Powder x-ray diffraction and Rietveld analysis of(C2H5NH3)2CuCl4?
Yi Liu(劉義) Jun Shen(沈俊) Zunming Lu(盧遵銘) Baogen Shen(沈保根) and Liqin Yan(閆麗琴)
1Beijing National Laboratory for Condensed Matter Physics,Institute of Physics,Chinese Academy of Sciences,Beijing 100190,China
2Key Laboratory of Cryogenics,Technical Institute of Physics and Chemistry,Chinese Academy of Sciences,Beijing 100190,China
3School of Physical Sciences,University of Chinese Academy of Sciences,Beijing 100190,China
4School of Material Science and Engineering,Hebei University of Technology,Tianjin 300130,China
Keywords: organic-inorganic hybrid,x-ray diffraction,(C2H5NH3)2CuCl4
1. Introduction
It is well known that a ferroelectric material undergoes a phase transition from a high-temperature phase that behaves as an ordinary dielectric to a low-temperature phase that has a spontaneous polarization. A primary requirement for the existence of ferroelectricity (FE) is a structural distortion from the prototypical high-symmetry phase that removes the center of symmetry and allows an electric polarization. There are 31 point groups that allow a spontaneous electric polarizationP.[1,2]
However,an ambiguous case has been ignored in organicinorganic hybrid system(C2H5NH3)2CuCl4(EA2CuCl4).[3–6]As a member of the family of compounds with composition(CnH2n+1NH3)2MCl4,whereMis a divalent metal(M=Mn2+, Cd2+, Fe2+, and Cu2+), its potentials are not only in hydrogen storage, catalysis, etc, but also attractive as a magnetoelectric, two-dimensional magnetocaloric, and electrocaloric material.[7–10]The layered cupric halide compound EA2CuCl4has been investigated as a ferroelectric material with a large remnant value 37 μC/cm2of electric polarizationPbyP–Eloop and pyroelectric current measurements below its FE transition temperatureTc≈247 K, whereas its space group of crystal structure has been reported as centrosymmetry below room temperature (RT: space group,Pbca;a=21.18 ?A,b=7.47 ?A,andc=7.35 ?A).[3,11]An electrically polar state is generally produced by the loss of inversion symmetry of the crystal structure,driven by spin order,displacive mechanism or hydrogen-bond ordering scheme in a ferroelectric material. Thus identifying the space group of EA2CuCl4is indeed an essential issue.
EA2CuCl4crystallizes in a layered perovskite structure consisting of nearly isolated layer of corner-sharing CuCl6octahedra separated by two layers of ethyl-ammonium cations (C2H5NH3)+at RT [see Fig. 3(b)], where the ethylammonium bilayer and the anionic inorganic layer are stacked ionically by Coulombic force. The hydrogen bonding between the ammonium head and halogens determines the orientation and conformation of the organic cation.[12]The crystal structure at RT is orthorhombic and belongs to the space groupPbca. The CuCl6octahedra are strongly deformed due to the in-plane Jahn–Teller (JT) distortion. The shorter and longer Cu–Cl bond lengths are~2.28 ?A and~2.98 ?A,respectively.[11]
Upon lowering temperature, EA2CuCl4undergoes five phase transitions atT1= 364 K (Bbcm),T2= 356 K(Bbcm→P21/c),T3=330 K (P21/c →rhombicPbca),T4=247 K,andT5=232 K.The previous investigations on crystal structure or phase transition evolution with temperature and pressure have been focused on the high temperature region atT1,T2, andT3by means of dilatometric, dielectric, V-UV absorption spectra,x-ray diffraction(XRD),hydrostatic pressure, etc.[11,13–16]However, available structural data are not sufficient to confirm the existence of phase transition from centro to non-centro symmetry atT4since the compound EA2CuCl4shows a phase transition from paraelectricity to FE.[3]Here we devote to the studies of structural phase transition at lower temperatureT4by XRD method.
