理論和方法[J]. 化學進展, 2009,21(2/3):350-358. Lei B L, Huang S B, Wang Z J. Theories and methods of ecological risk assessment [J]. Progress in Chemistry, 2009,21(2/3):350-357.
[46] Liu N, Jin X W , Wu F C, et al. Ecological risk assessment of fifty Pharmaceuticals and Personal Care Products (PPCPs) in Chinese surface waters: A proposed multiple-level system [J]. Environment International, 2020,136:105454.
[47] Liu N, Wang Y Y, Jin X W, et al. Probabilistic assessment of risks of diethylhexyl phthalate (DEHP) in surface waters of China on reproduction of fish [J]. Environmental Pollution, 2016,213:482-488.
[48] 王冬梅.城市污水處理過程中消毒副產物HAAs及其前體物的研究[D]. 西安:西安建筑科技大學, 2013:7-11. Wang D M. Research of HAAs formation and its precursors in municipalsewage treatment process [D]. Xi’an:Xi’an University of Architecture and Technology, 2103.
[49] Jin X W, Wang Z, Giesy J, et al. Do water quality criteria based on nonnative species provide appropriate protection for native species [J]. Environmental Toxicology and Chemistry, 2015,34(8):1793-1798.
[50] 劉 娜,金小偉,王業耀,等.生態毒理數據篩查與評價準則研究[J]. 生態毒理學報, 2016,11(3):1-10. Liu N, Jin X W, Wang Y Y, et al. Review of criteria for screening and evaluating ecotoxicity data [J]. Asian Journal of Ecotoxicology, 2016, 11(3):1-10.
[51] 金小偉,雷炳莉,許宜平,等.水生態基準方法學概述及建立我國水生態基準的探討[J]. 生態毒理學報, 2009,4(5):609-616. Jin X W, Lei B L, Xu Y P, et al. Methodologies for deriving Water quality criteria to protect aquatic life (ALC) and proposal for development of ALC in China: a review [J]. Asian Journal of Ecotoxicology, 2009,4(5):609-616.
[52] 梁 霞,周軍英,李建宏,等.物種敏感度分布法(SSD)在農藥水質基準推導中的應用[J]. 生態與農村環境學報, 2015,31(3):398-405. Liang X, Zhou J Y, Li J H, et al. Application of species sensitivity distribution (SSD) to derivation of water quality criteria for pesticides [J]. Journal of Ecology and Rural Environment, 2015,31(3):398-405.
[53] Zhou S B, Paolo C D, Hollert H, et al. Optimization of screening-level risk assessment and priority selection of emerging pollutants – The case of pharmaceuticals in European surface waters [J]. Environment International, 2019,128:1-10.
[54] Loekle D. The Effects of chlorinated hydrocarbons on carassius auratus (Goldfish) [D]. Binghamton:State University of New York, 1987.
[55] Bringmann G, Kuhn R. Testing of substances for their toxicity threshold: model organisms microcystis (Diplocystis) aeruginosa and scenedesmus quadricauda [J]. Int Ver Theor Angew Limnol, 1978,21: 275-284.
[56] Zhu Y H, Jiang G J. Combined toxic effects of typical mutagens - dimethylphenol, tribromethane and dinitroaniline, on unicellular Green Algae dunaliella salina [J]. Journal of Food Safety and Food Quality, 2009,29(1):1-13.
[57] Bantle J A, Finch R A, Fort D J, et al. FETAX validation using 12compounds with and without an exogenous metabolic activation system [J]. Journal of Applied Toxicology, 1999,19(6):447-472.
[58] Felicio A A, Crago J, Maryoung L A, et al. Effects of alkylphenols on the biotransformation of diuron and enzymes involved in the synthesis and clearance of sex steroids in juvenile male tilapia (Oreochromus mossambica) [J]. Aquatic Toxicology, 2016,180:345-352.
[59] Applegate V C, Howell J H, Smith M A, et al. Toxicity of 4346chemicals to larval lampreys and fishes [J]. Specific Science Reproduction Wild, 1957,207:157.
