Static and dynamic studies of adsorption by four macroporous resins to enrich oridonin from Rabdosia rubescens
Ling Meng ,Xia Gui,Zhi Yun
1 College of Chemical Engineering,Nanjing Tech University,Nanjing 211816,China
2 State Key Laboratory of Generic Manufacture Technology of Chinese Traditional Medicine,Lunan Pharmaceutical Group Co.,Ltd.,Linyi 273400,China
Keywords:Rabdosia rubescens Oridonin Macroporous resin Adsorption Kinetics Thermodynamics
ABSTRACT Oridonin,one of the active ingredients in Rabdosia rubescens (R.rubescens),has been reported to induce cell apoptosis and cell cycle arrest in many cancers.Conventional extraction methods tend to result in unsatisfied enrichment and poor quality of oridonin present in a given biomass.This paper aims to evaluate the performance and separation characteristics of four different macroporous resins to arrive at the most suitable methodology for the isolation and purification of high-quality oridonin.Static absorption kinetics,thermodynamic and dynamic adsorption were evaluated.HP-20 was selected for further study due to its high adsorption capacity of 32 mg﹒g-1 and desorption ratio with 98.5%.The pseudo-secondorder model was considered to be the most suitable for kinetic results,and Langmuir model was chosen to better describe the absorption thermodynamics.Under optimum conditions (flow rate of 4 ml﹒min-1,bed depth with 6 cm and initial concentration of 2.15 mg﹒ml-1),the effective content of oridonin increased from 33.9% to 79.1% in the dry extract with a recovery of 81% and the purity of oridonin improved from 76% to 93%.The results confirm that HP-20 provides an efficient method to purify most oridonin from R.rubescens.
1.Introduction
Rabdosia rubescens (R.rubescens),a well-known Chinese medicine herb,contains diterpenoids,flavonoids,phenolic acids,triterpenoids and volatile oils[1].For hundreds of years,R.rubescens has been considered to be a beneficial treatment for variety of ailments including stomach aches,pharyngitis,cough and sore throat,as well as certain cancer types[2].Diterpenoids,earlier known as oridonin,are bioactive constituent isolated from R.rubescens,and can be described as a compound which possess chemopreventative and antitumor effects [3].Clinical studies demonstrated that oridonin could exhibit significant potential anticancer activity against leukemia [4],colorectal cancer [5],liver cancer [6],lung cancer [7],and so on.
R.rubescens extracts are commonly highly complex mixtures of active compounds and consequently their purification becomes a particularly challenging task.In recent years,numerous reports of natural product purifications using thin layer chromatography(TLC) method,silica column chromatography and counter-current chromatography (CCC) techniques have been published [8–10].However,these methodologies are tedious,laboratory-intensive,less productive,solvent and time consuming,especially for extracting a single active