2. Experimental details
Crystals of EA2CuCl4are obtained by a solvothermal condition method from an aqueous solution, which contains stoichiometrically C2H5NH2·HCl and CuCl2·2H2O. The solution is put in a 100 ml flat beaker then heated for 3 days at
70?C in a thermostat. Then the beaker is placed at RT for several days for crystallization by the method of slow evaporation. The obtained crystals are transparent yellow square plates, typically 5×5 mm2(abplane) in area and 0.25 mm in thickness. The crystals are stored in Ar protective atmosphere. Differential scanning calorimetry (DSC) measurement as an aid to determine the characteristic temperatures of the EA2CuCl4are performed in air atmosphere at a heating rate of 10 K/min. The XRD experiments of powder are performed at various temperatures with increasing temperature using a Rigaku x-ray diffractometer (SmartLab 9 kW)with Cu Kα1radiation (λ=1.541 ?A). Data are collected by step scanning over the angular range 20?≤2θ ≤60?and analyzed by the Rietveld method implemented in the program FULLPROF.Powder XRD patterns have confirmed the structure and phase purity of the obtained samples, revealing the single phase perovskite structure with space groupPbcaat RT,which is in agreement with literature.[17]The single-crystal XRD pattern suggests that the crystal is naturally grown along[100]direction.[10]
3. Results and discussion
The phase transition temperaturesT4of 218 K and 243 K are indicated by DSC measurements during cooling and heating processes, as shown in Figs. 1(a) and 1(b), respectively.The warming and cooling curves show a big thermal hysteresis (25 K), implying a first-order phase transition atT4. The magnitude of heat flow signal ofT4is quite small compared to that ofT2(space group:BbcmtoP21/cwith coolingT). It may imply that the crystal structure is transformed by simple symmetry operator or the incomplete phase transition occurs.It is worth noting that the DSC curves do not show other trace of phase transitions.
Figure 2 shows the powder XRD patterns with 2θ=20?to 60?for the temperatures of 10,70,100,170,200,220,240,260,and 300 K.It is clear that the diffraction single peaks of 24?,29?,36?,and 49?split into double peaks with increasing temperature, which indicates that a structure phase transition has occurred aroundT4. To determine accurate lattice parameters and atomic positions,Rietveld analyses are carried out for all the temperatures. It is found that the structure phase with space group-Pbcaand the initial sets of Cu at 4a(0,0,0)where all Cu sites are crystallographically equivalent changes into a phase with space groupAba2and the sets of Cu at 4a(z,0,0)atT4. The symmetry group on“Cu2+”site is operated from“?1”to“2”. Rietveld analyses of the XRD data at 10 K and 300 K are summarized in Table 1.
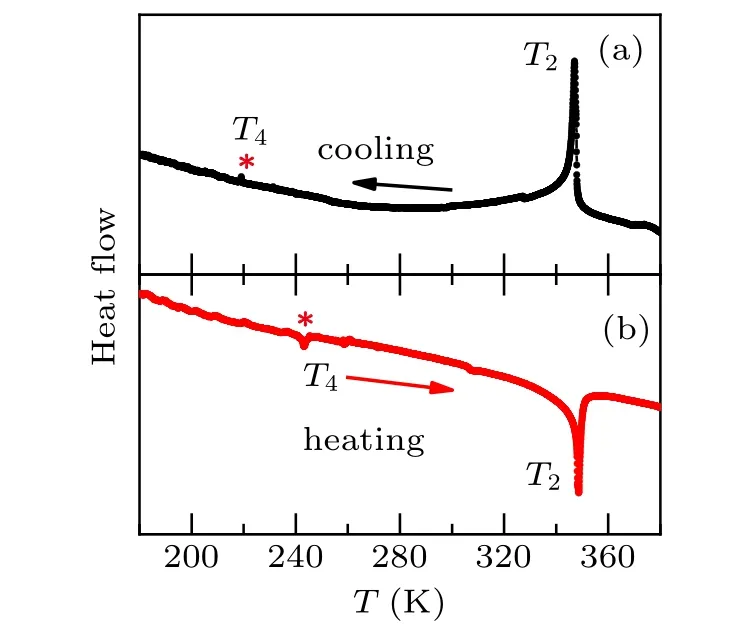
Fig. 1. Experimental DSC curves during cooling (a) and heating (b)processes for EA2CuCl4. The symbol“*”indicates a hysteresis phase transition temperature T4 of 218 and 243 K.