[60] Roberts J F, Price O R, Van Egmond R. Toxicity of halo acetic acids to freshwater algae [J]. Ecotoxicology and Environmental Safety, 2010, 73(1):56-61.
[61] Calabrese E J, Chamberlain C C, Coler R, et al. The effects of trichloroacetic acid, a widespread product of chlorine disinfection, on the dragonfly nymph respiration [J]. Environmental Health Science, 1987,22(4):343-35.
[62] Correa M, Calabrese E J, Coler R A. Effects of trichloroacetic acid, a new non-Tamiment found from chlorinating water with 0rganic Material, on dragonfly nymphs [J]. Bulletin of Environmental Contamination and Toxicology, 1985,34(2):271-274.
[63] Jin X W, Wang Y Y, Wang Z J, et al. Eclogical risk of nonylphenol in china surface waters based on reproductive fitness [J]. Environmental Science& Technology, 2014,48:1256-1262.
[64] Jin X W, Zha J M, Xu Y P, et al. Derivation of aquatic predicted no-effect concentration (PNEC) for 2,4-dichlorophenol: comparing native species data with non-native species data [J]. Chemosphere, 2011,84(10):1506-1511.
[65] Solomon K, Giesy J, Jones P. Probabilistic risk assessment of agrochemicals in the environment [J]. Crop Protection, 2000,19(8): 649-655.
[66] Wang Y Y, Zhang L S, Meng F S, et al. Improvement on species sensitivity distribution methods for deriving site-specific water quality criteria [J]. Environmental Science and Pollution Research, 2015, 22(7):5271-5282.
Occurrence and ecological risk of typical DBPs in Chinese surface water.
LUO Ying1,2,3, LIU Na3,SUN Shan-wei1, HOU Song1, GUO Chang-sheng1,3*, XU Jian1,3
(1.State Environmental Protection Key Laboratory of Ecological Effect and Risk Assessment of Chemicals, Chinese Research Academy of Environmental Sciences, Beijing 100012, China;2.College of Water Sciences, Beijing Normal University, Beijing 100875, China;3.State Key Laboratory of Environmental Criteria and Risk Assessment, Chinese Research Academy of Environmental Sciences, Beijing 100012, China)., 2021,41(4):1806~1814
Trihalomethanes (THMs) and haloacetic acids (HAAs), the disinfection by-products (DBPs) in surface water, have given rise to major concern in recent years. In this study, exposure and ecotoxicity data of 4 DBPs (trichloromethane, tribromomethane, dichloroacetic acid and trichloroacetic acid) were collected from literature published in China and abroad, and a multiple-level environmental risk assessment was performed. THMs and HAAs were collected from previous studies, with concentrations range from n.d. to 51μg/L. Predicted no effect concentration (PNEC) for trichloromethane (TCM), tribromomethane (TBM), dichloroacetic acid (DCAA) and trichloroacetic acid (TCAA) was based on acute and chronic toxicity data. The PNEC of TCM, TBM, DCAA and TCAA derived from acute and chronic toxicity data which based on endpoints of survival, reproduction and development, with concentrations range from 0mg/L to 44.88mg/L, 0.006mg/L to 0.956mg/L, respectively. Besides, risk quotient (RQ) and probabilistic ecological risk assessments (PERA) were calculated by exposure data, and PNEC of acute and chronic toxicity data. The results showed that RQ were less than 1. TCM of probabilities of exceeding NOEC based on chronic toxicity for 1% of the species were 78.86%. And TCAA of probabilities of exceeding NOEC based on chronic toxicity for 1% of the species were 20.61%. Based on these results, the ecological risks of TCM and TCAA in Chinese surface water were low.
disinfection by-products;surface water;ecological risk assessment
X171.5;X824
A
1000-6923(2021)04-1806-09
羅 瑩(1992-),女,新疆奎屯人,北京師范大學博士研究生,主要研究方向為生態毒理及風險評價.發表論文4篇.
2020-08-04
中央級公益性科研院所基本科研業務專項(2020-JY-003; 2019YSKY-022)
* 責任作者, 副研究員, guocs@craes.org.cn