component from many hundreds.Macroporous resin offers to the natural product scientists a different mode of operation to conventional processes.Recent studies found that macroporous resin has been widely employed to separate bioactive compounds from natural plant.Gao et al.used XAD-7HP resin to isolate procyanidins from cranberry [11].According to Zhong et al.,the cocoa polyphenol content improved from 2.23% to 62.87% following treatment with LX-17 resin [12].The recovery of anthocyanins from roselle extract,rosavin from Rhodiola rosea and flavonoids from Glycyrrhiza glabra were also achieved using macroporous resin [13–15].The method benefits from many advantages compared with traditional separation methods,such as providing a large surface area,high separation selectivity,low cost,prone to regeneration and suitable for large-scale production.Nevertheless,few studies have addressed the adsorption process of oridonin on macroporous resin in detail.He et al.employed resin to separate R.rubescens extract preliminarily and then used silica column chromatography [9].Ye et al.optimized the adsorption and desorption conditions,but they did not investigate the adsorption process and mechanism [16].Therefore,it is necessary to select a suitable resin to isolate oridonin and investigate its adsorption behavior further.
In this study,four resins D101,DM130,HP-20 and NKA-9 were employed to isolate oridonin from R.rubescens.Adsorption kinetics and thermodynamics were systematically investigated and fitted by different models to analyze the adsorption process.In addition,parameters were optimized to obtain the optimal dynamic adsorption conditions.Samples were analyzed by high performance liquid chromatography (HPLC).
2.Experimental
2.1.Reagent
Dry R.rubescens leaves were purchased from Ji Shi Pharmaceutical Co.Ltd (Ji Yuan,China) and were milled into a fine powder using an electric grinder.The powder was sifted through a 60-mesh screen sieve with a mean particle size about 0.4 mm.Oridonin (purity >97%) was purchased from Ji Shi Pharmaceutical Co.,Ltd (Ji Yuan,China).Ethanol (CH3CH2OH) and methanol (CH3-OH) were purchased from Aladdin Industrial Corporation (Shang Hai,China) with the purity of 95% and 99.8%.Analytical grade NaOH (96%) and HCl (36%) were purchased from Nanjing Reagent Co.and Lingfeng Chemical (Shang Hai,China) respectively.The purity was determined by HPLC and all reagents were used without any further purification.Resins used in this study were D101(Macklin Biochemical Co.Ltd,Shanghai,China),NKA-9 (Huakai Biochemical Co.Ltd,Jining,China),DM130(Bon Adsorbed Technology CO.Ltd,Cangzhou,China),HP-20(Huakai Biochemical Co.Ltd,Jining,China).The physical properties of four resins are shown in Table 1.PVDF membrane (φ13 mm/0.45 μm) was purchased from Chemical Bay Company (Nanjing,China).Deionized water was treated with a Milli-Q water purification system (TGI Pure Water Systems,Greenville,SC,USA).