Fig.2. XRD patterns for EA2CuCl4 at various temperatures. The splitting peaks’position with increasing temperature is indicated by symbol“*”.
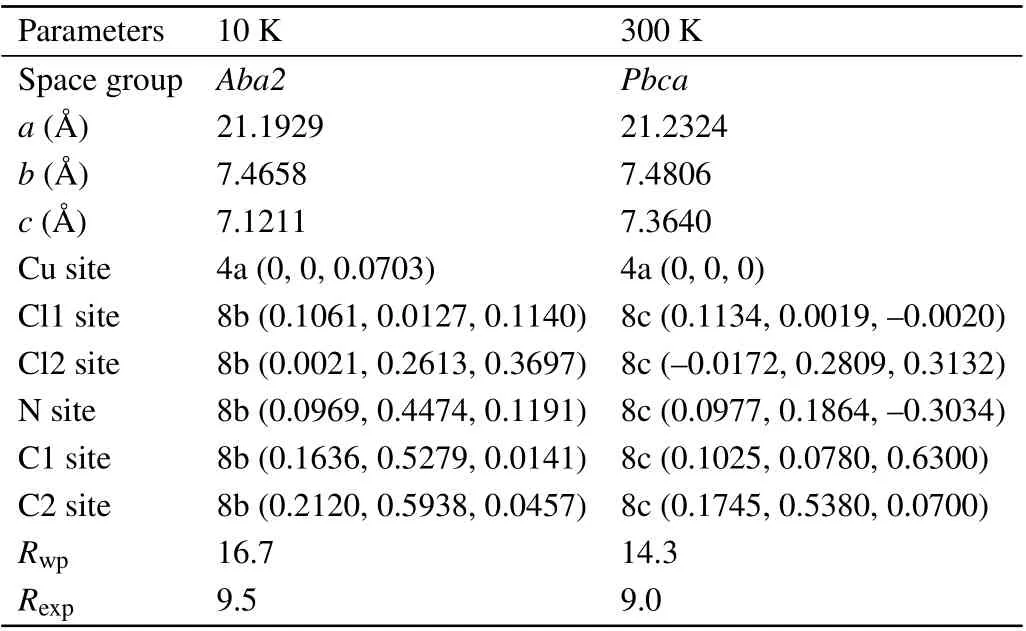
Table 1. Structural parameters and crystallographic positions determined from the Rietveld profile refinements of the powder XRD patterns for EA2CuCl4 at 10 K and 300 K, respectively. Here Cl1 refers to the apical chloride,Cl2 refers to the basal chloride which lies in the plane of the layer.
Figures 3(a)–3(d)show the representative XRD Rietveld refined patterns and the corresponding sketches of crystal structures. XRD patterns at RT [Figs. 3(a) and 3(b)] are almost identical to the data collected by Steadmanet al.[17]The slightly largercaxis at RT may originate from the different crystal growth condition or chemicals.The structure can be refined in the space groupAba2with lowerRwpfactor than that inPbcabelow 240 K [Figs. 3(c) and 3(d)]. From Fig. 3(c),one can see that the secondary phase with space groupPbcais detected with minor trace indicated by the symbol “*” at 10 K despite a perfect fitted structure(Aba2)is revealed withRwp=16.7%. Similarly, at other temperatures below 240 K,the minor second phase (Pbca) is also obtained based on the XRD patterns that show extra diffraction peak [600]. This indicates that the phase transition into main phase with noncentro symmetry(Aba2)from centro symmetry(Pbca)atT4is incomplete, which is in agreement with the behavior of DSC results. Both the incomplete phase transition and simple modification of symmetry operator atT4stunt the intensity of DSC.