Table 1 Physical properties,adsorption capacity and desorption ratio of four resins
2.2.Experimental procedures
2.2.1.Pre-treatment of the resin
Resins (D101,DM130,HP-20 and NKA-9) were soaked in 95%ethanol for 24 h followed by washing with distilled water.Subsequently,resins were treated with 2% (g﹒g-1) NaOH and 2% (g﹒g-1)HCl solutions to remove the monomers and porogenic diluents.Finally,resins were rinsed by deionized water to neutrality and stored in 2 °C refrigerator.
2.2.2.Preparation of R.rubescens extracts
R.rubescens powder (20 g) and ethanol solution (160 ml,95%)were added into a sealed 250 ml cylindrical flask at room temperature and with a stirring speed of 150 r﹒min-1.After 2 h,the extract was centrifuged(2000 r﹒min-1,5 min,Shanghai Machinery Manufacture,Shanghai,China).The filtrate was concentrated under reduced pressure at 55 °C until the volume became one-ninth of the extract.Appropriate amounts of deionized water were added to the extract and centrifuged to prepare aqueous oridonin solution.
2.2.3.Static adsorption and desorption test
Oridonin aqueous solution (30 ml,initial concentration 1.483 mg﹒ml-1) and 1 g (wet weight) of resin (D101,DM130,HP-20,NKA-9) were mixed in grinding mouth of flask.Experiments were conducted at room temperature and at 150 r﹒min-1(Yuhua Instrument Manufacture,Gongyi,China).The amount of adsorption was determined after 6 h.The adsorption capacity was quantified as follows [17]:

where Qeis adsorption capacity at equilibrium;C0and Cerepresent the initial and equilibrium concentrations of oridonin in solution (mg﹒ml-1);V0is the volume of solution (ml);W is the wet weight of resin (g);and A is the adsorption ratio of resins.
After arriving at the adsorption equilibrium,the resin was washed by distilled water.30 ml of 95% ethanol was added to the grinding mouths of flasks containing resins.Desorption conditions were consistent with adsorption,after 6 h,the concentration of oridonin was measured and desorption ratio was calculated as follows:

where Cdis the desorption concentration (mg﹒ml-1);Vdis the volume of desorption solvent (ml);Deis the desorption ratio of resins.
2.2.4.Adsorption and desorption kinetics
Adsorption kinetics were determined at 25°C and 150 r﹒min-1.Samples were taken at 0,30,60,90,120,150,180 and 210 min,respectively.Three kinetic models including pseudo-first-order,pseudo-second-order and particle diffusion were employed to explain the adsorption process [18,19].The operating conditions of desorption kinetics were consistent with those of the static desorption test,but the desorption amount was determined at intervals of 30 min.
Pseudo-first-order model.