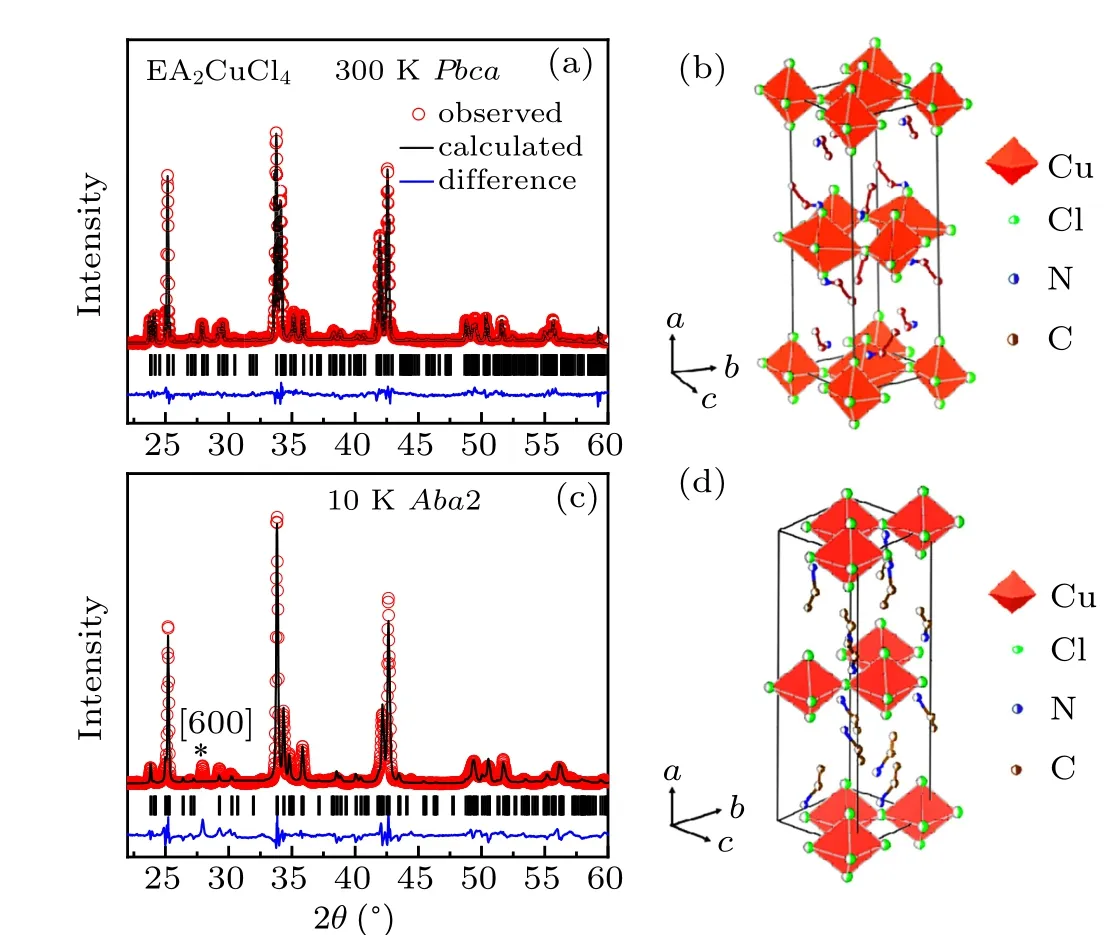
Fig. 3. Representative Rietveld refined patterns and the corresponding sketches of crystal structures for EA2CuCl4 at (a), (b) 300 K and(c),(d)10 K.Observed(dots),calculated(black line),diffraction peaks(vertical lines),and the difference profile from FULLPROF analysis are shown. The symbol“*”in(c)indicates the[600]direction of residual secondary phase with space group Pbca.