Pseudo-second-order model

Particle diffusion model

where Qe(mg﹒g-1) is the adsorption capacity at equilibrium;Qt(mg﹒g-1) is the adsorption amount at time t;K1,K2,K3represent the rate constants of pseudo-first-order,pseudo-second-order and particle diffusion models.C is the constant of particle diffusion model.
2.2.5.Adsorption isotherms and thermodynamics on selected resin
The selected resin (1 g) was added to a series of 30 ml of oridonin aqueous solutions with five different concentrations(0.582,0.74,1.07,1.33 and 1.5 mg﹒ml-1).Adsorption was measured at three different temperatures (25,35 and 45 °C) in 100 ml of grinding mouth of flask at 150 r﹒min-1.The adsorption isotherms of oridonin were determined using Langmuir and Freundlich equations [20].
Langmuir equation:

Freundlich equation:

where Qm(mg﹒g-1)is the theoretical maximum adsorption amount;Qe(mg﹒g-1) and Ce(mg﹒ml-1) are adsorption capacity and concentration at equilibrium;KL,KFare constants of Langmuir equation and Freundlich equation;1/n is a constant that represents the adsorption driving force.Langmuir equation can be characterized by RL,which is a dimensionless constant that is defined as the separation factor or equilibrium parameter.RLis calculated by the following equation:

RLrepresents the type of Langmuir adsorption isotherm.RL=0,0
Enthalpy change(ΔH)and entropy change(ΔS)of selected resin were calculated by the Van’t Hoff equation as follows [19,21]:

The Gibbs free energy change (ΔG) was calculated as follows:

where KLis constant of Langmuir equation;R is the gas constant(8.314 J﹒mol-1﹒K-1);T is the absolute temperature (K).
2.2.6.Dynamic adsorption test
Enrichment oridonin was carried out in a glass column(I.D.×L:20 mm×200 mm)packed with HP-20.The effects of flow rate(Q:ml﹒min-1),initial concentration and bed depth (h:cm) on adsorption performance were investigated at room temperature.oridonin solution (2.15 mg﹒ml-1) was pumped to the column (bed depth of 6 cm) by a peristaltic pump (Shenchen Pump Industry Co.,Ltd.,Baoding,China) at 4,6 or 8 ml﹒min-1.Three bed depths of 4,6 and 8 cm operated at flow rate of 4 ml﹒min-1under the initial concentration mentioned above.Three concentrations 1.693,2.15 and 2.49 mg﹒ml-1were tested under flow rate at 4 ml﹒min-1and bed depth of 6 cm.
2.3.High-performance liquid chromatography (HPLC) analysis
HPLC (Agilent 1100) analysis was performed using a ZORBAX Eclispse XDB C18(4.6 mm×150 mm,5 μm particle size,Hangzhou Ruixi Co.Ltd,Hangzhou,China) analytical column and a UV detector.Mobile phase was composed of 50% (v/v) methanol (eluent A)and 50% (v/v)water(eluent B) operating at a constant flow rate of 1.0 ml﹒min-1.The column was rinsed using mobile phase for 1 h prior to each analysis.The injection volume was 5 μl at 35°C with a UV detection wavelength of 238 nm.oridonin was identified by comparing the retention time with standard product and determined by the calibration curve (Y=6247X -21.7,Y:response value,X:concentration) with R2=0.9995.0.45 μm PVDF membrane was employed to filter samples before analysis.
3.Results and Discussion
3.1.Adsorption and desorption capacity
Ma et al.[14].have reported that adsorption and desorption characteristics of resin are affected by specific surface area,pore diameter,polarity of adsorbents,the structure and property of adsorbates.The structure of oridonin possesses four hydrogen groups,as shown in Fig.1.The adsorption of oridonin on macroporous resin occurs through hydrogen bonds or van der Waals forces.Considering the unique structure and molecular weight of oridonin,four resins with different surface areas and pore diameters,ranging from non-polar to polar,were selected as adsorbents and listed in Table 1.
Adsorption and desorption capacity of four resins were shown in Table 1.Results indicated that the adsorption capacity of the four resins was HP-20 >D101 >DM130 >NKA-9.The desorption ratios of D101 and HP-20 were higher than those of DM130 and NKA-9.NKA-9 had the lowest adsorption capacity.This was related to its small surface area which was half that of HP-20 and D101.The surface areas of DM130 and D101 were higher than HP-20,however,HP-20 had the highest adsorption capacity which indicated that surface area was not the only factor that affect adsorption capacity.As the polarity of four resins (NKA-9,DM130,D101,HP-20) decreases,the adsorption capacity increased as shown in Table 1.This revealed that the polarity of a resin could affect its adsorption capacity.Furthermore,the adsorption and desorption capacities of HP-20 were slightly higher than D101,which might be due to the pore diameter.Xu et al.[22]reported that it was easy to restrict the diffusion of an adsorbent when the pore diameter is too small.
3.2.Adsorption and desorption kinetics
Adsorption kinetics curves of four resins were shown in Fig.2(a).Adsorption process could be split into three categories:fast reaching adsorption equilibrium,rapid adsorption and slow adsorption[23].DM130 was subjected to the fast reaching adsorption equilibrium type.This type required less time,while the adsorption amount was relatively low at equilibrium.D101 and HP-20 were classified as undergoing the rapid adsorption type.Adsorption of these two resins increased quickly at initial stages and the adsorption capacity was highest at equilibrium.NKA-9 was slow adsorption type as its adsorption process took a long time and only a small adsorption amount occurred.Therefore,D101 and HP-20 were chosen for the following experiments.

Fig.1.Structure of oridonin.

Fig.2.Adsorption and desorption kinetics.(a)Adsorption kinetics of four resins.(b)Desorption ratio of D101 and HP-20 resins.
Desorption kinetics curves of D101 and HP-20 were shown in Fig.2(b).Desorption ratio of HP-20 was higher than D101.Physical adsorption during the process of desorption was shown to be reversible.The surface area of D101 was higher than HP-20,but pore diameter was the lowest,thus more irreversible adsorptions might exist on D101 resin.This could explain why the desorption capacity of HP-20 was higher than D101.Based on an overall consideration of adsorption and desorption capacities,HP-20 was selected to purify oridonin as an efficient and economical resin.
Pseudo-first-order was commonly used to predict the initial period of adsorption,while the behavior over entire adsorption was predicted by pseudo-second-order.The parameters of three kinetic models were listed in Table 2.Pseudo-second-order was shown to be more suitable for HP-20 adsorption based on its higher regression coefficient.The model fitting of pseudo-secondorder of HP-20 was demonstrated in Fig.3(a).According to the slope of fitting curve,the calculated Qmwas higher than that calculated with pseudo-first-order and was close to experimental value.

Table 2 Dynamic parameters of pseudo-first-order,pseudo-second-order and particle diffusion of HP-20 resin
Although the particle diffusion kinetics model had difficulty representing the whole adsorption process,it could be employed to predict the mechanisms of adsorption at certain stages [24].Adsorption in a macroporous resin was found to have three distinct zones:(1) almost completely saturated in outer zone of microspheres;(2) the middle zone,where adsorption took place at a given time;(3)the inner area where adsorption does not take place[25].The saturated zone thickens as the adsorption process expanded which lead to the intraparticle diffusion.Furthermore,macroporous particles including macropores and micropores were considered to owe a ‘‘double-dispersed”pore structure.Therefore,particle diffusion model might be controlled by intraparticle diffusion or film diffusion.The fit of particle diffusion model was demonstrated in Fig.3(b),it could be clearly observed that the slope and intercept were 1.42,9.66 respectively,which indicated that the adsorption process was not only controlled by intraparticle diffusion rather than film diffusion.This could be explained by the fact that the fitting curve does not through origin[26].In addition,the positive of intercept demonstrated that adsorption process was fast-dynamic.This strongly confirmed the abovementioned conclusion.
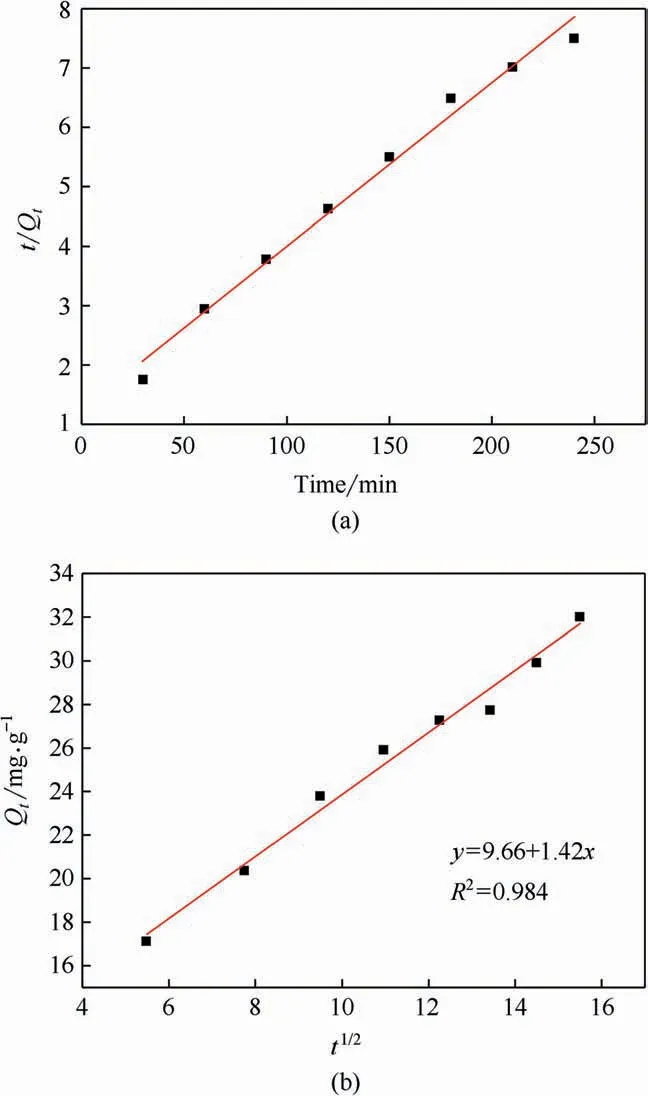
Fig.3.(a) Model fitting of pseudo-second-order of HP-20;(b) Linear regression of HP-20 resin employing particle diffusion model.
3.3.Adsorption isotherms and thermodynamics
Adsorption isotherms represent the relationship of equilibrium adsorption amount and equilibrium concentration of adsorbate at a constant temperature[27].The equilibrium adsorption isotherms of oridonin on HP-20 resin at 25,35 and 45°C were shown in Fig.4(a).Results indicated that the adsorption capacity of HP-20 resin increased with increasing the initial concentrations.In addition,the adsorption capacity decreased when temperature was increased from 25 to 45 °C,which demonstrated that adsorption of oridonin on HP-20 was an exothermic process.