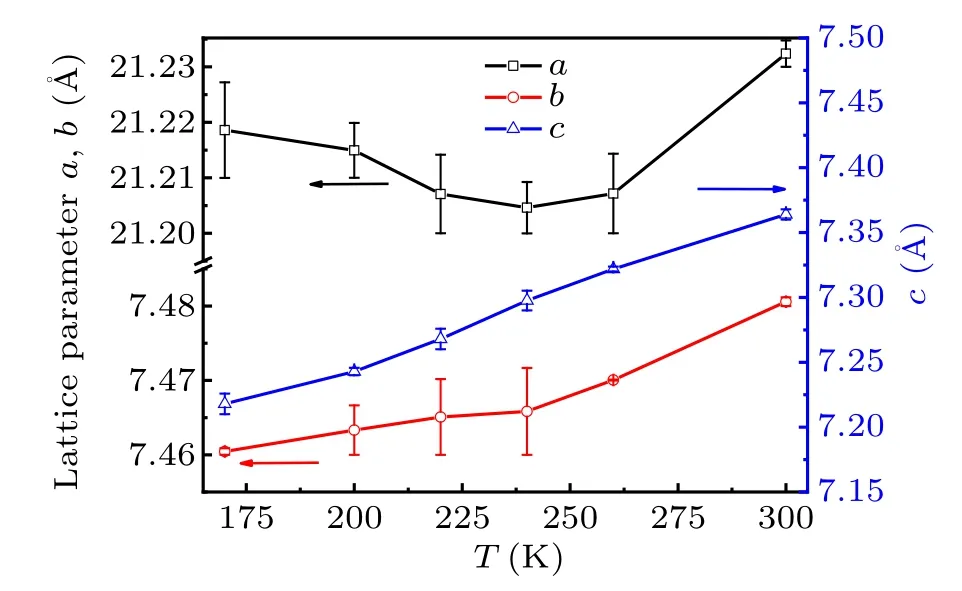
Fig.4. Temperature dependence of the lattice parameters a,b,and c.
The temperature dependence of the lattice parametersa,bandcis shown in Fig.4. It can be seen thatbandcdecrease with the lowering temperature monotonously whileashows a minimum value atT4. It may originate from the release of inplane JT distortion of CuCl6octahedron, as shown in Fig. 5.Upon lowering temperature,the distance of bond Cu–Cl1(outof-plane) almost maintains constant whereas the distance of Cu–Cl2(1)(in-plane)is abruptly squeezed and Cu–Cl2(2)(inplane) is elongated atT4. Figure 5 shows that the bond distances of Cu–Cl2(1) and Cu–Cl2(a) of the CuCl6octahedron converge to the value of Cu–Cl1 bond atT4then maintain almost unchangeable with decreasing temperature. It implies that the organic chain of EA between CuCl6layers is modified alongaaxis and contributes to the expansion ofaaxis belowT4due to the phase transition. At the same time,one can see a distinct distortion which still occurs just belowT4.
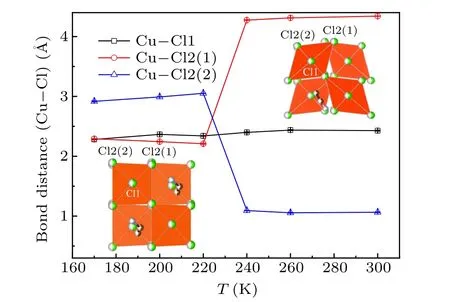
Fig.5. Temperature dependence of the bond distances of Cu–Cl1,Cu–Cl2(1) and Cu–Cl2(2) for CuCl6 octahedron. The insets illustrate the in-plane(bc plane)schematic structures of CuCl6 octahedron for clarification.
Such a structural change could influence the electronic or spin state of the system. Cu2+is a strong JT ion and JT effect (in-plane distortion) of CuCl6octahedron is significantly released. The behavior of the reduction of JT distortion may active the reorientation of EA chains among four equivalent orientations inside the cavity of CuCl6octahedron and induces an order–disorder transition of hydrogen bonds thus leading to FE.[3,13,18,19]The reported dielectric and electric polarization measurements do show appreciable anomalies around 240 K.[10]This experimental result proves that there is coupling between the electrical property and the order parameter.An interpretation on FE emergence aroundT4is addressed.
4. Conclusion
We have observed a structural phase transition fromPbcatoAba2of EA2CuCl4crystal atT4by XRD analysis. An inplane JT distortion of octahedron CuCl6is released and the organic chain is modified at 240 K.This structural phase transition is solidly evidenced to couple the electric property of EA2CuCl4.
- Chinese Physics B的其它文章
- Coarse-grained simulations on interactions between spectrins and phase-separated lipid bilayers?
- Constraints on the kinetic energy of type-Ic supernova explosion from young PSR J1906+0746 in a double neutron star candidate?
- Computational model investigating the effect of magnetic field on neural–astrocyte microcircuit?
- Gas sensor using gold doped copper oxide nanostructured thin films as modified cladding fiber
- Exact explicit solitary wave and periodic wave solutions and their dynamical behaviors for the Schamel–Korteweg–de Vries equation?
- Suppression of ferroresonance using passive memristor emulator