Fig.4.(a) Adsorption isotherms of HP-20 resin;(b) Langmuir model for HP-20 resin;(c) Freundlich model for HP-20 resin.
Langmuir model and Freundlich model are widely used in all isothermal equations [28].Langmuir model supposes that the adsorption energy is uniform with an identical solute affinity at all adsorption sites,and that it forms a monolayer on the free surface.Freundlich model is suitable for non-ideal systems which hardly forms a monolayer.Langmuir model and Freundlich model for HP-20 resin were shown in Fig.4(b) and (c).The important parameters of Langmuir model and Freundlich model were summarized in Table 3.For Langmuir model,KLrepresents the affinity of the binding sites.The values of KLreduced as the temperature increased from 25 to 45°C which indicated the adsorption capacity was higher at low temperature.Table 3 showed that the value of RL was between 0 and 1,which indicated that adsorption of oridonin by HP-20 resin had favorable isotherms.KFand n are important parameters of adsorption capacity and intensity of the resins in Freundlich models [29].Generally,the values of n between 1 and 10 suggest that the adsorption process is beneficial.In this study,the values of n were 4.35,4.17 and 3.7 at 25,35 and 45°C respectively.As shown in Table 3,the n values decreased as the temperature increased,which indicated that adsorption process was not favorable.Furthermore,the linear correlation coefficient of Langmuir model ranged from 0.994 to 0.995,while the Freundlich model was from 0.924 to 0.998.The linear of correlation coefficient of Freundlich model was slightly higher than Langmuir model at 25 °C,but the Langmuir model was higher than Freundlich model at 35 and 45°C.Given that the scope of Freundlich model was narrower than Langmuir model,Langmuir model was considered to be the better model for adsorption of oridonin on HP-20 resin.
Adsorption thermodynamics represent the mechanism of adsorption process [30].Thermodynamic parameters of HP-20 at different temperatures during the adsorption process were shown in Table 4.Results demonstrated that the ΔG was less than zero which showed that the adsorption process was spontaneous.In addition,the value of ΔG increased with an increasing temperature,indicating that the adsorption became more favorable at lower temperature.According to the Van’t Hoff equation,the ΔH was calculated to be -30.6 kJ﹒mol-1which indicated that the adsorption process was exothermic.This was consistent with the conclusion drawn for adsorption isotherms.Meanwhile,the absolute values of ΔH and ΔG for HP-20 were less than 43 kJ﹒mol-1and 20 kJ﹒mol-1respectively,indicating that the adsorption process of oridonin on HP-20 resin was dominated by physical mechanisms rather than chemical mechanisms[19,31].Therefore,25°C was selected as the suitable temperature for adsorption.

Table 3 Parameters of Langmuir and Freundlich models of HP-20 resin

Table 4 Thermodynamic parameters of HP-20 during the adsorption process.
3.4.Effects of bed depth,flow rate and iinitial concentration on adsorption
Breakthrough curves are obtained by plotting Ct/Coversus time or volume of the effluent where Cois the initial concentration of adsorbate and Ctis concentration of adsorbate at time t.The breakthrough curve is a step function as the concentration of adsorbate makes an instantaneous jump at the moment when the column capacity is reached [32].The performance of packed beds is demonstrated through the concept of breakthrough curves which are important for determining the operation and the dynamic response of an adsorption column.Therefore,factors affecting breakthrough curves were studied in depth.
The breakthrough curves at different bed depths are shown in Fig.5.It can be found that with an increasing bed depth,oridonin had more time to contact with HP-20 resin which resulted in higher adsorption capacity.In addition,more binding sites for adsorption were provided by high bed depths [33].The slope ofbreakthrough curve decreased as the bed depth increased which led to a broadened mass transfer zone.Breakthrough (Ct/C0=0.2)occurred after 50 min when h=4 cm,which might have been induced by the short contact time.However,the breakthrough time had no great difference between h=6 cm and h=8 cm.This due to the fact that channeling exited easily at high bed depth.Therefore,the suitable bed depth is h=6 cm.

Fig.5.Effect of different bed depths on oridonin adsorption.

Fig.6.Effect of different flow rate on oridonin adsorption.
In Fig.6,the breakthrough curves at different flow rates demonstrate that the breakthrough occurred faster with a higher flow rate.From Q=4 ml﹒min-1to Q=8 ml﹒min-1,the slope of breakthrough curve increased.Saturation time of breakthrough increased significantly with a decrease in the flow rate.At low flow rate,oridonin had sufficient time to spread from solvent into the pores of adsorbent.Thus,Q=4 ml﹒min-1was selected as optimal flow rate.
The breakthrough curves were obtained at different initial concentration as shown in Fig.7,which revealed that the breakthrough time decreased as the concentration increased.Breakthrough curves were dispersed and occurred slowly at lower concentration,which was due to a decrease in the diffusion coefficient or mass transfer coefficient induced by low concentration.As concentration increased,sharper breakthrough curves were acquired,which was due to that high concentration difference provided a high driving force for the adsorption process.These facts indicated a change of concentration could affect the saturation rate and breakthrough time [34].Breakthrough time was shorter than the others when C0=2.49 mg﹒ml-1.This could be explained that binding sites for adsorption became covered as concentration increased.In this study,breakthrough (Ct/C0=0.2) occurred at 90 and 100 min when concentration was 2.15 and 1.693 mg﹒ml-1respectively.Given the driving force of adsorption and breakthrough time,C0=2.15 mg﹒ml-1was selected for further study.
3.5.HPLC analysis

Fig.7.Effect of different initial concentration on oridonin adsorption.

Fig.8.HPLC chromatograms of oridonin before(a)and after(b)purification by HP-20 resin.(mAu=mabsorbance unit).
HPLC chromatograms of oridonin before and after purification by HP-20 were displayed in Fig.8(a) and (b).From the pictures,it can be clearly observed that many high polarity impurities were removed and the relative area peak of oridonin increased significantly.After treatment with the HP-20 resin,the effective content of oridonin increased from 33.9%to 79.1%based in the dry extract,and the purity of oridonin was improved from 76% to 93%.All experiments were performed in triplicates and the average recovery of oridonin was 81%with a relative standard deviation of 0.28%.Ye et al.optimized the flow rate,concentration and injection volume conditions and the final recovery of oridonin was 78.4%which was slightly lower than that obtained in this work [16].This confirmed that the method proposed in this work was effective.
4.Conclusions
The purification of oridonin from R.rubescens was conducted with four different resins.HP-20 exhibited much higher adsorption and desorption capacities.A maximum oridonin effective content of 79.1%was achieved in the dry extract with a purity of 93%under the following conditions:adsorption time:1.5 h,flow rate:4 ml﹒min-1,bed depth:6 cm and concentration:2.15 mg﹒ml-1.On average,81%of oridonin was recovered with a relative standard deviation of 0.28%.In addition,adsorption kinetics and thermodynamics were systematically investigated and fitted by different models to explain adsorption mechanism.Results indicated that adsorption kinetics were well-fitted to pseudo-second-order model (R2=0.987),meanwhile,both intra-particle diffusion and film diffusion were shown to be rate-limiting steps of the system.Thermodynamics studies indicated that Langmuir model was the best model to describe adsorption process (R2=0.994) and the optimal temperature was 25 °C.Based on the results of ΔH (-30.6 kJ﹒mol-1) and ΔG (-9.3 kJ﹒mol-1),adsorption was a physical and exothermic process.All results indicated that this purification process by HP-20 had good potential for utilization and commercialization of R.rubescens.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research is supported by the National Natural Science Foundation of China (21676145) and Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD,China).
 Chinese Journal of Chemical Engineering2021年4期
Chinese Journal of Chemical Engineering2021年4期
- Chinese Journal of Chemical Engineering的其它文章
- Structure-dependent re-dispersibility of graphene oxide powders prepared by fast spray drying
- Synthesis and optimization of high surface area mesoporous date palm fiber-based nanostructured powder activated carbon for aluminum removal
- Preparation and antibacterial properties of polycaprolactone/quaternized chitosan blends
- Synthesis of zinc oxide nanoparticles reinforced clay and their applications for removal of Pb (II) ions from aqueous media
- Synthesis and characterization of high strength polyimide/silicon nitride nanocomposites with enhanced thermal and hydrophobic properties
- Modeling viscosity of methane,nitrogen,and hydrocarbon gas mixtures at ultra-high pressures and temperatures using group method of data handling and gene expression programming techniques
